Clozapine remains the gold-standard antipsychotic for treatment-resistant schizophrenia, but its use comes with a significant number of potentially life-threatening side-effects (Taylor Reference Taylor2017). Among the most prominent of these are blood dyscrasias, diabetic ketoacidosis and myocarditis, for each of which there are strictly enforced monitoring guidelines (Cohen Reference Cohen, Bogers and van Dijk2012). Clozapine can also cause constipation (the difficult, incomplete or infrequent evacuation of dry hardened faeces), which affects 30–60% of patients (De Hert Reference De Hert, Dockx and Bernagie2011a; Shirazi Reference Shirazi, Stubbs and Gomez2016; Taylor Reference Taylor, Barnes and Young2019; also, clozapine SmPC: Box 1).
Historically, clinicians have viewed antipsychotic-induced constipation as a troublesome but mild adverse effect of antipsychotic therapy. However, today we are overwhelmed with evidence that constipation can in fact lead to ileus, intestinal obstruction, bowel ischaemia, gastrointestinal necrosis or toxic megacolon, the consequences of which can be fatal (Schwartz Reference Schwartz and Frisolone1993; Erickson Reference Erickson, Morris and Reeve1995; Hayes Reference Hayes and Gibler1995; Drew Reference Drew and Herdson1997; Levin Reference Levin2002; Townsend Reference Townsend and Curtis2006; Rondla Reference Rondla and Crane2007; Palmer Reference Palmer, McLean and Ellis2008; Hibbard Reference Hibbard, Propst and Frank2009; De Hert Reference De Hert, Dockx and Bernagie2011a; Flanagan Reference Flanagan2011; Nielsen Reference Nielsen and Meyer2012; Oke Reference Oke, Schmidt and Bhattarai2015; Every-Palmer Reference Every-Palmer and Ellis2017a; West Reference West, Rowbotham and Xiong2017).
Constipation caused by clozapine-induced gastrointestinal hypomotility (CIGH) is much more common than clozapine-induced blood dyscrasias and has a higher mortality rate (Cohen Reference Cohen, Bogers and van Dijk2012; Every-Palmer Reference Every-Palmer, Nowitz and Stanley2016; Ingimarsson Reference Ingimarsson, MacCabe and Sigurdsson2018). When prescribing clozapine, strict criteria must be followed to assertively monitor, detect and treat related blood dyscrasias (clozapine SmPC: Box 1). No such framework exists for the monitoring of constipation, even though CIGH may also lead to death. In a recent and large pharmacovigilance study in Australia and New Zealand, 160 patients (37/10 000) on clozapine were reported as having serious gastrointestinal hypomotility. Of these, 29 (7/10 000) are known to have died – a fatality rate of 18% (Every-Palmer Reference Every-Palmer and Ellis2017a). The case-fatality rate reported by the US Food and Drug Administration (De Hert Reference De Hert, Hudyana and Dockx2011b) was nearly identical to this, at 21.9% (7 of 32 patients). Despite the prevalence and significant mortality associated with CIGH, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK does not mention CIGH specifically in its drug information for prescribers (clozaril: http://www.mhra.gov.uk/spc-pil/); rather, the summary of product characteristics for the drug lists intestinal obstruction/paralytic ileus/faecal impaction as ‘very rare’ (Clozapine SmPC last accessed 2019). The term ‘very rare’ is defined as ‘isolated case reports or occurring in less than one in 10 000 users’.
BOX 1 Summaries of product characteristics (SmPCs)
On the electronic Medicines Compendium (eMC), searching for drugs under their generic names often retrieves the proprietary brands available in the UK. The SmPCs listed below are for generic or example proprietary brands. All urls shown were accessed in March 2019.
Clozapine: Clozaril 25mg tablets (https://www.medicines.org.uk/emc/product/4411/smpc)
Lactulose: Duphalac (https://www.medicines.org.uk/emc/product/5525/smpc)
Macrogols (polyethylene gycols): Movicol Plain 13.7 g sachet, powder for oral solution (https://www.medicines.org.uk/emc/product/257/smpc)
Orlistat: (https://www.medicines.org.uk/emc/product/8703/smpc)
Senna: (https://www.medicines.org.uk/emc/product/9458/smpc)
Sodium docusate: Dulcoease 100 mg capsules (https://www.medicines.org.uk/emc/product/212/smpc)
Sodium picosulfate: Dulcolax (https://www.medicines.org.uk/emc/product/905/smpc)
Aside from the fatal consequence of constipation caused by CIGH, it has been repeatedly shown that chronic constipation can have a negative impact on the patient's quality of life (Damon Reference Damon, Dumas and Mion2004; Dennison Reference Dennison, Prasad and Lloyd2005; Wald Reference Wald, Scarpignato and Kamm2007). Symptoms of delayed colonic transit time include reduced frequency of defecation, lack of urgency to defecate, abdominal distension, bloating and feelings of incomplete evacuation (Koch Reference Koch, Voderholzer and Klauser1997). One study reported that median colonic transit time for patients prescribed clozapine was 104.5 h, which is over four times longer than for those on other antipsychotics (23 h; P < 0.0001) (Every-Palmer Reference Every-Palmer, Nowitz and Stanley2016). Eighty per cent of clozapine-treated patients had colonic hypomotility, compared with none of those treated with other antipsychotics (olanzapine, risperidone, paliperidone, aripiprazole, zuclopenthixol or haloperidol).
