Introduction
Animals housed in zoos are exposed to various stimuli that can impinge upon their welfare (Birke Reference Birke2002; Cooke & Schillaci Reference Cooke and Schillaci2007; Clark et al. Reference Clark, Fitzpatrick, Hartley, King, Lee, Routh, Walker and George2012; Maia et al. Reference Maia, Volpato and Santos2012). Among these stimuli there is exposure to visitors (Carder & Semple Reference Carder and Semple2008; Clark et al. Reference Clark, Fitzpatrick, Hartley, King, Lee, Routh, Walker and George2012; Farrand et al. Reference Farrand, Hosey and Buchanan-Smith2014), which has been connected to the ‘zoo-visitor effect’ (e.g. Davey Reference Davey2007). Such effect may be assessed through changes in behaviour and/or physiological responses of the animals, when exposed to zoo visitors (Davey Reference Davey2006). However, research on visitor effect may lack scientific rigorousness as a result of constraints regarding the control of variables related to visitor presence (Farrand et al. Reference Farrand, Hosey and Buchanan-Smith2014). Animals may perceive the presence of visitors via a variety of different perception channels: visual, olfactory and auditory (Young Reference Young2003). While visual and olfactory stimuli are difficult to measure, accurate quantification of auditory stimuli is feasible. Zoo visitor-noise pollution has been referred to as having a negative effect on animal welfare (Owen et al. Reference Owen, Swaisgood, Czekala, Steinman and Lindburg2004; Powell et al. Reference Powell, Carlstead, Tarou, Brown and Monfort2006). Besides the effects of noise on the stress-response system (e.g. Bowles & Eckert Reference Bowles and Eckert1997; Ward et al. Reference Ward, Stehn, Erickson and Derksen1999; Owen et al. Reference Owen, Swaisgood, Czekala, Steinman and Lindburg2004; Wysocki et al. Reference Wysocki, Dittami and Ladich2006), noise has been reported to cause DNA damage, alterations in gene expression and numerous cellular processes with effects on neural, developmental, immunological and physiological functioning (Kight & Swaddle Reference Kight and Swaddle2011).
Studies on visitor noise have reported detrimental effects on various species: pumas (Puma concolor: Maia Reference Maia2009; Maia et al. Reference Maia, Volpato and Santos2012), gorillas (Gorilla gorilla: Clark et al. Reference Clark, Fitzpatrick, Hartley, King, Lee, Routh, Walker and George2012), chimpanzees (Pan troglodytes) and spectacled bears (Tremarctos ornatus) reduced feeding, and increased locomotion (Noga Reference Noga2010); Japanese monkeys (Macaca fuscata) increased reaction time during a cognitive task (Cronin et al. Reference Cronin, Bethell, Jacobson, Egelkamp, Hopper and Ross2018), and bush dogs (Speothos venaticus) increased exhibition of stereotypies (Corat & Chierregatto Reference Corat and Chierregato2015). Koalas (Phascolarctos cinereus) (Larsen et al. Reference Larsen, Sherwen and Rault2014) increased downtime and alertness; Western grey (Macropus fuliginosus fuliginosus) and red kangaroos (Macropus rufus) increased vigilance behaviours (Larsen et al. Reference Larsen, Sherwen and Rault2014; Sherwen et al. Reference Sherwen, Hemsworth, Butler, Fanson and Magrath2015a). Physiological effects, in tandem with such behavioural responses, have supported the interpretation of these responses as detrimental. For example, increased exhibition of stereotypies concomitant with increased glucocorticoid concentrations have been reported in several studies (e.g. Malmkvist et al. Reference Malmkvist, Jeppesen and Palme2011; Shepherdson et al. Reference Shepherdson, Lewis, Carlstead, Bauman and Perrin2013; Pizzutto et al. Reference Pizzutto, Sgai, Lopes, Pessutti, Nunes, Furtado, Oliveira and Guimaraes2015; for a review, see Mason Reference Mason1991). Giant pandas (Ailuropoda melanoleuca) showed increased locomotion, agitation, scratching, and glucocorticoid concentration when exposed to loud noise (Owen et al. Reference Owen, Swaisgood, Czekala, Steinman and Lindburg2004). Increased glucocorticoid concentration triggered by acoustic stressors may cause immunosuppression, insulin resistance, cardiovascular disease, catabolism (molecular decomposition), and intestinal problems (Spreng Reference Spreng2000).
