one Glass as a Material A Technological Background in Faience, Pottery and Metal?
Glass is a thing in disguise, an actor, is not solid at all, but a liquid, that an old sheet of glass will not only take on a royal and purplish tinge but will reveal its true liquid nature by having grown fatter at the bottom and thinner at the top, and that even that it is frail as the ice in a Parmatta puddle, it is stronger under compression than Sydney sandstone, that it is invisible, solid, in short, a joyous and paradoxical thing, as good a material as any to build a life from.
1.1 Glass as a Material
Glass was the first man-made translucent ‘solid’. Those who first created it must have been impressed and greatly mystified by the way the glowing red-hot liquid cooled and appeared to ‘freeze’ into a block of ‘solid’ glossy material that reflected and refracted light. A quintessential characteristic of early glass was its colour, which could be used to imitate semi-precious stones such as lapis lazuli and turquoise, and there was even the potential to modify it. Indeed, the first appearance of glass is likely to have been unexpected in a high-temperature environment, leading inquisitive minds to question how it formed. Even if produced adventitiously, it may have been highly coloured and is likely to have been attributed a ritual significance. Some of the earliest glass, made from plant ash and silica, was certainly intended to imitate semi-precious stones and was attributed apotropaic properties. As the scale of glass production increased and the roles that glass played in society changed over time, the processes of its production became less mysterious and less enveloped in ritual. Nevertheless, even today in Murano, the famous centre for glass production in the Venetian lagoon in Italy, glassmaking families retain closely guarded technical secrets.
Clearly, glass production involved a series of inter-related aspects. The more practical aspects included the selection and use of raw materials; the collection of enough of the appropriate fuel types; the production of bricks and construction and use of particular furnace types; observation of the glass being melted; the production of a range of vessel and bead forms by blowing, moulding and winding; the decoration of vessels and beads and the relationship between glass technology and other industries, with the potential for sharing knowledge and raw materials. The ethnicity of all of the groups of people connected to various aspects of production, whether they were responsible for gathering glass raw materials, preparing them, building furnaces and ancillary structures, making crucibles and moulds and blowing and shaping glass, for example, would have had an impact on the object forms and decorative styles produced and on how they were used. The forms and colours of glass vessels and beads produced were a reflection of the period in which they were made. Depending on the social contexts in which they were used, glass colours may have been significant in a variety of ways. For example, during the sixteenth and seventeenth centuries, native Indians in North America believed that glass properties could be symbolic of the mind, knowledge and life with white glass equating with good things, including peace and a desire for understanding and red glass with war, intense experience, animation and fire heat (Turgeon Reference Turgeon and Murray2004, 34–5).
Well before the first glass was made, a naturally occurring glass, obsidian, was worked into tools by percussive flaking. Obsidian can be grey, dark green, or apparently black, but the colours do not even come close to the beautiful range that can be achieved in man-made glass, and obsidian could not be worked at the temperatures achieved by ancient man. Man-made glass could also be flaked, but evidence of this is relatively rare (Fig. 1.1). The reason both glass and obsidian can be flaked as if they were stone is that both are amorphous materials. The word amorphous has a specific meaning to material scientists; it is a characteristic that helps define a material that lacks long-range structural order and can be described as a state of matter (Brill Reference Brill1962). Crystalline silica, as opposed to glass, is composed of silicon and oxygen atoms arranged in a regular way; glass is arranged in a far less regular way, with the bridges between atoms being broken and the other components, such as sodium and calcium atoms, distributed in a relatively random way. Most glasses are composed of a network of silica, SiO2 (the network former) and other metallic oxides. In pure crystalline silica, each silicon atom is bonded with four oxygen atoms, forming what is known as a tetrahedron (SiO4)4+. When arranged into a three-dimensional network, with the adjacent tetrahendra sharing one oxygen atom, it forms pure silica glass (with a melting point of c. 1700°C, unattainable by ancient man). A typical network modifier, such as soda (Na2O), bonds ionically with oxygen within the network and disrupts bridging atoms within the silica tetrahedral network. Network stabilisers (such as calcium oxide, CaO), another type of network modifier, increase the durability of the glass: the bond strength of Ca+ is twice that of sodium (Na+) and strengthens the structure. Trivalent alumina (Al2O3) and iron oxide (Fe2O3) can act as either stabilisers or fluxes.
