BACKGROUND
Concussions were reported to be responsible for 133,000 visits to Ontario’s Emergency Departments (EDs) in 2009.Reference Fu, Jing and McFaull 1 Children with concussions represented 90% of the hospitalization days among all children admitted for all levels of traumatic brain injury.Reference Kraus, Fife and Conroy 2 Between 55% to 90% of patients who sustained a concussion, also suffered from post-concussion symptoms at one week following the concussion, and approximately 30% had symptoms at one month.Reference King, Brughelli and Hume 3 - Reference Zemek, Barrowman and Freedman 7 These symptoms include cognitive (memory loss, attention deficit, etc.), somatic (headache, fatigue, nausea) or psychological (depression, irritability, etc.) in nature.
Most patients requiring medical attention following a concussion are initially evaluated in the ED. According to current guidelines, the standard of care for concussion is limited to the recommendation of a period of activity restriction (physical and cognitive rest) until full resolution of symptoms related to the injury.Reference McCrory, Meeuwisse and Aubry 8 - Reference Purcell 13
Over the past few years, there has been a growing trend among pediatric ED physicians to prescribe ondansetron for children with concussions who present with vomiting.Reference Sturm, Simon and Khan 14 , Reference Sturm, Pierzchala and Simon 15 A retrospective study reported that in children who sustained a mild traumatic brain injury and had normal head computed tomography (CT), the use of was associated with a significantly reduced risk of a return visit to the ED in the following 72 hours.Reference Sturm, Simon and Khan 16 Reduction in nausea and vomiting symptoms in the first hours could improve rest in the early days following concussion and promote faster recovery. Clinical practice is changing and, despite the lack of clear supporting evidence, ondansetron is more frequently used in the management of children with concussion. For example, one ED in the USA reported an increase in the use of ondansetron in children with concussion form 3% to 23% between 2003 and 2010.Reference Sturm, Simon and Khan 14 Considering its increased use over the past 10 years,Reference Sturm, Simon and Khan 14 the presumed positive effect of ondansetron needs to be properly evaluated in a quantitative manner before it becomes commonly used.
The primary objective of this pilot study was to evaluate the feasibility of a randomized controlled trial (RCT) evaluating the effect of ondansetron in comparison to placebo on the persistence of post-concussion symptoms at one week and one month following concussion in children.
METHODS
Ethics
The study protocol was approved by the Sainte-Justine Research Institute review board and received a non-objection letter form Health Canada. To participate, all children and a parental authority provided written informed consent before randomisation. The study was registered at the ClinicalTrials.gov website (#NCT01815125).
Design
This was a pilot study for a blinded, randomized controlled trial of one dose of either ondansetron or placebo in children who visited a single ED in the 24 hours following a concussion.
Setting
The study was conducted at Sainte-Justine University Health Centre, a tertiary care pediatric hospital with approximately 70,000 visits annually. Recruitment occurred during the presence of research assistants or nurses in 2013 and 2014. Research assistants were present from 9 AM to 4 PM during week days and uncommonly present during week-end at the same schedule.
Participants
To be included in the study, children had to meet all inclusion criteria and have none of the exclusion criteria.
Inclusion criteria (all needed):
-
1) Children aged between 8 and 17 years old. We limited our study to this small spectrum of age because this is the age group for which our measurement tool was validated.
-
2) Occurrence of a concussion as defined by the presence of a head trauma, a Glasgow Coma Scale of 13 to 15 and at least one of the three following criteria 17 , Reference McCrory, Meeuwisse and Johnston 18 :
-
∙ Any period of loss of consciousness.
-
∙ Any loss of memory for events immediately before or after the accident.
-
∙ Any alteration in mental state at the time of the accident (e.g., feeling dazed, disoriented).
And the absence of the following criteria:
-
∙ Glasgow Coma Scale <13, 30 minutes post-accident.
-
3) The trauma occurred in the preceding 24 hours.
Exclusion criteria (none present):
-
1) Inability to obtain proper written informed consent (language barrier, absence of a parental authority, developmental delay, intoxication, patient too confused to consent according to the treating physician).
-
2) Known allergic reaction or intolerance to ondansetron.
-
3) Known rhythm disturbance or cardiac pathology, or history of sudden death in the proximal family.
-
4) Patients who were taking medication which could increase the QT interval.
-
5) Patients who received ondansetron in the previous 24 hours.
-
6) Any abnormality on radiological studies, including any bleeding in the brain or skull fracture.