Zoo-animal responses to visitors may depend upon species- and individual-characteristics, the nature of visitor-animal interactions, and enclosure design (Woolway & Goodenough Reference Woolway and Goodenough2017; Learmonth et al. Reference Learmonth, Sherwen and Hemsworth2018). Compared to traditional facilities (in which visitors remain outside), walk-through enclosures have greater potential to affect the welfare of animals, since contact with visitors (auditory, visual) is magnified, due to greater physical proximity and/or the absence of physical barriers between animals and visitors (Learmonth et al. Reference Learmonth, Sherwen and Hemsworth2018). However, such enclosures are increasingly popular, and their impacts on animal welfare are understudied (Sherwen et al. Reference Sherwen, Magrath, Butler and Hemsworth2015b). The few studies addressing this type of housing report that visitors have an effect on animals: quokkas (Setonix brachyurus) spent more time hidden when the enclosure was open to visitors (Learmonth et al. Reference Learmonth, Sherwen and Hemsworth2018) and squirrels (Sciurus vulgaris: Woolway & Goodenough Reference Woolway and Goodenough2017) moved more and fed less. In contrast, Jones et al. (Reference Jones, McGregor, Farmer and Baker2016) pointed to a positive effect on crowned lemurs (Eulemur coronatus) via a decrease in aggression among conspecifics with an increase in numbers of visitors. The scarcity of data indicates the need for investigations into the effects of such enclosures on the welfare of animals.
The two-toed sloth (Choloepus didactylus) is a mid-sized, nocturnal tree mammal found in the rainforests of South America, which spends most of its time in the treetops (Eisenberg & Redford Reference Eisenberg, Redford, Eisenberg and Redford1999; Nowak & Walker Reference Nowak and Walker1999; Bezerra Reference Bezerra, da Silva Souto, Halsey and Schiel2008; Peery & Pauli Reference Peery and Pauli2012). Having very low metabolic rates (roughly half those of other placental mammals) and feeding on a low-energy diet, they require up to 14 h of daily inactivity, and locomotion occurs slowly (Montgomery & Sunquist Reference Montgomery, Sunquist, Golley and Medina1975; Nagy & Montgomery Reference Nagy and Montgomery1980). In this study, we investigated the possible effects of visitor noise on the behaviour of two-toed sloths, housed in a walk-through zoo enclosure.
Materials and methods
Ethical permission
All procedures here were evaluated and approved by the Ethics Committee for Animal Use from the Pontifical Catholic University of Minas Gerais (Permit n 007/2014).
Study protocol
One male and one female two-toed sloth, housed in a walk-through enclosure in a zoo in the UK were the subject of this study. Visitors were unable to touch the sloths in the enclosure,with animals remaining above them (at a height of approximately 6 m) for the majority of the time, i.e. hanging from ropes, without any barriers to isolate the animals from visitor noise. The enclosure was indoors, made of concrete and round in shape (approximately 11 m in diameter). The animals were fed in the mornings, prior to the admittance of visitors. Food was made available through environmental enrichment: feeding items were spread over the floor, and within the branches of trees in the enclosure. The animals were filmed for 24 h per day, for three consecutive days a week (Fridays, Saturdays and Sundays), during the first three weeks of July 2017. The videos were analysed through focal sampling with continuous recording of behaviour, using the Solomon Coder programme (Copyright 2006–2017 by András Péter). Behavioural observations were based on an ethogram (Table 1) adapted from Hayssen (Reference Hayssen2011), Silva et al. (Reference Silva, Clozato, Moraes-Barros and Morgante2013), and Clark and Melfi (Reference Clark and Melfi2012). Sound measurement was performed four times per day using the SVAN 977A sound level meter (SVAN 977A, SVANTEK, Poland). The equipment was fixed on a tripod, in the visitor space, facing the enclosure, 1.5 m above the floor and a good distance clear from the boundaries of the enclosure. Each session lasted 15 min, distributed between morning (0930–0945h; with the zoo still closed to visitors), mid-morning (1045–1100h), midday (1200–1215h) and afternoon (1500–1515h). During each session, the number of visitors passing through the enclosure was also assessed. Since there have been no studies to date assessing which aspects of sound have the greatest effect on animals, we chose to evaluate three parameters, using the A-weighting filter: Equivalent Continuous Noise Level (LAeq), Maximum Sound Pressure Level (LAmax), and Peak Value (LApeak). The LAeq is used to measure the average sound pressure levels and calculate the average noise level (energy) to which an environment is exposed (Duarte et al. Reference Duarte, Vecci, Hirsch and Young2011), LAmax is the maximum level of noise (mean square root) during a certain period and LApeak is the highest point of raw sound pressure, without considering time. A-weighting was chosen because this filter has been tested previously, and was shown to correlate to sloths’ behaviour more than filter Z. This correlation suggests their acoustic sensitivity may be similar to ours (Queiróz Reference Queiróz2018).
