No CrossRef data available.
Article contents
Diffraction Maxima Positions in Two Types of Liquid Organosilicon Compounds
Published online by Cambridge University Press: 06 March 2019
Abstract
A study of two homologous series of liquid oiganosilicates showed a position change of the diffraction maximum of the halo. The first series consisted of polymeric silicic acid esters of the general formula SinOn-1(OC2H5)2n+2 with the following arrangement, where n = 1,2,3,…
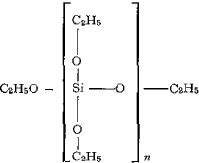
For these compounds, an increase of the d-spacings was observed with increasing length of the –Si–O–Si– chain. The d-spacings observed require coiled molecules.
The second series consisted of orthoesters of silicic acid with the general formula Si(OR)4, where R is the aliphatic hydrocarbon chain. Here, an increase of the d-spacings was observed with increasing number of carbon atoms in the hydrocarbon chain. The molecules have close to spherical symmetry. The hydrocarbon chains provide a very efficient shielding of the [SiO4] tetrahedra, so that only very little intermolecular interference occurs. A similar relation exists also for the refractive indices of these compounds which increase with increasing length of the –Si–O–Si– chain in Series 1 and with increasing number of carbon atoms in Series 2. A plot of silicon concentration vs. d-values or refractive index shows both minimum values for ethyl orthosilicate.
D. B. Nash could show that for silicate glasses, the d-values increased with increasing SiO2 content. For liquid organosilicon compounds, not the silicon content but the size of the molecule (total number of atoms except hydrogen) is responsible for both the change of the d-values and the refractive indices. The size of the “ordered regions” in these liquids is related to the size of the molecules only. The interaction of molecules is minimized by the shielding effect of the organic groups,
- Type
- Research Article
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 1964