This article will highlight the need for enhanced identification and treatment of constipation associated with clozapine and will challenge the status quo of reserving treatment for patients with identified or reported clozapine-induced constipation. We will emphasise the importance of prophylactic use of laxatives in patients who are taking clozapine, describing both commonly prescribed laxatives and less well-known agents.
Despite the recommendation that monitoring and treatment of constipation caused by CIGH should be brought in line with the strict framework in place to detect blood dyscrasias (Cohen Reference Cohen, Bogers and van Dijk2012), there has been no such coordinated provision. Although we uphold the need for a systematised approach to monitoring clozapine-induced constipation, we recommend interim improvement to local practice.
Given the life-threatening severe nature of severe clozapine-induced constipation, readers’ attention is drawn to Box 2, which lists symptoms of suspected acute CIGH. A low threshold for referral to an emergency department is warranted, as there are cases in which death occurred only hours after the first symptom was reported and the patient had no previous symptoms of constipation.
Risk factors for constipation in schizophrenia
People with schizophrenia typically have pre-existing risk factors for constipation: a sedentary lifestyle, obesity, poor diet, reduced fibre intake and dehydration, to name but a few. Prospectively identifying individuals who are suffering from constipation caused by CIGH is further limited as symptoms are often not reported and are not easily objectively recognised by clinicians, given the private nature of patients' bowel functions. Patient under-reporting could also be due to inherent cognitive deficits, core symptoms of schizophrenia, reduced pain sensitivity or reduced ability to communicate (Bickerstaff Reference Bickerstaff, Harris and Leggett1988; Dworkin Reference Dworkin1994; De Hert Reference De Hert, Correll and Bobes2011c; Stubbs Reference Stubbs, Thompson and Acaster2015). Clozapine itself causes side-effects that can exacerbate such pre-existing risk factors: sedation (through antihistaminergic properties), increased appetite and hypersalivation (which can worsen dehydration) (clozaril at http://www.mhra.gov.uk/spc-pil/; clozapine SmPC: Box 1). Clozapine is thought to reduce gastrointestinal motility through its gut peripheral muscarinic anticholinergic effects and antagonism at serotonin receptors (Meltzer Reference Meltzer and Nash1991; Chengappa Reference Chengappa, Pollock and Parepally2000; Crowell Reference Crowell2001; Abrams Reference Abrams, Andersson and Buccafusco2006). These multifaceted risk factors for constipation – schizophrenia itself, inherent under-reporting and under-detection, clozapine side-effects, together with reduced gastric motility – put this subgroup of patients at a degree of risk that necessitates either a challenge to the ethics behind most current ‘wait and treat’ constipation guidance or an overhaul of the way clinicians view the prescribing of laxatives.
Opinions differ about the relationship between clozapine dose, plasma levels and rates of CIGH or ileus. A subgroup analysis in a 22-year bi-national pharmacovigilance study in Australia and New Zealand showed that those with fatal outcomes had significantly longer duration of clozapine treatment (median 4.2 years, IQR = 1.5–8.6 years) than the rest of the group (median 1.9 years, IQR = 0.2–5 years) (Every-Palmer Reference Every-Palmer and Ellis2017a). The odds ratio of a fatal outcome increased by 1.21 years (95% CI 1.02–1.44) for every 2 years on clozapine. Age, female gender, dose and receiving other constipating medications had a positive, but non-significant association with fatal outcomes. A study focusing on ileus alone (Nielsen Reference Nielsen and Meyer2012) found an increased risk with increasing age (OR = 1.03, 95% CI 1.01–1.04), female gender (OR = 1.60, 95% CI 1.10–1.23) and duration of clozapine treatment (OR = 1.99, 95% CI 1.21–3.29). The onset of ileus occurred on average more than 3 years after the prescription of the offending drug, and a recent Icelandic study (Ingimarsson Reference Ingimarsson, MacCabe and Sigurdsson2018) found a mean time to ileus of 13.7 years. However, a review of the literature (West Reference West, Rowbotham and Xiong2017) lists risk factors for CIGH as older age, male gender, the first 4 months of treatment, co-prescription of constipating agents, higher doses and previous CIGH. Given the conflicting results in the literature, identifying patients who are at a higher risk of developing CIGH or more sinister gastrointestinal complications such as ileus remains challenging.
