Introduction
During periods of snow accumulation and melt, physical, chemical, and biological transformations occur within snow-packs. These transformations can alter the concentration and distribution of solutes in the snow-pack, and also lead to the release of the majority of these solutes as an ionic pulse of short duration during the initial phase of snow melt. Snow-pack melt waters with relatively high ionic concentrations are then followed into run-off by relatively dilute ionic solutions. Numerous field and laboratory studies have documented this phenomenon (Reference Johannessen and HenriksenJohannessen and Henriksen, 1978; Reference ColbeckColbeck, 1981; Reference SuzukiSuzuki, 1982; Reference Cadle, Dasch and GrossnickleCadle and others, 1984; Reference Stein, Jones, Roberge and SochanskaStein and others, 1986; Reference Rascher, Driscoll and PetersRascher and others, 1987).
The understanding of the manner in which snow melt contributes to the chemistry of surface waters in alpine basins is complicated by the rugged and variable terrain, since large topographical differences within short distances result in spatial and temporal variations in the onset of snow melt within a given watershed. Such spatial and temporal differences in snow-melt onset may exaggerate the apparent magnitude and duration of an ionic pulse, or conversely may attentuate the ionic pulse by dilution.
In this study, we examine the way in which variation in the onset of snow-melt run-off affects the magnitude and timing of ionic pulses in sub-basins of an alpine basin. By comparing the solute concentrations of streams draining sub-basins with those of the outflow from the whole basin, we are able to determine whether variation in the onset of snow melt between sub-basins exaggerates or diminshes magnitude and duration of an ionic pulse throughout the entire basin. Our study was conducted in the alpine basin of Emerald Lake (ELB) located in Sequoia National Park, southern Sierra Nevada, California, U.S.A. ELB is representative of alpine catchment zones composed predominately of intrusive igneous rock and subjected to a maritime climate. Stream and lake waters in alpine basins, such as ELB, are amongst the most chemically dilute and weakly buffered in the world (Reference LandersLanders and others, 1987), and are sensitive to any increase in acidic deposition (Reference Melack, Stoddard and DawsonMelack and others, 1982). Anthropogenic increases in acidity are primarily due to nitric and sulfuric acids, and in this paper we discuss only the anions, ![]() and
and ![]() associated with those strong acids. In this paper, temporal variations of the anions are emphasized.
associated with those strong acids. In this paper, temporal variations of the anions are emphasized.
Site Description
The study site is located on the upper Marble Fork of the Kaweah River drainage system in the southern Sierra Nevada of California, U.S.A. (36°35′ 49′N, 118°40′ 30′W). ELB is a north-facing glacial cirque drained by a stream network into Emerald Lake, which itself has a single outflow (Fig. 1). The elevation of the basin is in the range 2780–3416 m a.s.1., and its topography is steep and rugged in character, with a median slope of 31°. Basin surface area is 120 ha, which is 47 times greater than the surface area of Emerald Lake. More than one-third of the basin is exposed bedrock; most of the remainder of the watershed is rock covered by a thin mantle of talus, colluvium, or poorly developed soil. Only 20% of the basin is classified as bearing soil. Precipitation is highly seasonal and variable, with about 90% of the total annual precipitation falling as snow in the period from October through to April. Almost 3000 mm of precipitation was recorded at Emerald Lake in the water year 30 September–1 October 1986, in contrast with the 1150 mm which fell in water year 1985.
Methods
Sampling in the Emerald Lake basin spanned the snow-melt seasons of 1985 and 1986. Snow-pit samples for chemical analysis were collected intensively at one site, the inlet, and were also collected periodically at other locations in the basin (Fig. 1). Concurrently with the snow sampling, we sampled the major inflows to Emerald Lake and the Emerald Lake outflow. Sampling sites for water chemistry were located immediately above the lake for all inflows, and immediately below the lake for the outflow.
