Introduction
Over the last decades, catheter ablation for the treatment of supraventricular tachycardia has been reported to be a safe and effective therapy in children with a high success rate and low complication rate. Reference Dubin, Jorgensen and Radbill1,Reference Van Hare, Javitz and Carmelli2 Catheter ablation is currently considered as an elective treatment option in children with supraventricular tachycardia as young as 5 years of age and in those patients with asymptomatic pre-excitation in case of short accessory pathway anterograde refractory periods. Reference Brugada, Blom and Sarquella-Brugada3 Data on safety and efficacy in the first large multicentre registries by the North American Pediatric Electrophysiology Society were limited to procedures using radiofrequency catheters and fluoroscopic imaging. Reference Van Hare, Javitz and Carmelli2,Reference Kugler, Danford, Houston and Felix4 Concerns that remained were the relatively long radiation exposure associated with increased risk of developing malignancies later in life and the risk – albeit small – of atrioventricular block. Reference Bacher, Bogaert, Lapere, De Wolf and Thierens5–Reference McFadden, Mooney and Shepherd7
Technology has advanced since that time with the most relevant changes being the standard use of three dimensional electro-anatomical mapping systems to reduce or even avoid fluoroscopy and the use of cryo-energy for slow pathway modification and ablation of septal accessory pathways to reduce the risk of atrioventricular block. Reference Dubin, Jorgensen and Radbill1,Reference Brugada, Blom and Sarquella-Brugada3,Reference Anderson, Rahman and Bradley8 Although several studies have reported the results of paediatric catheter ablations for supraventricular tachycardia using electro-anatomical mapping systems, there are few studies that compare these procedures with those using only fluoroscopic imaging and standard electrophysiology recordings. Reference Anderson, Rahman and Bradley8–Reference Pass, Gates, Gellis, Nappo and Ceresnak11
The aim of this study is to compare the safety and efficacy of radiofrequency and cryoablation performed in a large group of children, in a single center by the same operators, before and after routine use of electro-anatomical mapping systems.
Materials and method
This single-centre study evaluated all paediatric patients (0–18 years of age) referred for catheter ablation of asymptomatic pre-excitation and/or supraventricular tachycardia from January 2014 up until December 2020 at Leiden University Medical Centre and their consecutive redo ablations. An official waiver of ethical approval was granted from the local ethical committee.
Patients were divided into two groups based on the year of the ablation: a group of patients who received a first ablation from 2014 till 2016 ablation and were performed with fluoroscopy, and a second group of patients who received a first ablation from 2018 till 2020 performed with electro-anatomical mapping (EAM). The year 2017 was a transition year in which the use of electro-anatomical mapping was introduced as a standard of care, therefore first ablations performed in 2017 were excluded. Patients in which only an electrophysiology study was performed without ablation, either because of non-inducibility of the tachycardia or in case of an unacceptable risk for damage of the atrioventricular node in asymptomatic patients, were also excluded.
For all patients, baseline characteristics like age, sex, weight, congenital heart disease (CHD), symptoms, and medication were collected. Furthermore, information on procedural outcomes, fluoroscopy time, dose area product, and procedure time was collected to evaluate the result of the procedure, together with the 12 months follow-up data.
The procedure
Depending on the age of the patient, either general anaesthesia (<16 years) or local anaesthesia with sedation was used (≥16 years). At the beginning of a standard procedure, two 6- and one 7-Fr sheath were inserted into the right femoral vein. Next, two quadripolar diagnostic catheters were positioned in the right ventricle and at the His bundle. A decapolar catheter was inserted into the coronary sinus. A standard electrophysiology study was performed to identify arrhythmia mechanism and target site of ablation, using standard burst pacing or extra stimulation pacing manoeuvres and standard atrioventricular ring mapping techniques. When needed, isoprenaline infusion (1–5 μg/minute) was used to induce tachycardia. Following this, detailed mapping and substrate localization were performed using fluoroscopy only in the first group or using the 3D electro-anatomical mapping system in the second group (Ensite Velocity System, St. Jude Medical, St. Paul, MN, USA, or CARTO; Biosense Webster Inc, Waterloo, Belgium). In case of a left-sided pathway, a transseptal puncture was performed guided by fluoroscopy and intra-cardiac echocardiography or trans-oesophageal echocardiography.