Table 1. Ethogram of Choloepus didactylus observed in this study*
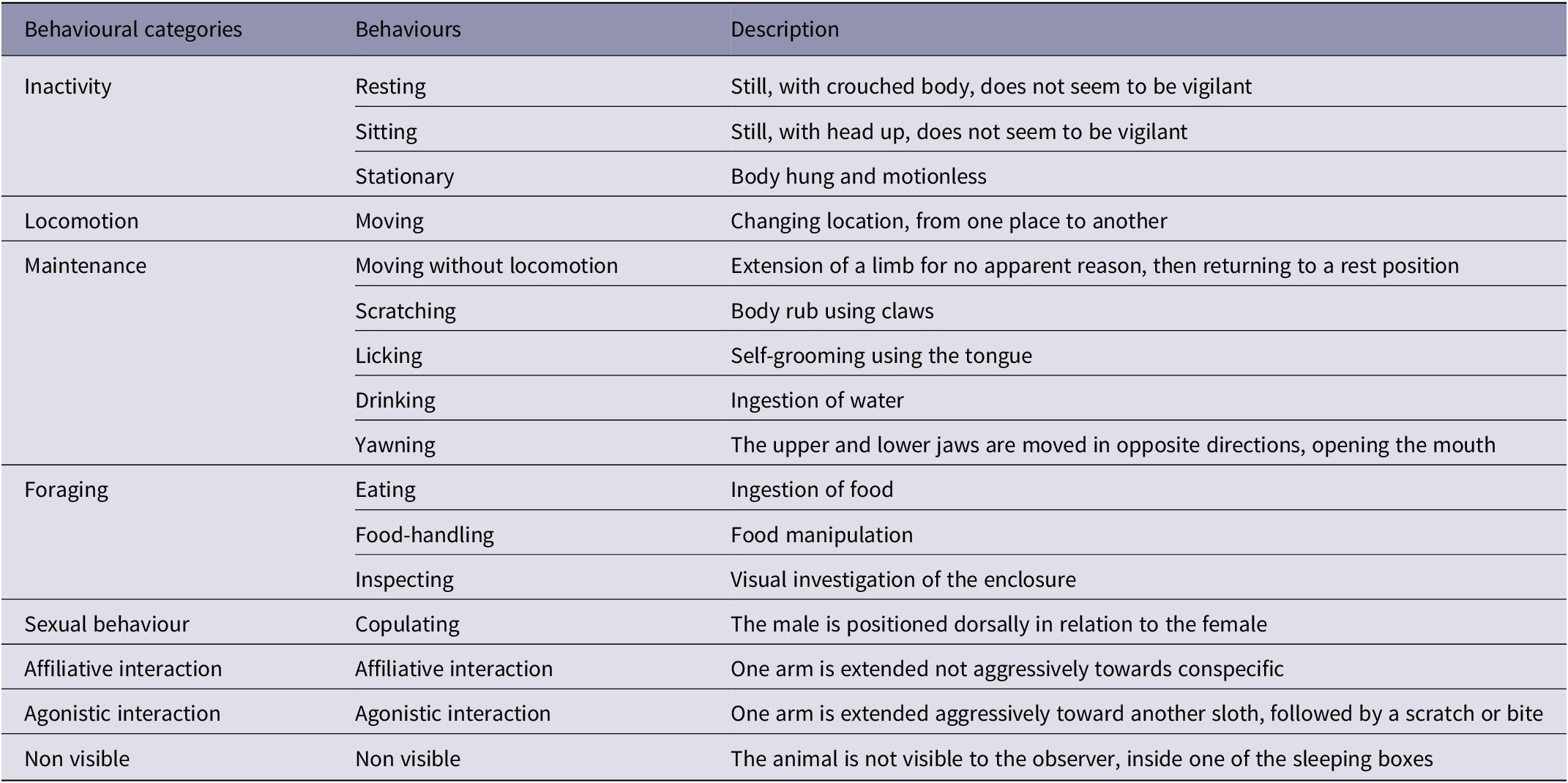
* Adapted from Hayssen (Reference Hayssen2011), Silva et al. (Reference Silva, Clozato, Moraes-Barros and Morgante2013) and Clark and Melfi (Reference Clark and Melfi2012).
Statistical analysis
For statistical analysis, we grouped the observed behaviours into the following categories: Inactivity; Locomotion; Maintenance; Foraging; Sexual behaviour; Affiliative interaction; Agonistic interaction; and Non-visible. Every behavioural category was correlated with each acoustic parameter (LAeq, LAmax, LApeak) using Pearson’s correlation test for parametric data or Spearman’s correlation test for non-parametric data (Zar Reference Zar2010). These analyses were performed for the behaviours exhibited during the 15-min of acoustic collection, and for the behaviours recorded during the subsequent 24 h, to assess a possible lasting effect of sound pressure. In the case of data correlation within 24 h of noise exposure, the behaviours recorded on video were correlated with the LAeq recorded on the previous day. The number of visitors passing in the enclosure during the 15-min sessions were also correlated to the acoustic parameters. Considering all behaviour data were correlated with three acoustic parameters, we applied Bonferroni correction, and considered P-values which were not greater than 0.017 as significant.