By using techniques such as X-ray diffraction spectroscopy and neutron diffraction spectroscopy a ‘structure’ of glass can be revealed (e.g. Hannon and Parker Reference Hannon and Parker2000), although this in no way approaches the tightly ordered lattice of metal. Greaves et al. (Reference Greaves, Gurman, Catlow, Cahrdwick, Howde-Walter, Henderson and Dobson1991) have shown that alkalis and nonbridging oxygen atoms are not arranged in a random way but tend to be concentrated in channels, giving rise to the term ‘modified random network model’. This model therefore does not quite fit that for a ‘liquid’. Indeed, the glassy state does not fit any of the three classical states of matter – solids, liquids and gases (Brill Reference Brill1962, 129). This has implications for the durability of ancient glass, as has the distribution of pores through the glass (Lester et al. Reference Lester, Hilal and Henderson2004).
Because glass is amorphous and assuming that there are no inclusions in it when it is struck, the ‘shock waves’ will pass through it in an unhindered way. In contrast, when a highly structured material like metal is struck, the ‘shock waves’ are prevented from being transmitted through it by the presence of ordered crystalline lattices consisting of repeated structural units; this is known as short-range order. When struck, the amorphous property of glass, obsidian and flint produces conchoidal (shell-like) fractured facets (see Fig. 1.1). However, the properties of glass that we are mainly concerned with here are not those that link it to other siliceous materials, like obsidian and flint, but those that come about when it is fused from raw materials, moulded, drawn, blown and wound to create objects and then cooled to a ‘solid’ state.
The apparently ‘solid’ property of glass is somewhat deceptive. Material scientists actually refer to glass as a ‘super-cooled liquid’ (Shelby 2005, 4–5). In 1956, Jones provided the following definition of glass: ‘an inorganic product of fusion which has been cooled to a rigid condition without crystallisation’ (Jones Reference Jones1956, 1). Transparency and translucency must have been considered important properties in the past, so it would have been necessary to avoid the formation of crystals as a result of cooling the glass at the appropriate rate. In relation to the clarity of glass, Al-Buhturi (820–897), a celebrated Arab poet, described a glass containing wine in the following way:
Its colour hides the glass as if it is standing in it without a container.
Another property that has been commented on is the brittleness and transitory nature of (some) glass. In his Pirotechnia, published in the sixteenth century, Vannoccio Biringuccio used these properties as a metaphor for man's own transitory existence:
Considering its brief and short life, it cannot and should not be given too much love, and it must be used and kept in mind as an example of the life of man and of the things of the world which, though beautiful, are transitory and frail.
However, the numbers of complete Hellenistic and Roman glass vessels that have survived intact are a testament to the durability of glass rather than its brittleness. This piece seems to be more of a commentary on the fragility of human life than on (most) glass! Glass came late to China. In the medieval period the Chinese were fascinated by the transparency of glass and at the same time they were puzzled by the fact that glass could be both hard and fluid. They compared it with ceramics, metal, precious stones (especially jade) and even water 'but were unable to find a satisfactory analogy to further people's understanding of the material'. Moreover glass did not fit their 5 element system (metal, wood, water, fire and earth) (Hsueh-Man Reference Hsueh-Man and Braghin2002a, 72–3).
The appearance of ancient glass, including some of the earliest, was deliberately altered to make it appear more like semi-precious stones, such as the rare mineral lapis lazuli, with a principal source in Afghanistan. This was achieved by rendering the glass opaque by adding crystalline materials or developing crystals out of the glass by reheating (striking) the glass.