Based on substrate characteristics, it was decided whether to use cryo- or radiofrequency energy; cryo-energy was typically used in atrio-ventricular nodal re-entry tachycardia (AVNRT) cases and in para-Hissian accessory pathways. Radiofrequency ablations were performed using 7-Fr steerable radiofrequency catheter (BIOTRONIK SE & CO, KG, Berlin, Germany) in combination with the Ensite Velocity System; or, in case the CARTO system was used, mapping and ablation were performed using a 3.5 mm irrigated-tip catheter (NaviStar Thermocool, Smarttouch, Biosense Webster Inc., CA, USA). During radiofrequency, energy applications had a target of 30–50 W and 60°C for a maximum of 60 seconds. The decision for an irrigated-tip catheter was made by the physician based on substrate characteristics and impedance during radiofrequency ablation. Cryoablations were performed using 7-Fr steerable catheter with a 6-mm-tip (Medtronic, Minneapolis, MN, USA), in combination with the Ensite Velocity System. Cryo energy applications had a target of −70°C for a maximum of 240 seconds. When successful ablation was achieved, there was a waiting period of at least 30 minutes to test for possible recurrences using pacing manoeuvres, with isoprenaline and adenosine if applicable. Procedure time was measured from the moment the patient entered the catheter laboratory till they left the catheter laboratory.
Success rate
Immediate success was defined as (1) retrograde and/or antegrade block in case of an accessory pathway; (2) maximum of 1 echobeat and non-inducibility of the tachycardia after slow-pathway ablation or modification; (3) non-inducibility of the tachycardia after ablation of a focal atrial tachycardia (AT) atrial focus; and (4) a confirmed block line and non-inducibility after ablation of an atrial flutter. Recurrence was defined as a documented recurrence of pre-excitation or tachycardia, or recurrence of symptoms requiring (re)start of medication. Regular follow-up consisted of an outpatient visit at our own centre 3–6 months after the ablation including a 12-lead ECG, if indicated additional ECG diagnostics (Holter registration, event recording, smart watch ECG recording) and strict instruction to get in contact in case of recurrence of symptoms. Overall success is defined as the combined success of first ablations and redo ablations within 12 months.
Statistical analysis
Statistical analysis was performed using the IBM SPSS software version 25. Continuous data that were normally distributed were analysed using an independent t-test and are always presented as mean ± standard deviation. Continuous data that were not normally distributed were analysed using a Mann–Whitney U test and are presented as median ± interquartile range. To compare the categorical data between groups, Chi-square test or Fisher’s exact test was performed. Categorical data are always presented as absolute count and/or percentage. A p-value of <0.05 was considered significant.
Results
Patient demographics
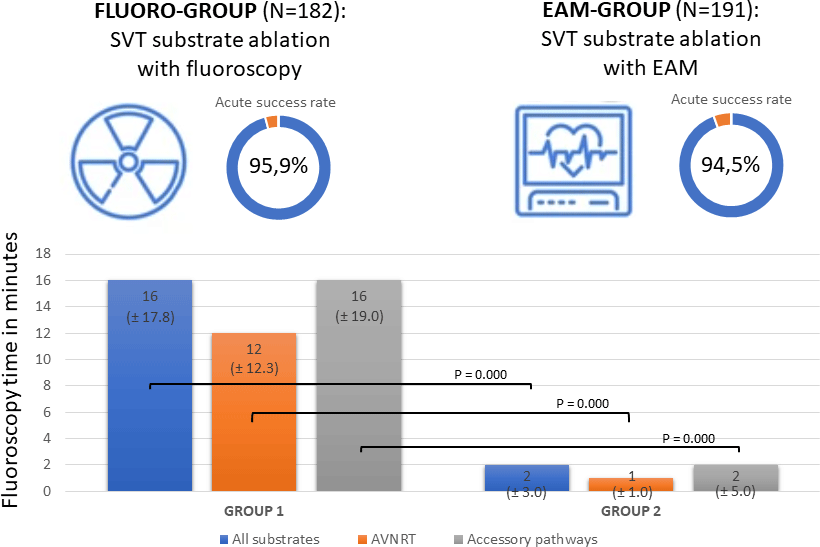
In the study period, 403 patients underwent a total of 425 electrophysiology procedures for asymptomatic pre-excitation or supraventricular tachycardia. Patients in which only an electrophysiology (EP) study was performed, and no ablation energy was used, were excluded (n = 52). The cohort of patients that was included (n = 351) was divided into two groups. The fluoro-group (170 patients) consists of 182 procedures in 2014, 2015, and 2016. The electro-anatomical mapping group (181 patients) contains 191 procedures in 2018, 2019, and 2020.