Integrated samples from the entire snow-pack were obtained by digging pits down to the ground and collecting duplicate, contiguous, vertical cores in increments of 0.4 m. Snow samples were collected using 50 mm diameter, 0.5 m long, PVC tubes, which had been washed with 10% HCl and carefully rinsed with de-ionized water. The snow cores were decanted into similarly prepared polyethylene bags. Snow samples were stored, frozen, from 3 to 9 months before analysis, and snow-water equivalence (SWE) was determined from the weight of the sample bag and volume of the core. Water samples were collected in acid-washed, linear, polyethylene bottles which had been rinsed with de-ionized water.

Fig. 1. Topographic map of the Emerald Lake alpine catchment zone. Sub-basins are as follows: A = east joint, Β = south-east gully, C = inflow No. 1, C + D = inflows No. 1 and No. 2, D = inflow No. 2, E = inflow No. 3, F = inflow No. 4, G = west joint. Sampling sites are as follows: 1, tower; 2, inlet; 3, bench; 4, ridge; 5, ramp; 6, pond; 7, hole; 8, cirque.
Snow samples were placed in covered polyethylene buckets and melted at room temperature. Sub-samples were filtered through pre-rinsed, 47 mm Gelman A/E glass-fiber filters, and then stored at 4°C for subsequent anion analyses. Sample pH was measured with combined electrodes, such as Sargent Welch S-30072-15 or Ross 8104 suitable for use in dilute solutions and a Fischer Acumet 805 pH meter. Anions were identified and their concentrations measured by ion chromatography with a Dionex instrument, Model 2010i. The standard deviation between replicate samples from snow pits was ![]() for
for ![]() and
and ![]() , and for the water sampled was
, and for the water sampled was ![]() for
for ![]() , and
, and ![]() dm–3 for
dm–3 for ![]() . Our analytical quality control and quality assurance procedures have been described in Reference Dozier, Melack, Marks, Elder, Kattelmann and WilliamsDozier and others (1987).
. Our analytical quality control and quality assurance procedures have been described in Reference Dozier, Melack, Marks, Elder, Kattelmann and WilliamsDozier and others (1987).
Snow melt for each sub-basin in 1986 was determined by the random selection of survey points from a 25 m grid overlain on a contour map with contour lines at 5 m intervals. Snow depth was then measured with probes in the field at the predetermined survey points. To minimize local variation in snow depth, measurements were taken at the center point and then in the four cardinal-point directions each at a distance of 4 m from the center point. All five measurements were then averaged to give a single-sample point depth, and then the depths of all sample points within a sub-basin were averaged to obtain a value for snow depth within that sub-basin. Sampling points for each survey varied from 100 to 180. Snow pits were dug at selected sites in order to make possible the measurement of density. Snow-water equivalence was calculated as the average snow depth in a sub-basin multiplied by the average snow-density value. Four surveys were conducted, with the first starting before the onset of snow melt at maximum snow accumulation.
Results
The magnitudes of the ![]() and
and ![]() pulses in the snow-pack run-off were determined by finding the differences between the percentage decrease in water content of the snow-pack and the percentage decrease in concentration of each type of ion in the snow-pack (Table I). The ridge site generated a four-fold increase in
pulses in the snow-pack run-off were determined by finding the differences between the percentage decrease in water content of the snow-pack and the percentage decrease in concentration of each type of ion in the snow-pack (Table I). The ridge site generated a four-fold increase in ![]() loss, a 2.50-fold increase in
loss, a 2.50-fold increase in ![]() loss, and no enhanced loss for H+ during the period from 14 April 1986 to 23 May 1986. From 5 to 23 May 1986, at the inlet site there was no enhanced H+ loss, but an increase in
loss, and no enhanced loss for H+ during the period from 14 April 1986 to 23 May 1986. From 5 to 23 May 1986, at the inlet site there was no enhanced H+ loss, but an increase in ![]() and
and ![]() loss in the range 2–2.5 fold was noted. At the inlet and bench sites from 10 to 23 May 1985, the
loss in the range 2–2.5 fold was noted. At the inlet and bench sites from 10 to 23 May 1985, the ![]() pulse ranged from 1.7 to 3.7-fold and
pulse ranged from 1.7 to 3.7-fold and ![]() was about 1.6-fold, and H+ about 1.7-fold (Melack and others, in press).
was about 1.6-fold, and H+ about 1.7-fold (Melack and others, in press).