-
7) Multi-system injuries with treatment requiring admission to hospital or procedural sedation in the ED.
Intervention
The intervention of interest was the administration of one dose of 8 mg of oral ondansetron.Reference Freedman, Adler and Seshadri 19 There was no previous study to determine the optimal duration of treatment. Previous studies have failed to show an impact of multiple doses in comparison to a single dose for other disease.Reference Freedman, Ali and Oleszczuk 20 The control group received an identical looking/tasting pill as placebo.
Randomization
A biostatistician not involved in the analysis generated a randomization table using a computer generated sequence according to the following request: children were stratified by the presence or absence of vomiting and by the delay between trauma and intervention (<12 hours, 12 to 23 hours).
Blinding
A research pharmacist, not involved in the treatment of the patients, prepared the study medication by putting either ondansetron or placebo in an opaque capsule. The study medication was coded in advance according to the list generated by the biostatistician.
Outcomes of the pilot study
Outcomes for the pilot study included: the proportion of concussed children who were screened and found eligible to participate, the proportion of eligible patients who were invited to participate and agreed to participate, and the proportion of study participants who were compliant with the intervention. Of those participants who were compliant with the intervention, the proportion who chose electronic follow-up and completed the one-week and one-month follow-up questionnaire.
Outcomes of the randomized controlled trial
The primary clinical outcome was the persistence of post-concussive symptoms at one week and one month following the injury. In our study, persistence of post-concussive symptoms was defined as an increase from pre-concussion baseline of at least three symptoms of the Post-Concussion Symptom Inventory (PCSI). The PCSI is a self-reporting tool evaluating the presence of 25 symptoms (on a 3-point Likert scale) for children 8-12 years of age or 26 symptoms (on a 7-point Likert scale) for children 13-17 years of age. An increase of two points or more from pre-injury in any symptom was considered clinically significant.Reference Barlow, Crawford and Stevenson 21 Secondary outcomes were the mean number of PCSI symptoms, the mean number of school days missed, the number of days of sport activity restriction, the time before full recovery, health care utilization, and side effects. PCSI scores were calculated differently for the 8-12 years and 13-17 years age groups. PCSI scores were standardized using percentage in order to be able to compare results from both age groups. Side effects included diarrhea and constipation, in addition to symptoms related to concussion (worsening headache, dizziness, sleepiness, etc.), abdominal pain on palpitation. All outcomes were measured at one week and one month following concussion.
Several independent variables were measured at baseline related to the patients’ age, sex, past medical history of traumatic brain injury (TBI) using the brain injury questionnaire, the type of accident (road, sports, fall, non-accidental, other) and the symptoms at time of randomisation (PCSI, vomiting, etc.).
Compliance and quality control
Compliance was measured by asking the parents/patients how many pills of the study medication were consumed. Several precautions were taken in order to minimize potential biases. A screening log was maintained to record the number of patients screened, excluded, missed, or not randomized for any other reason. The diagnosis and reason for exclusion (ineligible or refused patients) were recorded in order to detect any selection bias.
Procedure
All children who fulfilled all of the inclusion criteria and met none of the exclusion criteria were invited to participate. After informed consent was obtained, children and parents were invited to complete a computerized questionnaire in order to provide baseline data (status pre-injury using the PCSI). Then they were asked to complete another standardized questionnaire to evaluate symptoms secondary to the concussion (PCSI at the moment of randomisation). Children received the study medication and standardized instruction regarding management of concussion at home. Study medication was provided as two pills of 4 mg of ondansetron/placebo per sample. The standardized instructions followed guidelines provided by the Canadian Pediatric Society based on the world consensus on concussion. 22
Patients and parents were asked to provide contact information, including phone numbers and email addresses prior to ED departure. Depending on their preferences and the availability of computer access at home, patients were contacted one week later to complete the follow-up questionnaire. Electronic follow-up questionnaires were sent to the parental email address one week and then at one month following the concussion. The same schedule and questionnaires were used for the families that opted for telephone follow-up. In the event of incomplete electronic survey within 24 hours of receipt, a second email was sent. In the absence of response, the family was contacted by telephone for a phone interview. The follow-up questionnaire asked children questions related to the persistence of symptoms using the PCSI, school/work absenteeism, duration of symptoms, and any side effects using a standardized datasheet. Answers to the questionnaires were provided by the children with the assistance of a parent.