Results
In total, 216 h of behavioural recording, and 36 × 15-min sessions of acoustic recording, were produced. The activity budget of male and female animals can be seen in the Supplementary material. The noise levels recorded ranged from 51.7 to 122.2 dBA (LApeak), with an LAeq of 85.5 dBA. The acoustic parameters from the mornings (first acoustic collections, without visitors) were: LAeq ranging from 58.2 to 74.7 dBA; LAmax ranging from 56.5 to 62.4 dBA; LApeak from 67.5 to 73.3 dBA (Figure 1[a]–[c]). The number of visitors inside the enclosure during each 15-min session (ranging from 2 to 322 people) correlated strongly and positively with the three acoustic parameters (LApeak P < 0.0001, Pearson = 0.92; LAmax P < 0.0001, Pearson = 0.92; LAeq P < 0.0001, Spearman = 0.87). During exposure to increasing noise levels, we recorded significant reduction in inactivity (P = 0.0016, r = –0.5141 LApeak; P = 0.0015, r = –0.5158 LAmax; P = 0.0011, r = –0.5273 LAeq), and increased locomotion (P = 0.0004, r = 0.5625 LApeak; P = 0.0004, r = 0.5626 LAmax; P = 0.0019, r = 0.5071 LAeq; Figure 2) and maintenance behaviours (P = 0.0081, r = 0.4403 LApeak; P = 0.0083, r = 0.4389 LAmax; P = 0.0149, r = 0.4082 