The onset of snow melt shifts temporally from sub-basins with a south-westerly aspect to sub-basins with a progressively more northerly aspect (Fig. 2). Snow melt in each of the ELB sub-basins was calculated by subtracting the SWE value from a survey specific from the maximum SWE in that sub-basin, dividing the result by the maximum SWE, and multiplying by 100. Since no survey was conducted before 16 April 1986, snow-melt values for the south-east gully (SEG; sub-basin Β in Figure 1) sub-basin prior to that date were based on the SWE difference between the SEG sub-basin and the adjacent sub-basin (inflow No. 1) on 16 April 1986. Sub-basin C + D is drained by a perennial stream dividing into inflow Nos. 1 and 2 about 200 m above the lake (Fig. 1). Before discharging into Emerald Lake, these streams are joined by waters from their respective catchment areas. Inflow No. 4 generally freezes during the winter months; in the spring of 1986 it began flowing sometime in early or mid-May. Precipitation and chemical flux from wet deposition to the basin, from the start of snow melt to the last snow survey were negligible, amounting to less than 1% of the SWE and solutes in the snow-pack at maximum accumulation. Relative ranking of annual discharge from the inflows is No. 2 > No. 1 > No. 4 > SEG > East joint (Fig. 1).
Table I. Snow-pit concentrations, spring melt, emerald lake basin

SWE is snow-water equivalence.
Standard deviation for both ![]() and
and ![]() was ±0.1 μeq dm−3.
was ±0.1 μeq dm−3.
January concentrations of ![]() and
and ![]() in the outflow water were also similar to those measured on 14 April, at levels of about 5.5 and 6 μeq dm−3, respectively. Concentrations of
in the outflow water were also similar to those measured on 14 April, at levels of about 5.5 and 6 μeq dm−3, respectively. Concentrations of ![]() and
and ![]() in the outflow also increased with the onset of snow melt in the watershed, and then decreased as the snow-melt season progressed. The maximum concentration of
in the outflow also increased with the onset of snow melt in the watershed, and then decreased as the snow-melt season progressed. The maximum concentration of ![]() measured in the outflow was 11 μeq dm−3, which was about half of the peak value of 18 μeq dm−3 observed in inflows Nos. 2 and 4. Maximum concentration of
measured in the outflow was 11 μeq dm−3, which was about half of the peak value of 18 μeq dm−3 observed in inflows Nos. 2 and 4. Maximum concentration of ![]() in the outflow occurred between the times at which the maximum values were measured in inflows Nos. 2 and 4.
in the outflow occurred between the times at which the maximum values were measured in inflows Nos. 2 and 4. ![]() concentrations in the outflow remained near their maximum, in the range of 10–11 μ eq dm−3, for the period of 1–18 May, but inflow No. 2 showed a rapid decline in
concentrations in the outflow remained near their maximum, in the range of 10–11 μ eq dm−3, for the period of 1–18 May, but inflow No. 2 showed a rapid decline in ![]() concentration during this period.
concentration during this period. ![]() followed a pattern similar to
followed a pattern similar to ![]() .
.
Discussion
Ablation results from the complex interplay of physiographic factors such as slope, aspect, latitude, and horizon, with energy-balance exchanges at the air/snow and snow/ground interfaces (Elder, in press). Thus, source areas of snow-melt run-off vary both spatially and temporally (Reference Woo and SlymakerWoo and Slay maker, 1975). The onset of snow melt in ELB shifts temporally from sub-basins with a south-westerly aspect to those with a progressively more northerly aspect. These ablation differences within a watershed cause a corresponding change in chemistry in both the snow-pack and the snow-pack run-off.

Fig. 2.
Stream water sampled in ELB sub-basins from January through to July had the highest values of ![]() and
and ![]() at the time of onset of snow melt in each sub-basin. Outflow exhibited a similar anionic pulse with the onset of snow melt in the basin.
at the time of onset of snow melt in each sub-basin. Outflow exhibited a similar anionic pulse with the onset of snow melt in the basin.