When glass is cooling, one particular temperature, the transition temperature (Tg), is critical to the formation of a glass rather than a crystalline silicate (Henderson 2000, 24, fig. 3.1). At this temperature, the properties of glass pass from those like liquids to those like solids, although ‘solidification’, as such, does not occur. The crystalline silicate has a lower volume than the equivalent glass, and it is at the transition temperature that an abrupt change of volume occurs in glass and a slowing down of the rearrangements in its structure. When glass cools, its viscosity increases, so much so that for a Roman soda-lime natron glass of a typical chemical composition (see Chapters 4 and 8), Brill (Reference Brill1988) showed that the glass could be gathered and marvered (rolled across a flat [metal] surface to regularise the shape of a gather) at between c. 1100°C and 1000°C and softened sufficiently at c. 1000°C to blow it. Most ancient glass is composed of three major components. The first is silica (SiO2), which is generally present at between c. 65% to 70%; the second is a flux, soda (Na2O), which reduces the melting temperature of silica from between c. 1710°C. and c. 1730°C. to c. 1150°C; the third is ‘lime’ calcium oxide (CaO), which provides durability to the glass. Without calcium oxide the glass would dissolve in water. An alternative alkali, potassium oxide (K2O), was also used. It has been found in glasses dating to as early as the tenth century b.c. in the Mediterranean, western Han Chinese glass and glass dating to the medieval period in northwestern Europe (see Chapter 4).
Wood or plant ashes and alkali-rich minerals are sources of glass fluxing materials (see Chapter 2). By changing the proportions of soda, lime and silica, the melting and working properties of glass also change. Therefore, the chemical composition of glass has a direct relationship to the ways in which it can be worked. Soda-lime-silica glass has a minimum liquidus temperature (the absolute melting temperature of the glass above which nuclei and crystals cannot form) at the ternary eutectic of 725°C for a composition of 21.9% soda, 5.1% calcium oxide and 73.1% silica (Morey Reference Morey1964, fig. 20, tables 13 and 33). A eutectic mixture of compounds is one that has the lowest freezing point of all possible mixtures of sodium, calcium and silicon oxides. The wide range of ancient glass chemical compositions that has been found is discussed in Chapter 4. Variations in the balance of each major (and some minor) component in the glass would have had a direct affect on its working properties.
1.2 The Formation of Glass: Of Volcanic Glass, Asteroids, Slags and Scums
When magma is spewed from volcano vents and then chilled, obsidian is formed. Nuclear explosions, such as the first atomic bomb test at the Trinity site in New Mexico in 1945, can lead to glass formation. More recently, the use of another natural glass has been suggested as the material used for the carved scarab that forms the decoration of a breastplate of King Tutankhamen: an asteroid. Asteroid impacts (Mayell Reference Mayell2005) can leave a ‘carpet’ of glass. One exploded 29 million years ago above the Sahara Desert, turning the sand into glass, with a heat that was equivalent to a 110-megaton bomb (see http://www.sandia.gov/news/publications/technology/2006/0804/glass.html). Impact metamorphism can produce ‘diaplectic glasses’ from quartz and feldspars (Heide and Heide Reference Heide and Heide2011, 28).
In considerably less dramatic contexts, glassy slags can be produced in virtually any high-temperature environment and it is these that probably constituted the first glassy material seen by ancient peoples. Glassy slags can be produced by burning cereals rich in silica-rich opal phytoliths, which provide rigidity to the plant structure (Dimbleby Reference Dimbleby1978, 129–30), and the combustion of haystacks (Baker Reference Baker1968). Folk and Hoops (Reference Folk and Hoops1982) found ‘attractive’ twelfth-century b.c. blue-green glass at Tel Yin'am in Israel, which they interpreted as being the adventitious fusion of silica-rich plant ash and silica. Even though vesicular (ibid., 460, fig. 14), the chemical composition and colour suggest that it was possibly man-made. Youngblood et al. (1978) published scientific analyses of glasses that were formed when the ramparts of Scottish Iron Age forts were ignited, the result of silica in the soil fusing with alkaline-rich materials in the fort ramparts. This actually led to a strengthening of the defences. ‘Bone-ash’ slags can be produced in cremations, and these also typically have a vesicular appearance (Henderson, Janaway and Richards Reference Henderson and Cunliffe1987); Photos-Jones et al. (2007) have shown that seaweed known as cramp fused to bone in Bronze Age burials also produced a kind of vitreous slag. Glassy (fuel ash) slags are also often produced in hot furnaces and kilns in which metals are smelted or pots fired; the ashes from the fuel interact with silica present in both the bricks used to build the furnaces and kilns and in crucibles containing hot metals (Biek and Bayley, Reference Biek and Bayley1979; Henderson 2000, 53). Indeed vitrification in pottery, which results from alkali-bearing minerals interacting with silica in the clay (Kingery et al. 1976, 490), can produce hard ceramics such as stoneware and porcelain (Henderson 2000, 133). Pottery wasters from kiln sites often resulted from pots being heated to such high temperatures that the clays became glassy, having started to bloat and the pots then lost their shapes (Henderson 2000, 133). Even glass production generates a range of glassy slags resulting from the interaction of the fuel ashes with the silica-rich bricks used to construct the glass furnace. Glass production can also generate vitreous ‘scums’ (the nonreactive ingredients of the glass batch) on the surface of glass melts, or they are deposited on the sides and lips of crucibles as the raw materials in the glass batch fuse and the whole melt contracts. Thus these very different formation environments led (and still lead) to the formation of vitreous slags of a range of distinctive chemical compositions, some of which are highly coloured.