Statistical analysis
All data were entered in an Excel database (Microsoft Inc., Richmond, WA) and analyzed with SPSS v21 (IBM Software Inc., Armonk, NY). To assess balance across arms, baseline demographic (i.e., gender, age) and clinical data (i.e., presence of vomiting, time from trauma, past history of TBI or other health problems) of patients were compared between arms. The primary analysis was acceptability proportion measured by dividing the number of children/families who accepted participation divided by the total number of children/families invited. Other primary analysis was the proportion of participants who were compliant and provided all necessary information to measure the persistence of post-concussive symptoms at one week and one month following the injury. Another analysis was performed to calculate the proportion of participants who opted for an electronic follow-up.
The exploratory analysis was the comparison between the two groups of the proportion of children who had persistent concussive symptoms at one week and one month post-injury using Fisher’s exact test and an intention-to-treat principle. Other analyses included: comparison of the mean duration of symptoms using linear regression; comparison of the mean number of school days missed using linear regression; comparison of the mean PCIS at one week and at one month; and the proportion of patient who presented side effects.
RESULTS
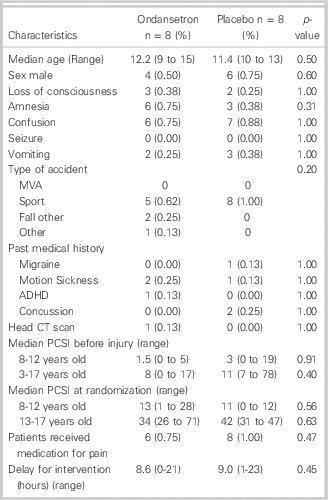
Between April 2013 and January 2014, a total of 281 children visited the ED for a concussion (Figure 1). Among them, 108 were screened for eligibility because they visited the ED during the presence of a research assistant. A total of 36 families were invited to participate in the study because they meet inclusion/exclusion criteria. The main reason for exclusions were: young age and delayed consult. Sixteen children/families (44%) agreed to participate and provided informed consent. The participants were all randomized to the study medication and were all compliant. They all completed the follow-up questionnaires both at one week and one month. Baseline demographics of the study participants were similar between the two study groups (Table 1). The median age of participants was 12 years and the median delay from injury to presentation was 4 hours (range 1-23 hours). The most common cause of concussion was a sport-related accident. Only two participants had had previous concussions and none received medication for vomiting before study recruitment.
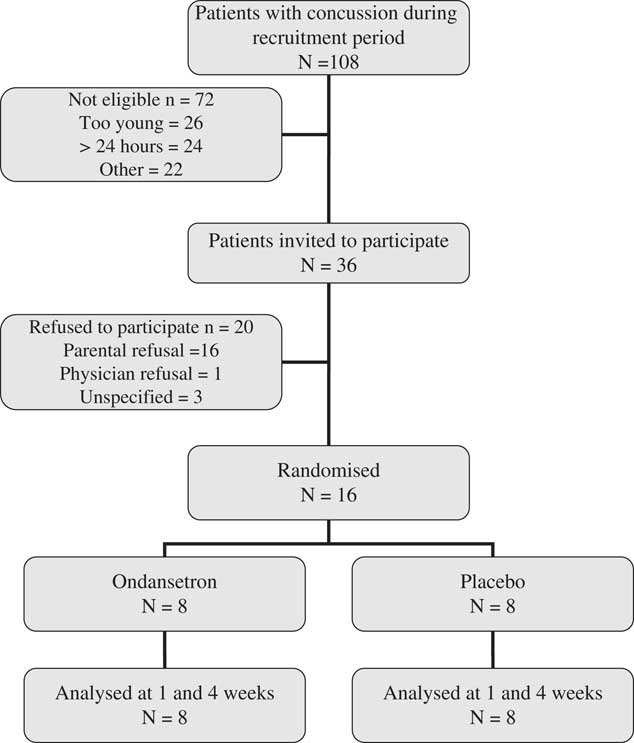
Figure 1 Flow-chart of the study participants in 8 months.
Table 1 Baseline demographics of randomized patients by intervention arm (N=16)
Thirteen of the 16 participants (81%) opted for the electronic follow-up questionnaire, and responded to both electronic questionnaires (one week and one month) at the first attempt without electronic or telephone reminder. All participants provided all necessary data for the measurement of the primary outcome at one week and one month (100% follow-up rate).