Long-term prophylactic and post-CIGH use of laxatives: limitations and side-effects
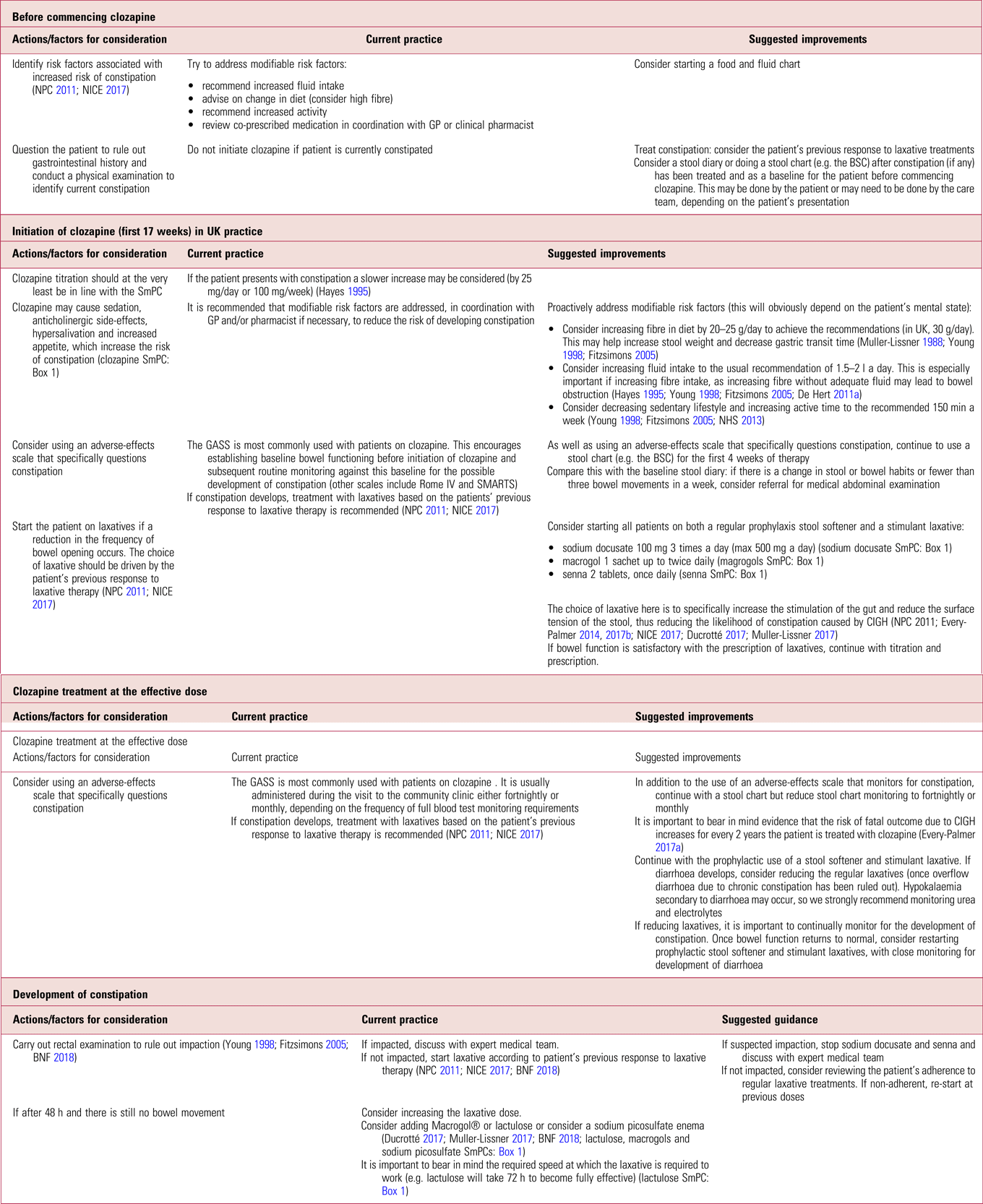
It is important to be aware of the limitations of prophylactic laxatives in completely eradicating gastrointestinal side-effects associated with clozapine, for example they will most likely have no effect in eliminating the risk of colitis or bowel ischaemia (Shammi Reference Shammi and Remington1997; Karmacharya Reference Karmacharya2005; Leong Reference Leong, Wong and Koh2007; Foxx-Orenstein Reference Foxx-Orenstein, McNally and Odunsi2008; McKinnon Reference McKinnon, Azad and Waters2009; Yu Reference Yu, Chen and Lee2013). There are also potential side-effects related to long-term prophylactic use, which we describe in more detail later in this article. Despite this, and in support of other authors (Every-Palmer Reference Every-Palmer, Newton-Howes and Clarke2014, Reference Every-Palmer and Ellis2017a, Reference Every-Palmer, Ellis and Nowitz2017b), we propose prophylactically treating all patients on clozapine, because the risks of not treating far outweigh the benefits of a ‘wait and treat’ practice. Table 1 summarises current practice in the prevention of clozapine-induced constipation and outlines suggested improvements.
Re-introducing clozapine in a patient following a potentially fatal side-effect is never without risk. Therefore, prophylactic laxatives at appropriate doses, along with dietary and lifestyle changes highlighted in Table 1, are recommended when re-introducing clozapine in a patient with a history of clozapine-induced constipation or CIGH. Cases of successful clozapine re-challenge following bowel perforation and bowel infarction, with notably smaller doses of clozapine alongside many lifestyle changes and closer monitoring of bowel habits, have been reported (McKinnon Reference McKinnon, Azad and Waters2009; Ikai Reference Ikai, Suzuki and Uchida2013).