This adventitious formation of glass may be regarded as somewhat prosaic in the context of ancient technologies. However, the brilliant red glassy material produced by the presence of reduced copper (Cu I) in metal smelting (Henderson 2000, 54) must have been impressive. Its blood-red colour would undoubtedly have had a ritual significance. Moreover, it is striking that one of the commonest early glass colours in Bronze Age Mesopotamia, Mycenaean Greece and in parts of Europe is the oxidised form of copper (Cu II), which is a turquoise green colour. So although the occurrence of copper in early glasses shows that a copper-rich colourant was available, it does not prove that metallurgy was the driving force behind the emergence of the first glass. As one of the last primary ancient inorganic materials to have been manufactured, it would be obtuse to suggest that only pottery or metal or faience technology led to the development of glass (see Figs. 1.2 and 1.3). Peltenburg (Reference Peltenburg, Bimson and Freestone1987, 20–2) has stressed the paucity of solid evidence for the link between glass and metal technologies. Nevertheless, copper-rich minerals did provide the critical colourant that allowed early glassmakers to imitate turquoise, a stone considered to have health-giving properties with ritual playing a dominant part in every aspect of ancient society. Moreover, early stone glazing was achieved by heating copper ore on the powdered surface of talc (Hodges Reference Hodges1970, 62). So the availability of copper can be regarded as one of a number of parameters that played a part in the emergence of early vitreous materials, including glass.
Thus, the adventitious production of glass can be regarded as especially significant in two ways: (1) if brilliantly coloured, it would have made a great impression on those who first observed it, and (2) its very formation would have been striking and almost certainly would have motivated those who saw it to manufacture glass deliberately.
Evidence for an apparent value for early vitreous slag is provided by the discovery of (unpublished) greenish glassy slag placed in an Akkadian inhumation burial (c. 2300 b.c.) at Tell Brak excavated by David and Joan Oates. This was characterised by elevated levels of phosphorus and calcium and is therefore probably a bone-ash slag. Although to our eyes it may appear insignificant, given that it was found in an area where the first glasses in the world are likely to have been made and dates to around the time when they were first made, it suggests that such materials may have played a part in the experiments involved in the production of the first glasses. It is, however, difficult to agree with J. B. Lambert's statement (1997, 105) that ‘Refinement of slag could have eventually led to glass manufacture’ simply because it is difficult to envisage how this could be achieved. Some of the earliest glass known, such as that from Eridu, Iraq (Garner Reference Garner1956a), dating to c. 2300 b.c., has a chemical composition that is similar to glasses found in archaeological contexts that date to a period covering a further 1,300 years (see Chapter 6). The chemical composition of the block of cobalt blue glass from Eridu indicates that it was made deliberately from a combination of plant ash and silica and coloured with a cobalt-rich material. There is no hint of a compositional link to ‘refined’ slag, and it is unlikely to be a by-product from an experiment. The technology of ancient glass production may therefore have been ‘fully formed’ by this time.
1.3 Production of the First Glasses
The first glass appeared c. 2500 b.c. in modern-day northern Syria and Iraq (Moorey Reference Moorey1994). Initially the glass made from plant ash and silica would have been fused in a crucible in relatively small volumes. Most of this early raw glass was then made into beads, and it was not for another 1,000 years or so that larger volumes of raw glass and greater quantities of glass objects, including the first (late Bronze Age) core-formed vessels, were produced. Therefore, there was a period of c. 1000 years during which only small quantities of glass were made (see Chapter 5).