LAeq; Figure 3) for the male. The female, when noise was higher, spent significantly more time inside a box (P = 0.0170, r = 0.4010 LApeak; P = 0.0170, r = 0.4006 LAmax; P = 0.0149; Figure 4). Within 24 h of noise exposure, the female showed no behavioural changes but the male tended to spend less time foraging when LAmax was higher the previous day (P = 0.0479, r = –0.6709; Figure 5). For all other behaviours recorded, the correlations with acoustic parameters were not statistically significant (for more details, please see Tables S1 and S2, in the Supplementary material).
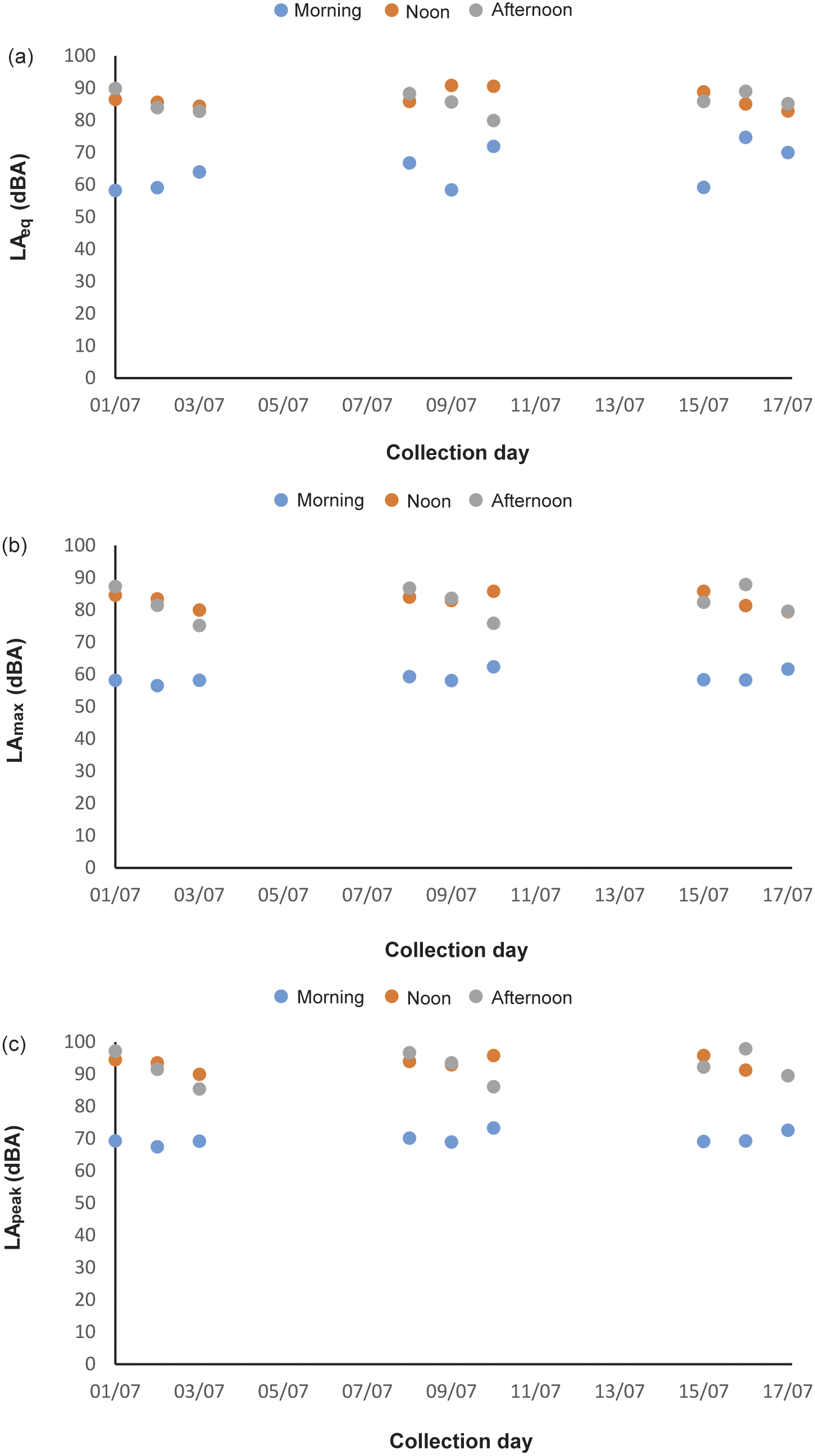
Figure 1. Mean acoustic parameters measured along three weeks, in three periods (morning, noon, and afternoon), in 15-minute sessions inside a walk-through enclosure of Choloepus didactylus, in a zoo in the United Kingdom. A – LAeq (equivalent continuous noise level); B – LAmax (maximum sound pressure level); C - LApeak (peak value).
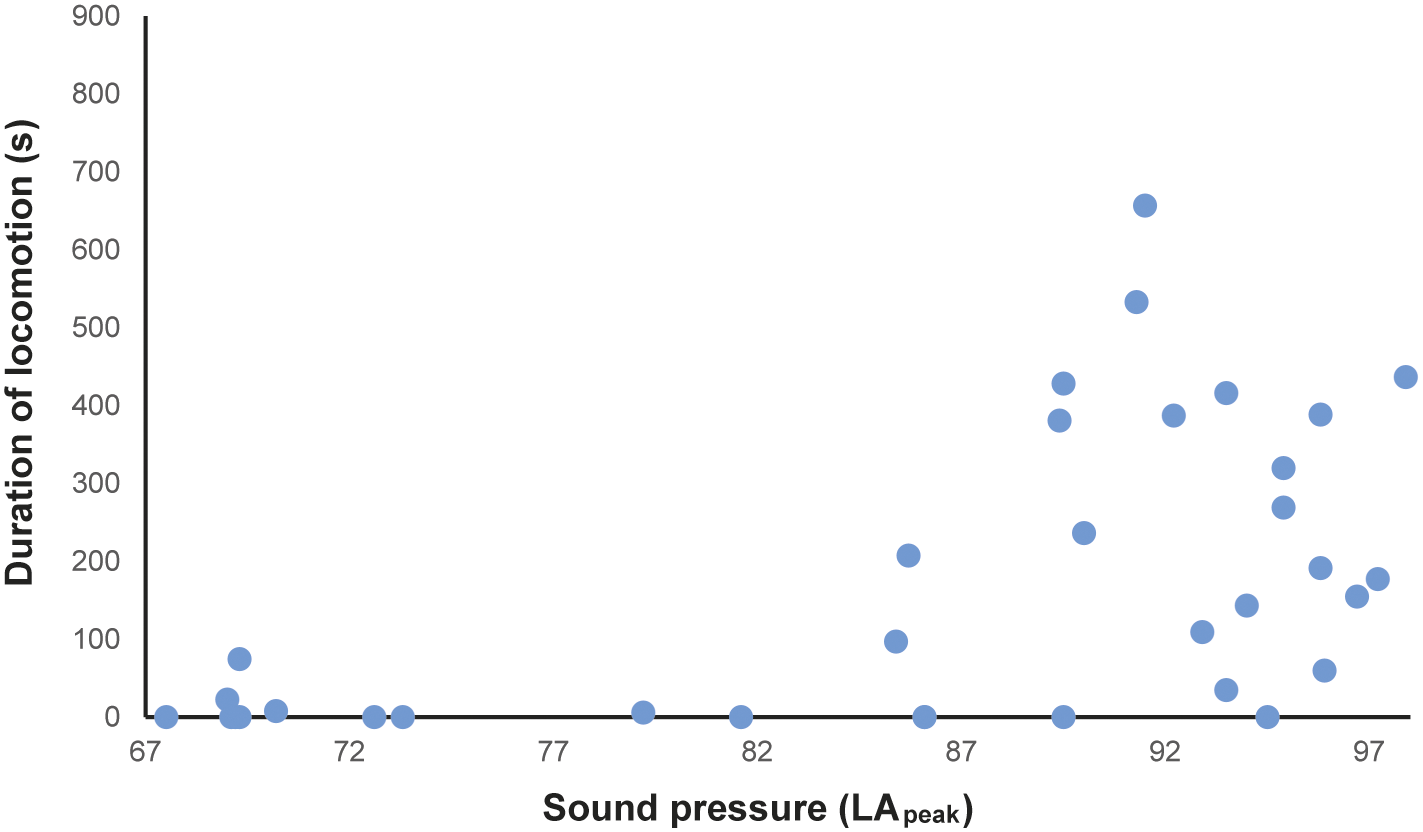
Figure 2. Duration (s) of locomotion of the male Choloepus didactylus housed in a walk-through enclosure, in a zoo in the UK, as a function of the noise level (LApeak) during the 15-min sessions of noise recording.