TABLE 1 Prevention of clozapine-induced constipation: current practice and suggested improvementsa
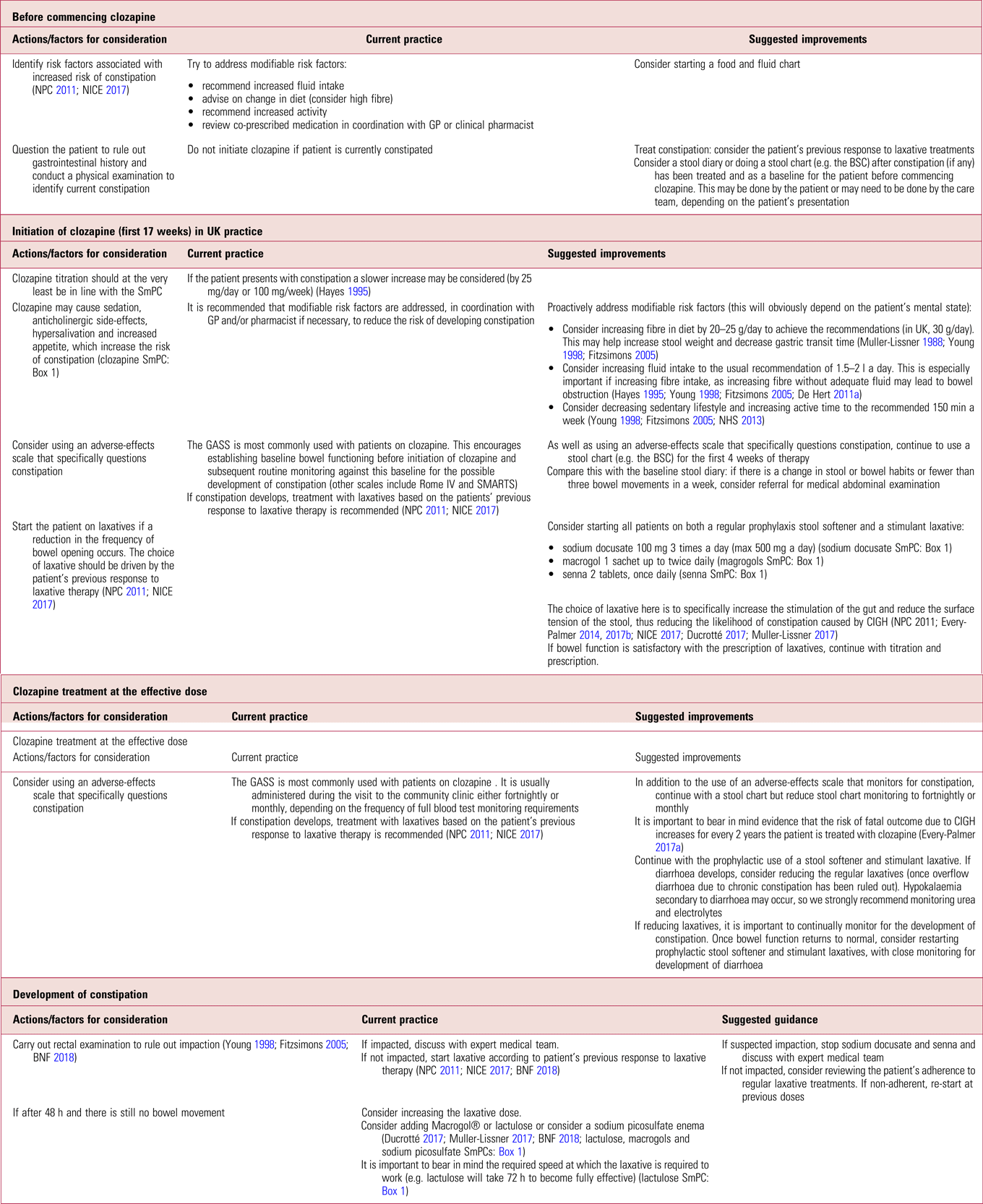
BNF, British National Formulary; BSC, Bristol Stool Chart (Lewis Reference Lewis and Heaton1997); GASS, Glasgow Antipsychotic Side-Effect Scale (Hynes Reference Hynes, Keating and McWilliams2015); GP, general practitioner; NICE, National Institute for Health and Care Excellence; NPC, National Prescribing Centre; Rome IV, the Rome IV Diagnostic Criteria for Functional Constipation (see Lacy Reference Lacy, Mearin and Chang2016); SMARTS, Systematic Monitoring of Adverse Events Related to Treatments (Haddad Reference Haddad, Fleischhacker and Peuskens2014); SmPC, summary of product characteristics.
Sources: Koch et al (Reference Koch, Voderholzer and Klauser1997); Every-Palmer et al (Reference Every-Palmer, Newton-Howes and Clarke2014, Reference Every-Palmer and Ellis2017a).
a. At all stages, clinicians should be alert for signs of acute CIGH (see Box 2). A low threshold for referral to an emergency department is warranted as death can occur within hours. This does not deviate from current recommended practice.