At one week, 50% of the children who received ondansetron reported persistence of post-concussive symptoms compared to 63% in the placebo group (Table 2). This did not reach statistical significance (difference: 13%; exact 95% CI: –40% to 69%). At one month, 2/8 children in the ondansetron group reported persistent post-concussive symptoms compared to 5/8 in the placebo group, but this failed to reach statistical significance (difference: 37%; exact 95% CI: –15% to 90%).
Table 2 Outcome measures for randomized patients by intervention arm (N=16)
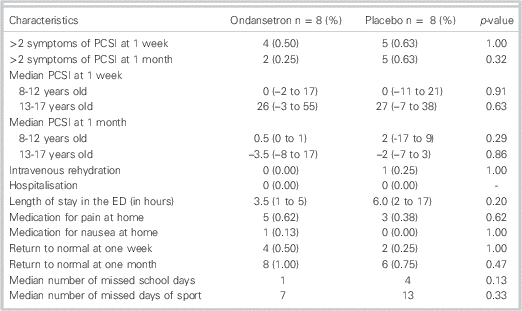
There were no differences between the groups for multiple secondary outcomes (Table 2). No patient was admitted (re-hospitalized) from either groups and only one patient received an intravenous infusion (normal saline) in the placebo group because he was kept in observation for 17 hours. The mean PCSI at one week and one month and the use of medication (for pain or nausea/vomiting) at home were similar between the two groups. Patients who received the intervention returned to school earlier (median 1 day v. 4 days), but there was no statistical difference for length of stay in the ED (3.5 hours v. 6 hours) and number of missed days of sport (7 days v. 13 days).
DISCUSSION
This study demonstrated the feasibility of a RCT to evaluate the impact of ondansetron for concussion symptoms in children. While the number of participants was small, the follow-up rate of 100% was reassuring in terms of the possibility of conducting a larger study using a similar design. Approximately 45% of the invited families agreed to participate; and this rate of participation should be considered when estimating sample size and feasibility of the study. The fact that 81% of the families decided to use the electronic follow-up method and that we had excellent response rates also supports the usefulness of this follow-up strategy. Because this was a pilot study, it was not powered to detect a statistical difference between the two groups.
In the past, few studies have evaluated potential treatments for patients suffering from concussion. Three systematic reviews reported only one clinical trial of a pharmacological intervention for concussion.Reference Borg, Holm and Peloso 23 - Reference Comper, Bisschop and Carnide 25 One study showed no effect of nasal vasopressin on cognitive symptoms secondary to mild TBI.Reference Bohnen, Twijnstra and Jolles 26 A systematic review identified studies investigating interventions initiated in the ED for short-term (one week) and medium term (one month) outcomes in adults and children who sustained mild TBI.Reference Gravel, D’Angelo and Carriere 24 The review identified 15 randomized controlled trials. Among them, one evaluated a pharmacological intervention (DDAVP),Reference Filipova, Jung and Filip 27 two evaluated activity restriction (full bed rest,Reference de Kruijk, Leffers and Meerhoff 28 hospitalizationReference Lowdon, Briggs and Cockin 29 ), one evaluated head tomodensitometry v. admission,Reference af Geijerstam, Oredsson and Britton 30 four evaluated an information intervention (pamphlet, information session at the ED)Reference Casey, Ludwig and McCormick 31 - Reference Hinkle, Alves and Rimell 34 and seven evaluated diverse follow-up interventions (in neuropsychology, phone follow-up, etc.).Reference Ponsford, Willmott and Rothwell 33 , Reference Elgmark, Emanuelson and Bjorklund 35 - Reference Wade, Crawford and Wenden 40 Since the publication of the last systematic review, a small randomized controlled trial reported fewer headaches associated with hypertonic intravenous saline v. normal saline for 44 concussed children who needed intravenous access for CT-scan.Reference Lumba-Brown, Harley and Lucio 41
Ondansetron usage remained limited to patients who suffered from nausea following chemotherapy until a decade ago when research began to emerge supporting its use in children with acute gastroenteritis.Reference Freedman, Adler and Seshadri 19 , Reference Reeves, Shannon and Fleisher 42 Although it is primarily used for children visiting the ED for gastroenteritis,Reference Kharbanda, Hall and Shah 43 it is not constrained to that group of children. A retrospective study of ondansetron in the ED reported that 38% (n=12,620) of prescriptions were for reasons other than gastroenteritis in the ED.Reference Sturm, Pierzchala and Simon 15 Other indications of ondansetron are post-operativem,Reference Fujii 44 - Reference Bolton, Myles and Nolan 46 chemotherapy, radiotherapy,Reference Lindley, Goodin and McCune 47 Reference Spitzer, Friedman and Bushnell 48 sedation,Reference Langston, Wathen and Roback 49 or during pregnancy.Reference Reichmann and Kirkbride 50 Recently, ondansetron has been prescribed for brain-related diseases like obsessive-compulsive disordersReference