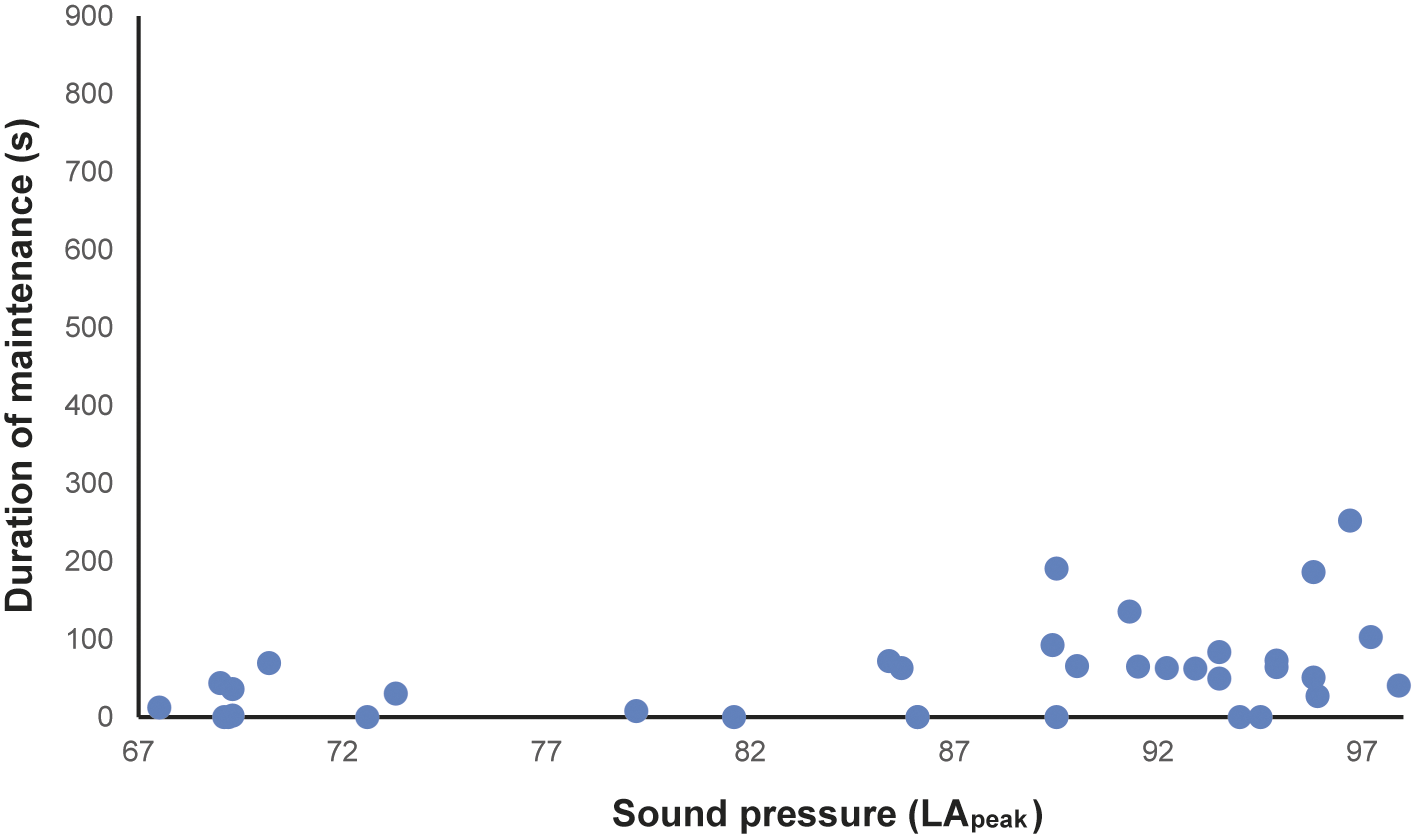
Figure 3. Duration (s) of maintenance behaviours of the male Choloepus didactylus housed in a walk-through enclosure, in a zoo in the UK, as a function of the noise level (LApeak) during the 15-min sessions of noise recording.