BOX 2 Warning signs of acute clozapine-induced gastrointestinal hypomotility (CIGH)
Seek urgent medical review if any of the following symptoms occur:
• moderate or severe abdominal pain lasting over an hour
• abdominal distension
• vomiting
• overflow diarrhoea or bloody diarrhoea
• absent or high-pitched bowel sounds
• metabolic acidosis
• haemodynamic instability
• leukocytosis
• signs of sepsis
These symptoms are signs of suspected acute CIGH, and a low threshold for referral to A&E is warranted as death can occur within hours.
Classes of laxative
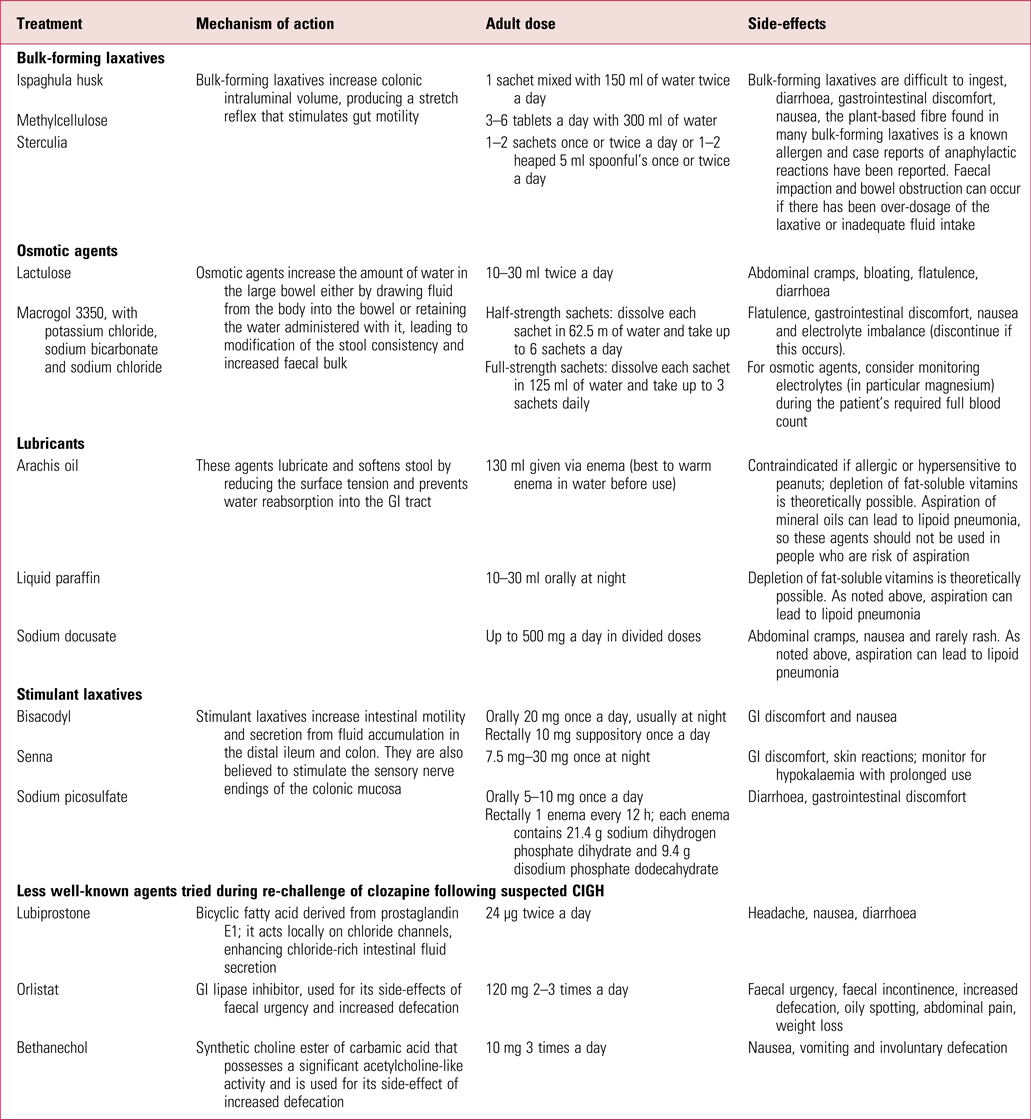
Intervention protocols such as that published by the Cochrane Collaboration (Every-Palmer Reference Every-Palmer, Newton-Howes and Clarke2014) offer useful advice on the different laxatives, and in Table 2 we give a summary of those that are currently available in the UK, including lesser-known medications that have been used in specialist situations by consultant psychiatrists during clozapine re-challenge following CIGH.