The baseline characteristics of the cohort are presented in Table 1. Overall, the groups were similar at baseline; patients in the electro-anatomical mapping- group were slightly older with a mean of 14.5 years versus 14.0 years (p = 0.041), and medication use prior to ablation was higher in the fluoro-group (50% versus 37%, p = .004). There were more males than females in both groups but no differences between the two groups in male – female ratio. Accessory pathways formed the largest group of substrates with 143 substrates in males and 70 in females (including redo-ablations in 9 patients). For other substrates, we found a more equal distribution in males and females. Indication for ablation were based on symptoms in 90% in both groups or asymptomatic pre-excitation.
Table 1. Baseline patient characteristics of the procedures in the fluoro-group and the EAM-group

Procedural outcomes
In general, an acute success rate of first ablations of 95.2% was observed in the total cohort of patients with 95.9% in the fluoro-group and 94.5% in the electro-anatomical mapping group (p = .539) as presented in Table 2. Ablations of AVNRT had an acute success rate of 96.4% in the fluoro-group and 100% in the electro-anatomical mapping group (p = .219), ablations of accessory pathways 96.1% in the fluoro-group versus 91.2% in the electro-anatomical mapping group (p = .152) and ablations of AT 88.9% in the fluoro-group versus 92.3% in the electro-anatomical mapping group (p = 1.000).
Table 2. Procedural outcomes, acute success rate was calculated based on all performed first ablations. Recurrence data were calculated based on the first ablations that were successful.

AVNRT = atrio-ventricular nodal re-entry tachycardia, AT = atrial tachycardia, AFL = atrial fultter.
The overall recurrence rate within 12 months after the first procedure was 6.1% in the fluoro-group and 6.4% in the electro-anatomical mapping group (Table 2). Redo procedures were not performed in all patients with a failed first ablation or a recurrence of the tachycardia, depending on the location of the electro-anatomical substrate and clinical burden of the tachycardia. In the patients who did receive a re-do procedure, the success rate was 83% in the fluoro-group and 80% in the electro-anatomical mapping group. This results in overall success rate of 95.9% in the fluoro-group and 92.8% in the electro-anatomical mapping group (p = 0.216) with an overall total of 94.3%. Ablations of AVNRT had an overall success rate of 98.2% in the fluoro-group and 93.7% in the electro-anatomical mapping group (p = .369), accessory pathways 95.1% in the fluoro-group versus 91.2% in the electro-anatomical mapping group (p = .390) and AT 88.9% in the electro-anatomical mapping group versus 92.3% in the electro-anatomical mapping group (p = 1.000). The overall success rate of atrial flutter (AFL) was 100% in both groups with no recurrences.
The procedure time was not reduced statistically, in the electro-anatomical mapping group, the procedure time was 179.00 minutes (interquartile range ± 60.00) versus of a mean of 184.50 minutes (interquartile range ± 81.50) in the fluoro group, as is shown in Table 3. However, the fluoroscopy time differed significantly between the two groups, with a median of 16.00 minutes (interquartile range ± 17.75) in the fluoro-group and 2.00 minutes (interquartile range ± 3.00) in the electro-anatomical mapping group (p = .000). In accordance, the dose area product was significantly reduced from a median of 210.5 µGym2 (interquartile range ± 249.3) in the fluoro-group to 32.9 µGym2 (interquartile range ± 78.6) in the electro-anatomical mapping group (p = .000). In 24 substrates in the electro-anatomical mapping group, no fluoroscopy was used at all.