Figure 4. Duration (s) of the time the female Choloepus didactylus spent out of view, in a walk-through enclosure, in a zoo in the UK, as a function of the noise level (LApeak) during the 15-min sessions of noise recording.

Figure 5. Duration (s) of foraging behaviour of the male Choloepus didactylus housed in a walk-through enclosure, in a zoo in the UK, as a function of the noise level (LAmax) within 24 h of noise exposure.
Discussion
In this study, we evaluated the behaviours exhibited by a pair of two-toed sloths housed in a walk-through enclosure, on days with different levels of sound pressure – measured as LApeak, LAmax and LAeq – due to human visitation. During exposure to higher sound pressure, the male moved more and spent longer time in maintenance behaviours while the female spent more time out of view. Within 24 h of experiencing intense noise exposure, the male displayed a tendency to reduce foraging.
An increase in locomotion, or in general activity in zoo animals – as observed in the male sloth in response to increased noise in this study – has been interpreted as indicative of improved welfare for certain species (e.g. lions [Panthera leo]: Novo & Santos Reference Novo and Santos2014, capuchin monkeys [Sapajus libidinosus]: Koether Reference Koether2017, jaguarundis [Herpailurus yagouaroundi]: Buhr et al. 2018, and Southern brown howlers [Alouatta guariba clamitans]: Muhle & Bicca-Marques Reference Muhle, Bicca-Marques, Ferrari and Rímoli2008). However, studies with other species have shown a correlation between increased activity/locomotion and acoustic stress; in pumas (Maia Reference Maia2009; Maia et al. Reference Maia, Volpato and Santos2012), gorillas (Clark et al. Reference Clark, Fitzpatrick, Hartley, King, Lee, Routh, Walker and George2012), chimpanzees and spectacled bears (Noga Reference Noga2010). In these cases, such increased activity was interpreted as an attempt by the animal to mitigate stress (Mitchell et al. Reference Mitchell, Tromborg, Kaufman, Bargabus, Simoni and Geissler1992; Boere Reference Boere2001; Hosey Reference Hosey2005), or simply as restlessness, caused by noise (Davey Reference Davey2006; Quadros et al. Reference Quadros, Goulart, Passos, Vecci and Young2014; Hashmi & Sullivan Reference Hashmi and Sullivan2020). These apparently contradictory interpretations point to the need for a careful study of data, based on species characteristics (Queiroz & Young Reference Queiróz2018) and, preferably, also on a joint evaluation based on technical measurements of sound pressure levels using appropriate equipment and protocols for a more accurate analysis (Quadros et al. Reference Quadros, Goulart, Passos, Vecci and Young2014; Jakob-Hoff et al. Reference Jakob-Hoff, Kingan, Fenemore, Schmid, Cockrem, Crackle, Bemmel, Connor and Descovich2019; Hashmi & Sullivan Reference Hashmi and Sullivan2020).
The same debate pertaining to the interpretation of increased activity in zoo animals also applies regarding maintenance behaviours; the correlation of such behaviours with stress is often uncertain. Giant pandas increased locomotion, maintenance, scent-marking, and stereotypies during noisy periods but, according to the authors, there was no sign of a decline in welfare (Powell et al. Reference Powell, Carlstead, Tarou, Brown and Monfort2006). However, white-handed gibbons (Hylobates lar) increased frequency and duration of scratching behaviour on days with more zoo visitors, especially children (Ribeiro Reference Ribeiro2016): in this case, scratching may be interpreted as a displacement behaviour (Roth & Cords Reference Roth and Cords2020), suggestive of stress.
Here, we collected behavioural and acoustic data to carefully evaluate possible connections between noise and behaviour. Taking the species’ characteristics into account (low metabolic rates, low-energy diet, inactivity during most of the day, and slow locomotion; Montgomery & Sunquist Reference Montgomery, Sunquist, Golley and Medina1975; Nagy & Montgomery Reference Nagy and Montgomery1980), the increased locomotion/activity and maintenance might be connected to acoustic stress. Another factor contributing to this interpretation is that the average noise level in the rainforest, natural habitat of the study species, is usually around 38 dB(A), considerably lower than the noise levels recorded in this study (Santos Reference Santos2012). Besides, a study on visitor effect in zoos found that herbivorous species and those from closed habitats, such as ours here, were more negatively impacted by visitors than species from open habitats (Queiroz & Young Reference Queiróz and Young2018). The fact that the male has increased locomotion in the moments the noise was more intense during the day, and the records during the subsequent 24 h did not point to such an increase suggests the animal adjusted its activity budget, by relocating locomotion from their natural time (at night) to day-time. Such an adjustment may have unpredictable impacts on the welfare of this individual.