TABLE 2 Summary of available laxatives used for constipation in the UK (including those less commonly prescribed following constipation caused by clozapine-induced gastrointestinal hypomotility)
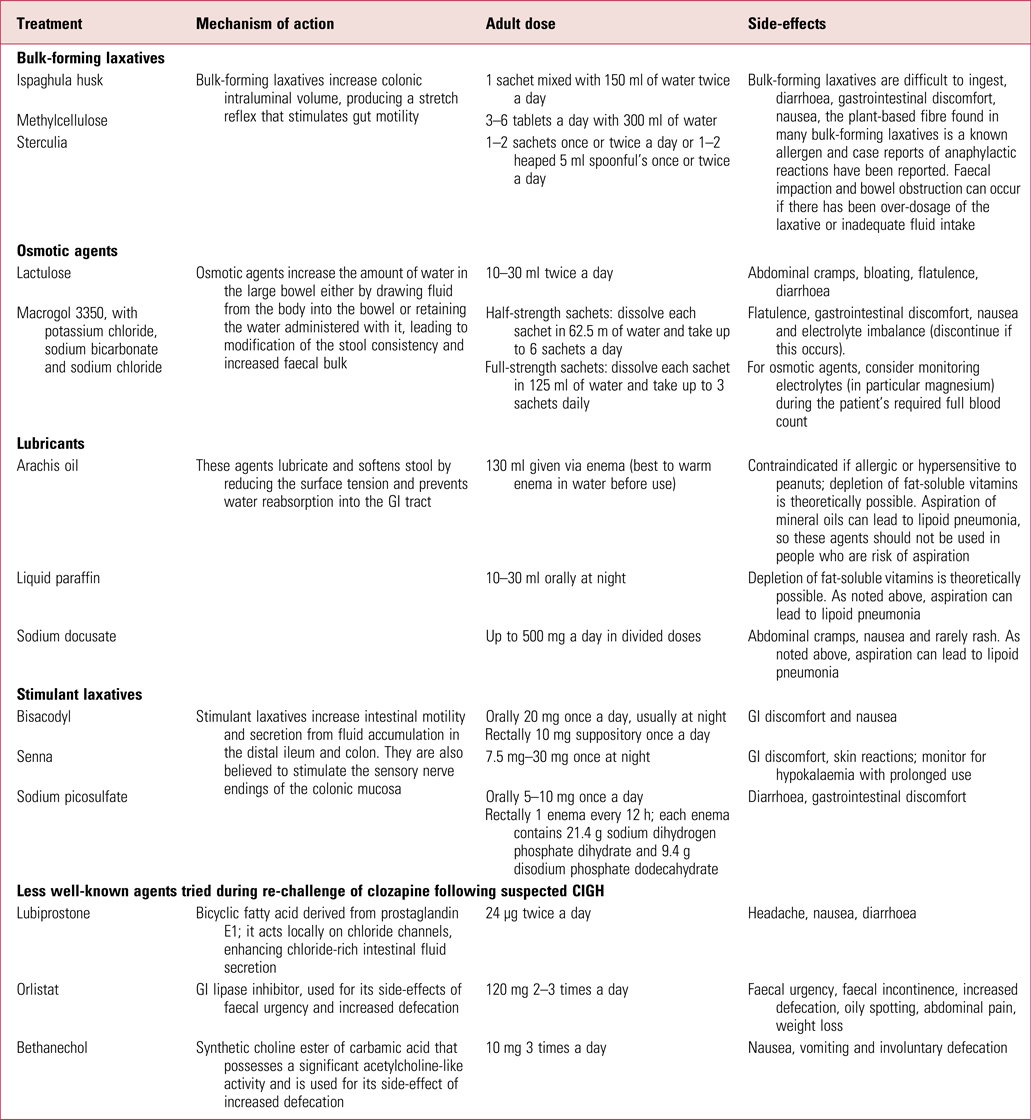
GI, gastrointestinal.
Sources: National Prescribing Centre (2011); Every-Palmer et al (Reference Every-Palmer, Newton-Howes and Clarke2014); National Institute for Health and Care Excellence (2017); British National Formulary (2018).
Laxative agents are typically broken down into four separate classes, namely bulking agents, osmotic agents, lubricants and stimulants. It is generally accepted that the first three classes are thought to be safe in long-term use.
Bulking agents
Bulking agents act by virtue of their water retaining properties, leading to increased gut motility; however, overdosage of bulk-forming laxatives or significant dehydration can result in faecal impaction and bowel obstruction. Adverse effects of bulk-forming laxatives, even in chronic use, are infrequent but psyllium, the plant-based fibre found in many bulk-forming laxatives, is a known allergen and there are case reports of anaphylactic reactions (Vaswani Reference Vaswani, Hamilton and Valentine1996; Khalili Reference Khalili, Bardana and Yunginger2003).
Given the primary mechanisms of clozapine-induced constipation, we would not recommend bulking agents as first-line treatment because of the risk of obstruction; this recommendation is in keeping with the Porirua protocol (Every-Palmer Reference Every-Palmer, Ellis and Nowitz2017b) and research findings (Meltzer Reference Meltzer and Nash1991; Chengappa Reference Chengappa, Pollock and Parepally2000; Crowell Reference Crowell2001; Abrams Reference Abrams, Andersson and Buccafusco2006).
Osmotic laxatives
Osmotic laxatives broadly work by drawing fluid into the bowel as well as promoting the retention of fluid in the lumen of the colon. Theoretically, the long-term use of such laxatives could lead to reduced absorption of fat-soluble minerals, although this has never been reported in the literature (Xing Reference Xing and Softer2001). A potential serious side-effect is electrolyte imbalance as a result of excess ion absorption, particularly in patients with compromised renal function. Assessing electrolytes is an established procedure before commencing clozapine. For patients co-prescribed long-term osmotic laxatives, and we would suggest that clinicians also consider monitoring electrolytes and magnesium levels alongside the mandatory monthly full blood count (macrogols and lactulose SmPCs: Box 1).