Table 3. Procedural parameters

* The sub-group of atrial flutter ablation procedures was too small to perform statistical analysis, therefore only the median is presented.
Other procedure-related parameters did not differ between the fluoro-group and electro-anatomical mapping group. The energy source used to perform the ablation was cryo-energy in 30% of the procedures; 25% in the fluoro-group versus 34% in the electro-anatomical mapping group (p = .146). In the fluoro-group, 60% of the accessory pathways were left sided versus 59% in the electro-anatomical mapping group (p = .386). These required transseptal puncture with intra-cardiac echocardiography or trans-oesophageal echocardiography guidance in absence of a foramen ovale.
In total, four complications occurred in the fluoro-group and none in the electro-anatomical mapping group (Table 3). Three patients had a transient atrioventricular block, which recovered during the procedure. One patient had a groin vessel injury that required treatment because of a pseudoaneurysm of the right common femoral artery. He was treated with thrombin injection and no late complications occurred.
Discussion
To our knowledge, this is the largest single centre paediatric study to show that the results of ablations of supraventricular tachycardia substrates in children remain excellent, with a high acute success rate of 94.5% and a low recurrence rate of 6.4%, while reducing the fluoroscopy time significantly by the use of electro-anatomical mapping systems from 16 to 2 minutes, in accordance with a significant reduction of the median dose area product from 210.5 µGym2 to 32.9 µGym2. Procedure time and complications decreased as well in the group using electro-anatomical mapping, however, not statistically significant. These results demonstrate that the use of electro-anatomical mapping is safe and very effective in the treatment of ablations in children with supraventricular tachycardia and associated with a significant decrease in fluoroscopy time and dose area product.
The baseline characteristics in the two groups of patients were the same, except for medication use prior to ablation which was higher in the fluoro-group. This might reflect that in the electro-anatomical mapping group ablation was more often the primary choice of treatment, instead of the start of anti-arrhythmic medication. There was no significant difference in sex distribution at baseline; however, there is a high male–female ratio in our patient population, with a total of 61.2% of the substrates found in males. Differences in male–female ratio have been previously described with accessory pathways being more common in males and AVNRT more common in females. Reference Anand, Rosenthal, Van Hare and Snyder12,Reference Rodriguez, de Chillou and Schlapfer13
The acute success rate in our study of 96.4% in AVNRT and 96.1% in accessory pathways in the fluoro-group is similar to results of large landmark study that used data from the Prospective Assessment after Pediatric Cardiac Ablation Cohort in 2004 (n = 2761). They found an acute success rate of 97–99% in AVNRT and 92–97% in AVRT without the use of electro-anatomical mapping (mean fluoroscopy time 38.3 ± 33 minute). Reference Kugler, Danford, Houston and Felix4 In the newer Multicenter Pediatric and Congenital EP Quality Initiative registry from 2019 (n = 1417), electro-anatomical mapping was used in 95% of the procedures (mean fluoroscopy time 7.0 ± 9.2 minute) with an acute success rate of 98% in AVNRT and 95–97% in AVRT. Reference Dubin, Jorgensen and Radbill1 The “EUROPA” Registry with 683 patients published in 2021 showed a success rate of 94% in AVRT and 99% in AVNRT. Electro-anatomical mapping was used in 628 patients with a mean fluoroscopy time of 4.9 ± 6.8 minutes. Reference Krause, Paul and Bella14 In addition, there are smaller studies that report on ablation outcomes in combination with the use of electro-anatomical mapping in paediatric populations, but often focus on only one type of arrythmia or one type of ablation energy. For example, a recent study by Jan et al with 62 patients (<19 years) examined the outcomes of ablations of AVNRT without any use of fluoroscopy specifically and found an acute success rate of 98% in their paediatric population. Reference Jan, Yazici and Kalinsek10 Another study from Elkiran et al focused on the outcomes of ablations of AT whilst using electro-anatomical mapping, with an acute success rate of 87%. Their study population consisted of 39 patients; the ablation was performed without fluoroscopy in 25 out of the 39 patients and the remaining fluoroscopy time was 5.53 ± 5.22 minutes. Reference Elkiran, Akdeniz, Karacan and Tuzcu9 These studies demonstrate the possibility of completely eliminating fluoroscopy.