Although we have found no data on the hearing capacities of C. didactylus, studies on the ancestors of the sloth extant species point to a possible need for great sound sensitivity in the species (Blanco & Rinderknecht Reference Blanco and Rinderknecht2012; Blanco & Jones Reference Blanco and Jones2016). Those authors base their argument on the short snouts of certain sloth species, which suggest that they possess more focused and specialised sight, and as a consequence, a need for good sound acuity. This greater hearing sensitivity could make the species more prone to developing stress reactions when exposed to high levels of noise – such as those recorded in this study (e.g. up to 122.2 dBA). Apart from that, the potential (non-measured) high levels of reverberation can be also a factor affecting the sloths’ welfare. Both the circular shape of the enclosure and the material with which it was constructed favoured reverberation. Reverberation has been associated with impaired cognitive processes in humans (Kjellberg Reference Kjellberg2004), and interferences in animal communication (Padgham Reference Padgham2004). The disturbing effects of reverberation may also contribute to animal stress. Different responses to stress in males compared to females have previously been reported (e.g. Vasconcellos et al. Reference Vasconcellos, Guimarães, Oliveira, Pizzutto and Ades2009; Quadros et al. Reference Quadros, Goulart, Passos, Vecci and Young2014). Such differing reactions might be due to diverse coping styles (e.g. Koolhaas et al. Reference Koolhaas, Korte, De Boer, Van Der Vegt, Van Reenen, Hopster, De Jong, Ruis and Blokhuis1999; Ferreira et al. Reference Ferreira, Mendl, Wagner, Araujo, Nunes and Mafra2016).
In contrast to the apparently contradictory results mentioned previously, avoidance/hiding behaviours – as reported for the female in this study – have been consistently linked with fear, stress or apathy (Young Reference Young2003; Forkman et al. Reference Forkman, Boissy, Meunier-Salaün, Canali and Jones2007; Sherwen et al. Reference Sherwen, Harvey, Magrath, Butler, Fanson and Hemsworth2015c). Little penguins (Eudyptula minor) increased their distance from the visitor area and spent more time hiding in the presence of visitors (Sherwen et al. Reference Sherwen, Harvey, Magrath, Butler, Fanson and Hemsworth2015c). Our data also corroborates studies with jaguarundis (Buhr Reference Buhr2018) and quokkas (Learmonth et al. Reference Learmonth, Sherwen and Hemsworth2018) in which hiding behaviours were correlated with visitor presence, and the noise they created.
Foraging behaviours are an important component of the behavioural repertoire of any species, since they are essential for survivorship (Young Reference Young2003). A reduction in the exhibition of foraging in response to visitor noise has been also reported in pumas (Ricci et al. Reference Ricci, Branco, Sousa and Titto2018) and tigers (Panthera tigris: Kerley et al. Reference Kerley, Goodrich, Miquelle, Smirnov, Quigley and Hornocker2002). On visiting days, a giant otter (Pteronura brasiliensis) showed lower frequency of eating, aquatic activity, playing and rolling, behaviours that tend to be associated with good welfare (Oliveira & Carpi Reference Oliveira and Carpi2016).
Although some behavioural alterations reported in this study could lead to conflicting interpretations if taken in isolation (increase in locomotion and maintenance) when seen as a whole, and considering species’ characteristics, our data suggest that our study animals were in a state of restlessness due to noise – a condition with the potential to impact negatively on welfare. Such results have already been reported in walk-through enclosures for squirrels (Woolway & Goodenough Reference Woolway and Goodenough2017). The possible restlessness, in conjunction with an increase in hidden (out of view) time for the female and the tendency for decreased foraging as a medium-term noise response for the male, suggests a stress response, which can be related to reduced welfare. Discomfort due to interactions with visitors has already been reported for sloths (brown three-toed sloths [Bradypus variegatus]), with individuals performing vigilance and limb stretching, behaviours either not reported for the species, or performed at lower rates in the wild (Carder et al. Reference Carder, Plese, Machado, Paterson, Matthews, McAnea and D’Cruze2018). Such results have also been interpreted by those authors as possible evidence of fear and stress.
Animal welfare implications and conclusion
Although our results cannot be generalised due to our small sample size, they suggest the maintenance of two-toed sloths in walk-through enclosures – without any acoustic control (i.e. sonic barrier or management of visitor behaviour) – might be detrimental to their welfare. Acoustic management of zoo enclosures could include shorter visitation times and/or control of the number of concomitant visitors.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/awf.2023.34.
Acknowledgments
We are grateful to the Foundation for Research of the State of Minas Gerais – FAPEMIG – for supplying financial support for this project. YGAR was funded by the Brazilian Council for Scientific and Technological Development (CNPq). We thank the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES) and CNPq for their continued support, and the anonymous referees for their comments and suggestions. We also thank the zoo for allowing this work to be carried out at its facilities, and the staff for their co-operation and assistance – especially in the camera installation process.
Competing interest
None.