Lubricant laxatives
Lubricant laxatives, often derived from liquid paraffin, decrease the absorption of water in the colon and act to soften the stool. Aspiration of such mineral oils can lead to lipoid pneumonia, highlighting that these agents should not be used in people who are risk of aspiration (Weinstein Reference Weinstein2001). It is of course worth highlighting here the elevated risk of aspiration pneumonia with clozapine (Taylor Reference Taylor, Barnes and Young2019).
Stimulant laxatives
Stimulant laxatives increase intestinal motility and secretion from fluid accumulation in the distal ileum and colon. They are also believed to stimulate the sensory nerve endings of the colonic mucosa. Given the proposed mechanism of action of constipation caused by CIGH, these have been used prophylactically in the treatment of clozapine-induced constipation and prevention of CIGH (Every-Palmer Reference Every-Palmer, Ellis and Nowitz2017b; bisacodyl, senna and sodium picosulfate SmPCs: Box 1).
Less well-known laxatives
If lesser-known laxatives are to be tried at clozapine re-challenge, it is essential that the clinician is familiar with the laxative chosen.
Lubiprostone, a bicyclic fatty acid, has been used for chronic constipation and opioid-induced constipation in both men and women and for the treatment of irritable bowel syndrome with constipation in women (Wilson Reference Wilson and Schey2015). It has also been used successfully where other laxatives have failed in a clozapine re-challenge following CIGH (Meyer Reference Meyer and Cummings2014).
Three case reports have shown that the side-effects of orlistat, a medication licensed for weight loss, led to the successful treatment of intractable opioid-induced constipation (Guarino Reference Guarino2005; orlistat SmPC: Box 1). In all three cases, the patient had not responded to bisacodyl, bulking agents, osmotic laxatives and senna, but orlistat 120 mg taken twice daily resulted in successful bowel management. A small randomised controlled trial involving individuals with clozapine-induced constipation thought to be due to CIGH reported a statistically significant improvement in the prevalence of constipation, diarrhoea and normal stools in those given orlistat for 16 weeks in comparison with placebo (Chukhin Reference Chukhin, Takala and Hakko2013). It is important to note, however, that 47 of the 54 patients also received conventional laxatives in addition to orlistat or placebo.
Bethanechol is a synthetic choline ester of carbamic acid which is licensed for use in urinary incontinence or reflux oesophagitis and has side-effects that include nausea, vomiting and involuntary defecation in overdose. Bethanechol 10 mg taken three times a day has been used to treat CIGH and was effective in reducing total amount of laxatives and enemas required to maintain regular bowel movements (Poetter Reference Poetter and Stewart2013).
Long-term side-effects associated with the use of stimulant laxatives
Although the chronic use of bulking, osmotic and lubricant laxatives has long been considered safe, there has historically been concern about the risk attached to long-term use of stimulant laxatives such as senna (Leng-Peschlow Reference Leng-Peschlow1992). This is of particular significance given our recommendations, in keeping with the Porirua protocol (Every-Palmer Reference Every-Palmer, Ellis and Nowitz2017b), for prophylactic use of senna and sodium docusate in those prescribed clozapine. Such concern has typically been focused on the potential for stimulant laxatives to cause structural changes in the colon that lead to intestinal dysmotility as well as the risk that chronic use might lead to the development of cathartic colon, melanosis coli or even, potentially, cancer. Stimulant laxatives exert direct toxic effects on the colonic mucosa, although this is of uncertain functional significance and is dependent on the weight percentage of sennosides in the preparation (Leng-Peschlow Reference Leng-Peschlow1992; Xing Reference Xing and Softer2001). The presence of neurotoxic compounds in preparations no longer available is thought to have influenced the conclusions of historical trials. From more recent research, there is no clear evidence that long-term use even of anthranoid-containing laxatives causes damage to the colonic myenteric system (Xing Reference Xing and Softer2001). Further, there is no convincing evidence linking use of stimulant laxatives to cathartic colon, a radiological term describing loss of haustration and dilated lumen (Morales Reference Morales, Hernández and Bustamante2009).
First mentioned in 1829, melanosis coli describes the abnormal pigmentation of the colon by lipofuscin and so is perhaps better termed pseudo-melanosis coli. More recent literature highlights that this phenomenon is a non-specific marker of epithelial apoptosis with a variety of causes, including idiopathic constipation, carcinoma and stimulant-laxative use (Byers Reference Byers1997). Although in vitro and animal studies suggest the potential carcinogenic effect of stimulant laxatives, the results of epidemiological studies in several countries have not supported this view. There is no robust evidence to suggest a link between chronic use of stimulant laxatives and the development of gastrointestinal tumours (Kune Reference Kune1993; Nusko Reference Nusko, Schneider and Schneider2000; Xing Reference Xing and Softer2001). Thus, the recent evidence suggests that the risks of chronic stimulant-laxative use have historically been overstated, which has potentially limited their use.
Should diarrhoea develop in the context of long-term use of a stimulant laxative there is a risk of hypokalaemia. We would therefore recommend ongoing monitoring of bowel habit change in patients taking clozapine and prophylactic laxatives and the cessation of laxatives, followed by clinical review, should diarrhoea occur.