Recently, Anderson et al. published a study in which they compared outcomes of paediatric ablations from a previously published Prospective Assessment after Pediatric Cardiac Ablation registry in 2004 using fluoroscopy, to the more recent Catheter Ablation with Reduction or Elimination of Fluoroscopy registry in which electro-anatomical mapping was used. Reference Anderson, Rahman and Bradley8 The acute success rate was similar in both registries, with a clear and significant reduction of fluoroscopy time (mean 38 minutes versus 1 minutes). Though this study has a large study population (n = 786), the authors conclude that there are a number of potential confounders in comparing a recent cohort to a previous registry, due to changes in treatment strategy other than the introduction of electro-anatomical mapping. For example, in the registry published in 2004 cryo-ablation was not used, but was frequently used in the more recent registry. The strength of the present single centre study is that the only difference between the groups was the introduction of routine use of electro-anatomical mapping. Otherwise, the two groups had similar patient characteristics and supraventricular tachycardia substrates and were both treated in recent years by the same two operators with the standard use of cryo-energy for AVNRT and septal pathways.
The success rates we present in this study are also comparable with the results in large studies in adults. A meta-analysis of 7693 patients calculated an acute success rate of 90.9% for AVRTs and 94.3% for AVNRTs and complication rate of 2.8% for AVRTs and 3.0% for AVNRTs with fluoroscopy. Reference Spector, Reynolds and Calkins15 Another large study (n = 3060) in adults compared a group of patients ablated with electro-anatomical mapping only (zero-fluoroscopy) to a group ablated with a conventional fluoroscopy approach (fluoroscopy with or without electro-anatomical mapping). Reference Chen, Wang and Proietti16 They found no significant difference between groups, the immediate success rate was 98.8% in the zero-fluoroscopy group and 99.2% in the conventional fluoroscopy group. The fluoroscopy time differed significantly, from 6.9 in the conventional fluoroscopy group to (per definition) 0 minutes in the zero-fluoroscopy group. However, in this study, patients were assigned to a group after they underwent transesophageal EP study and during the procedure some patients even changed groups. Razminia et al presented a single centre retrospective analysis of five hundred adult patients with supraventricular tachycardia and premature ventricular complex/ventricular tachycardia. Procedures were performed without fluoroscopy, with the use of electro-anatomical mapping in combination with intra-cardiac echocardiography. The reported acute success rate was high, almost 100%. Reference Razminia, Willoughby and Demo17
Limitations
Limitations of this study are its retrospective observational design in which two subsequent cohorts of patients were compared. Data on the number of applications and ablation time were not documented during the first years and therefore could not be included in this study. However, since it is a single centre study with the same two electrophysiologists in both cohorts and with no other changes in treating method than the introduction of electro-anatomical mapping as standard of care, we do feel that this study provides clear insight in the value of electro-anatomical mapping in reducing fluoroscopy time in a paediatric population. Another limitation of this study could be the follow-up duration of 1 year. Recurrences that may have occurred after 12 months are not taken into account.
Conclusion
This study shows the results of the largest single-center study, in which catheter ablation remains a highly effective and safe treatment therapy for children with supraventricular tachycardia substrates after the introduction of electro-anatomical mapping as a standard of care. The fluoroscopy time and dose area product are reduced significantly, while the success rate is still high, and recurrence and complication rate remain low.
Acknowledgements
None.
Financial support
This investigator-initiated study did not receive any grants or funding.
Competing interests
The authors have nothing to disclose.
Ethical standard
An official waiver of ethical approval was granted from the local ethical committee.