In stream water, the ![]() pulse may be due to sources other than an ionic pulse from snow-melt run-off. Ground water, soil water, and organic horizons in the soil may all contribute
pulse may be due to sources other than an ionic pulse from snow-melt run-off. Ground water, soil water, and organic horizons in the soil may all contribute ![]() to this run-off, although the small percentage of soils and high percentage of bedrock characteristic of ELB make this explanation for the observed ionic pulses unlikely. Furthermore, biological activity of vegetation, soils, and phytoplankton utilizes
to this run-off, although the small percentage of soils and high percentage of bedrock characteristic of ELB make this explanation for the observed ionic pulses unlikely. Furthermore, biological activity of vegetation, soils, and phytoplankton utilizes ![]() as a nutrient source, which would tend to reduce the amplitude of the
as a nutrient source, which would tend to reduce the amplitude of the ![]() pulse in stream waters. Coincident with the
pulse in stream waters. Coincident with the ![]() pulse there was also a
pulse there was also a ![]() pulse, and for both the 1985 and the 1986 snow-packs
pulse, and for both the 1985 and the 1986 snow-packs ![]() was eluted in greater concentration than
was eluted in greater concentration than ![]() . Anionic concentrations in stream waters follow a pattern similar to that for snow-pack elution; maximum concentrations of
. Anionic concentrations in stream waters follow a pattern similar to that for snow-pack elution; maximum concentrations of ![]() in streams at the onset of snow melt were about 100% greater than the corresponding January concentrations, while
in streams at the onset of snow melt were about 100% greater than the corresponding January concentrations, while ![]() concentrations at snow-melt onset were about 40% greater than the matching January concentrations. Finally, measurements of
concentrations at snow-melt onset were about 40% greater than the matching January concentrations. Finally, measurements of ![]() concentrations in snow-pack melt water in 1987 before contact with the ground showed concentrations five times greater than bulk snow-pack concentrations.
concentrations in snow-pack melt water in 1987 before contact with the ground showed concentrations five times greater than bulk snow-pack concentrations.
There is an apparent sequential shift in the generation of ![]() and
and ![]() pulses through ELB corresponding with the onset of snow melt in each sub-basin. The period of time during which the whole of the alpine basin experienced these anionic pulses was thus greater than that for any single sub-basin. Lake-water concentrations of
pulses through ELB corresponding with the onset of snow melt in each sub-basin. The period of time during which the whole of the alpine basin experienced these anionic pulses was thus greater than that for any single sub-basin. Lake-water concentrations of ![]() and
and ![]() may respond to ion pulses by remaining elevated for a longer period of time than those for individual sub-basins, or alternatively may remain unaltered because concentrated ionic solutions in melt water from a sub-basin just starting to undergo melt may be balanced by dilute melt-water ion solutions from a sub-basin in the late stages of snow melt. Ion concentration in water from the lake outflow indicates how the alpine catchment area as a whole was influenced by sub-basin differences in anionic concentration.
may respond to ion pulses by remaining elevated for a longer period of time than those for individual sub-basins, or alternatively may remain unaltered because concentrated ionic solutions in melt water from a sub-basin just starting to undergo melt may be balanced by dilute melt-water ion solutions from a sub-basin in the late stages of snow melt. Ion concentration in water from the lake outflow indicates how the alpine catchment area as a whole was influenced by sub-basin differences in anionic concentration.
Maximum concentrations of ![]() and
and ![]() measured in inflow samples were between 1.5 and 1.1 times those in the sampled outflow. The level of the anionic pulse generated by the inflows was attenuated when these flows were combined in the outflow. What is surprising is that the percentage increases in
measured in inflow samples were between 1.5 and 1.1 times those in the sampled outflow. The level of the anionic pulse generated by the inflows was attenuated when these flows were combined in the outflow. What is surprising is that the percentage increases in ![]() and
and ![]() concentrations in the outflow at the onset of spring melt were similar to those in the inflows, namely about 100% for
concentrations in the outflow at the onset of spring melt were similar to those in the inflows, namely about 100% for ![]() and 40% for
and 40% for ![]() . Furthermore, outflow concentrations remain elevated to near-maximum values for a much longer period of time than was noted for any of the individual inflows. For the alpine catchment zone as a whole, Figure 2 illustrates how decrease in both
. Furthermore, outflow concentrations remain elevated to near-maximum values for a much longer period of time than was noted for any of the individual inflows. For the alpine catchment zone as a whole, Figure 2 illustrates how decrease in both ![]() concentration and
concentration and ![]() concentration in inflow No. 2 appears to be balanced by contributions from inflow No. 4, such that
concentration in inflow No. 2 appears to be balanced by contributions from inflow No. 4, such that ![]() concentration and
concentration and ![]() concentration in the outflow remained elevated for a longer time span than was observed for any individual sub-basin.