Fontenelle, Oostermeijer and Harrison 51 or drug/alcohol addictions,Reference Brackins, Brahm and Kissack 52 - Reference Swift 54 suggesting that it may influence other symptoms than vomiting. Ondansetron may improve recovery from concussion by decreasing early symptoms of nausea and vomiting, decreasing energy demands, and enhancing brain rest. Nausea and vomiting at initial presentation is associated with persistence of post-concussion symptoms at three months.Reference Babcock, Byczkowski and Wade 55 Limitation of vomiting, therefore, may theoretically decrease energy demands and improve rest. This could improve recovery because it has been previously demonstrated that persistence of concussion symptoms is inversely related to rest quality.Reference Boutis, Cogollo and Fischer 56 , Reference Moser, Glatts and Schatz 57 Although there is a paucity of literature specifically describing the use of ondansetron for patients with head trauma, there is evidence for two patients successfully treated with ondansetron for vomiting following neurosurgical brain trauma.Reference Kleinerman, Deppe and Sargent 58
Our study demonstrated the willingness of families to respond to an electronic follow-up questionnaire. This is consistent with previous studies using a similar follow-up strategy for concussed children,Reference Zemek, Barrowman and Freedman 7 , Reference Zemek, Clarkin and Farion 59 , Reference Pasek, Locasto and Reichard 60 Using this strategy has many advantages including the option for children to answer the questionnaire when they desire, the immediate transfer of data to a database, and inherent cost savings. However, it may be associated with limitations related to the absence of supervision by a professional to ensure that responses are appropriate. Another important aspect of our study is the promising findings on the persistence of post-concussion symptoms. As mentioned, most children have persistent symptoms at one week post-concussion and one third of children at one month;Reference Carroll, Cassidy and Peloso 5 , Reference Zemek, Barrowman and Freedman 7 and there is no proven effective treatment for any outcome following concussion.Reference Gravel, D’Angelo and Carriere 24 Concussion has clinical, societal, and financial impacts. Our results suggest that ondansetron could potentially be useful to limit persistence of post-concussion symptoms. This would improve quality of life for children while decreasing societal cost resulting from work/school absenteeism.
There are limitations to this study. The study was conducted without financial support, hence research assistant coverage was very low. This explains why only 108/281 children with head trauma were assessed for eligibility. The small sample size limited our ability to show statistical differences or equivalences between the two groups. However, this was not the primary objective of the study. The study was performed in a single setting. The feasibility demonstrated in this setting may not be reproducible in another setting. Children were not evaluated by a physician at follow-up and participants may have under or over-estimated their ability to return to normal activity. Children received only one dose of study medication. While this permitted excellent compliance, more doses may have been a better treatment option to decrease long-term symptoms. Finally, there was no standardization of co-interventions.
CONCLUSION
In conclusion, this pilot study demonstrated the feasibility of a RCTto evaluate the impact of ondansetron for concussion in children. Approximately one-third of children who sustained a head concussion were eligible for the study and one-half of eligible patients agreed to participate. Also, more than 80% chose an electronic follow-up method for the assessment of outcomes. Based on these results, a larger, more statistically powered study is required and feasible.
Acknowledgement
The investigators would like to thank the following medical students who volunteered as research assistants for the project: Chadey Assane, Delphine Bélanger, Liliann Bérubé-Thibeault, Gabrielle Bibas, PhilippeJoly, Camille Bédard-Gauthier, Annie-Jade L’Espérance, Christophe Salois and Patrick Smallhorn
Contributors statement: JG conceived the study and designed the trial. All collaborators contributed to the design and provided suggestions to improve the study. JG, BC, and AD were responsible for data collection. JG and BM performed the analysis of the data. JG drafted the manuscript and all collaborators contributed to its revision. JG takes responsibility for the paper as a whole. Each author listed on the manuscript has seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript.
Competing interests: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors’. All authors report no conflict of interest.