Conclusions
Constipation is already more common in people with schizophrenia than in the general population, owing to multiple factors, including sedentary lifestyle, obesity, poor diet, reduced fibre intake and dehydration. Clozapine-induced gastrointestinal hypomobility (CIGH) leading to constipation is a common, and potentially life-threatening, side-effect in a population where this medication's use is often the only means of maintaining mental well-being. It is more common than blood dyscrasias and has a higher mortality rate. There is a lower likelihood that patients will report this side-effect. It is therefore imperative that CIGH and constipation are given a higher profile by regulatory agencies and that clozapine manufacturers' literature mirrors the most recent data. A more stringent monitoring framework in national and local prescribing guidelines is also warranted.
We recommend the routine use of adverse-effects rating scales that include constipation as a side-effect, the use of stool monitoring charts and the operation of a low threshold for the referral of patients on clozapine who develop constipation to specialist medical attention. We challenge the status quo of reserving laxative use for those patients with identified or reported clozapine-induced constipation. Given the prevalence of clozapine-induced constipation and the high mortality rate associated with this side-effect, we are of the view that it is ethically sound to present an alternative: prophylactic use of laxatives throughout the patient's entire treatment with clozapine. We recommend review of regular laxatives only if diarrhoea develops or a change in frequency of bowel movements necessitates their withdrawal. This is especially important for patients attending UK community clozapine clinics, where often clozapine treatment is managed by senior non-medical staff.
We acknowledge that, if this recommendation is followed, there will be a proportion of clozapine-treated patients prescribed laxatives who do not have constipation secondary to CIGH. However, when taking into consideration the fact that CIGH can occur at any time throughout the course of a patient's treatment with clozapine and can have a potentially fatal outcome, the safety of long-term laxative use, the recommendation to stop laxatives if diarrhoea develops and the latitude of clinicians to provide individualised care, we are of the clear view that prophylactic use of laxatives in patients taking clozapine is ethical. Similar prophylactic use of laxatives is considered ethical and appropriate in other situations, such as patients taking opioids (Bell Reference Bell, Panchal and Miaskowski2009).
Existing protocols for the use of prophylactic laxative treatment recommend the first-line choice of sodium docusate or macrogol and senna, because of the reported pathological mechanism of CIGH. The risks of long-term stimulant laxative use have been overstated historically and we would correspondingly recommend their prophylactic use unless the patient develops diarrhoea. Monitoring of bowel-habit change in the context of long-term laxative use should be included in any protocols for their prophylactic use. The addition of regular electrolytes monitoring, including magnesium levels, should be considered when long-term osmotic laxatives are required.
Acknowledgement
We thank Colonel G.J. Attard FRCS (ret.) for his advice in the writing of this article.
MCQs
Select the single best option for each question stem
1 Which of the following about the relationship between clozapine and gastrointestinal motility is false?
a clozapine is thought to reduce gastrointestinal motility through its effect on peripheral muscarinic acetylcholine receptors at gastrointestinal sites
b the relationship between clozapine and gastrointestinal motility is poorly understood
c clozapine is thought to reduce gastrointestinal motility through its agonistic effect on peripheral dopamine receptors at gastrointestinal sites
d clozapine is thought to reduce gastrointestinal motility through its antagonistic effect on serotonin receptors at peripheral gastrointestinal sites
e clozapine is thought to reduce gastrointestinal motility by causing hypersalivation.
2 In patients prescribed clozapine, median colonic transit time is:
a not affected by the antipsychotic
b quicker than with other antipsychotics
c over four times longer than with other antipsychotics
d twice as long as that with other antipsychotics
e twice as quick as that with other antipsychotics.
3 In terms of the gastrointestinal side-effects of clozapine, which of the following statements is false?
a constipation is much more common than blood dyscrasias
b clozapine-induced gastrointestinal hypomotility (CIGH) often leads to bowel ischaemia
c a recent study demonstrated a fatality rate of 18% among patients with severe CIGH
d CIGH has a higher mortality rate than clozapine-induced blood dyscrasias
e prophylactic laxatives may reduce the adverse consequences of CIGH.
4 Which of the following statements about people who take clozapine is false?
a symptoms of constipation are not easily recognised by clinicians
b a patient's under-reporting of constipation could be due to inherent cognitive deficits
c people with schizophrenia may have a reduced pain sensitivity
d most patients with schizophrenia will openly report symptoms of constipation
e people with schizophrenia may have a reduced ability to communicate.
5 Which of the following statements about the treatment of refractory constipation is false?
a lubiprostone has been used successfully to treat constipation in people who have previously experienced CIGH where other laxatives have failed
b the weight-loss agent orlistat may be helpful in treating intractable constipation
c there is little evidence to substantiate claims that anthranoid-containing laxatives such as senna lead to intestinal dysmotility
d using arachis oil may lead to depletion of fat-soluble vitamins
e bethanechol is ineffective in treating refractory constipation.
MCQ answers
1 c 2 c 3 b 4 d 5 e





eLetters
No eLetters have been published for this article.