concentration in the outflow remained elevated for a longer time span than was observed for any individual sub-basin.
Conclusion
Spatial and temporal variations in the onset and intensity of snow melt appear to affect the water chemistry of alpine basins. Concentrations of ![]() and
and ![]() in melt water during spring run-off in alpine basins vary considerably both spatially and temporally, necessitating sampling from all sub-basins adequately to characterize inputs to lake systems in this season. Spatial and temporal variations in onset of snow melt do not appear to reduce the percentage increase in
in melt water during spring run-off in alpine basins vary considerably both spatially and temporally, necessitating sampling from all sub-basins adequately to characterize inputs to lake systems in this season. Spatial and temporal variations in onset of snow melt do not appear to reduce the percentage increase in ![]() and
and ![]() pulses in alpine basins; rather these ablation differences appear to increase notably the period over which such areas are subjected to raised concentrations of
pulses in alpine basins; rather these ablation differences appear to increase notably the period over which such areas are subjected to raised concentrations of ![]() and
and ![]() , and also to extend the period during which these more concentrated solutions are transported into down-stream aquatic systems.
, and also to extend the period during which these more concentrated solutions are transported into down-stream aquatic systems.
Acknowledgements
K. Tonnessen was helpful in all aspects of our study. D. Clow, R. Kattelmann, and K. Elder shared many a cold snow pit. D. Marks provided direction and enthusiasm as project manager of the snow part of the research. J. Sickman, M. Williams, and H. Hardenburg collected and analyzed water samples; F. Setaro developed our QA and QC protocol for snow-chemistry analysis. We thank S. Hamilton for editorial comments. Funding was provided by California Air Resources contracts A3-103-32 and A3-096-32.




 and sulphate ion
and sulphate ion  concentrations in streams were correlated with the amount of snow melt in each sub-basin. Inflows to Emerald Lake had elevated concentrations of
concentrations in streams were correlated with the amount of snow melt in each sub-basin. Inflows to Emerald Lake had elevated concentrations of 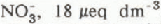 , and
, and 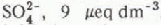 , which corresponded temporally with the initiation of snow melt in each sub-basin. Concentrations of both anions then decreased as snow melt progressed. The onset of snow melt shifted temporally from sub-basins with a south-westerly aspect to basins with a progressively more northerly aspect. The
, which corresponded temporally with the initiation of snow melt in each sub-basin. Concentrations of both anions then decreased as snow melt progressed. The onset of snow melt shifted temporally from sub-basins with a south-westerly aspect to basins with a progressively more northerly aspect. The 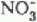 and
and  pulses also moved, with time, from the sub-basins with south-westerly aspects to those with more northerly aspects. The time span over which the basin experienced these anionic pulses was longer than that for any single sub-basin. Maximum concentrations of
pulses also moved, with time, from the sub-basins with south-westerly aspects to those with more northerly aspects. The time span over which the basin experienced these anionic pulses was longer than that for any single sub-basin. Maximum concentrations of 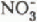 and
and  in the lake outflow, which also occurred at the onset of snow melt, were elevated by 100 and 40%, respectively, above their January concentrations in line with increases in inflow, but were sustained for a longer period than that of any inflow increase.
in the lake outflow, which also occurred at the onset of snow melt, were elevated by 100 and 40%, respectively, above their January concentrations in line with increases in inflow, but were sustained for a longer period than that of any inflow increase.