The first appearance of glass, and subsequent technological developments, seem to fit Cyril Stanley Smith's contention that ‘the discovery of the materials, processes, and structures that comprise technology almost always arose out of aesthetic curiosity, out of the desire for decorative objects and not, as the popular phrase would have it, out of preconceived necessity’ (Smith Reference Smith1981, 347). When seen for the first time, the shiny, coloured, translucent, refractive and smooth properties of glass must have been both exciting and inspiring. As Smith went on to say, ‘discovery is art, not logic, and new discoveries have to be cherished for reasons that are far more like love than purpose’ (Smith Reference Smith1981, 347). By stating this, Smith, who had a worldwide reputation as an Massachusetts Institute of Technology–trained material scientist, was removing the predictable aspects that characterise much contemporary positivist material science research, consisting of a mechanical and inevitable series of established procedures in the primary manufacture, formation and shaping of materials. For example, the subtle and progressive changes in the colour of glowing iron as a blacksmith works it is a reflection of its changing crystalline structure and something that has little relevance to modern industry. Such characteristics are therefore more connected to the art of creation (and in ancient contexts also to ritual and religion) than to logic.
Although the first glass may not have been deliberately coloured, the process of creating and then modifying the glass eventually led its creators to produce glass opacity, involving the incorporation of masses of tiny crystals (Henderson 2000, 35–8) in imitation of semi-precious stones (Fig. 1.4). Pale blue glass was produced in imitation of turquoise; a deep cobalt blue colour was produced in imitation of lapis lazuli; opaque yellow perhaps in imitation of gold and opaque red in imitation of blood. Most of these colours first started to be made from about the mid-fifteenth century b.c. when the first glass vessels were produced in Mesopotamia (Nolte Reference Nolte1968; Moorey Reference Moorey1994, 193). The Mesopotamian concept of raw material sources was that most had to be imported from specific 'mountains', some more mythical than others (Oppenheim Reference Oppenheim, Brill, Barag and Von Saldern1970, 9). For example there was a 'cedar mountain', a 'gold mountain', a ‘silver mountain’ and a 'lapis lazuli mountain'. The wide range of terms used to describe the wide range of shades of lapis lazuli, such as 'beet coloured', 'wild donkey coloured' and 'star-like, starry' [the latter relating to flecks of pyrite in lapis lazuli] is a reflection of how lapis lazuli colours were the most cherished.
There are important questions concerning the manufacture of the first glass that we may never be able to answer adequately. Any evidence for the earliest phases of primary glass production is likely to be on a small scale. It is possible that the first glasses were made deliberately using a range of raw material proportions so as eventually to optimise the process. Perhaps the relatively common production of vitreous slags prompted early glassmakers to somehow establish that plant ash and silica were the primary raw materials. The successful production of glass or glassy materials would, however, be limited by the proportions that could successfully lead to glass production, a ratio of 2:1 by weight of plant ash to silica. Rehren (Reference Rehren2000, 15; 2008, 1353) has argued that glass melts follow minimum eutectic troughs, leading to relatively narrow glass compositions. Evidence for experimentation could potentially be shown scientifically if the chemical compositions of contemporary vitreous materials and glasses found on a production site occupy a range or a continuum of connected compositions. The compositional continuum would reflect the use of different proportions of raw materials in the glass batch (ideally mixed in a ratio of 2:1 by weight of alkali to sand) and reveal which were unsuccessful. Experiments with different proportions of beech ash and sand have revealed how the all-important melting behaviour of the resulting glasses is affected (Smedley and Jackson Reference Smedley and Jackson2002). By plotting proportions of one component against another, a mixing line – the ‘dilution’ of one major component by another – would be produced (see Chapters 10 and 11). The caveats to the possible discovery of early experimental glasses are, first, that the chemical compositions of some partially reacted raw materials would be altered by weathering and, second, that the chemical compositions of the glassy materials may not be a direct reflection of the raw materials used to make them (Turner Reference Turner1956b; Rehren 2008). Some ‘unsuccessful’ glass batches (the combination of raw materials in the crucible) are likely to have been vitreous materials containing unfused or partially fused silica. Returning to Cyril Stanley Smith's contention, we should remind ourselves that the actual driving force (rather than the physical evidence) for the earliest glass is likely to have been the excitement of creating it!
During the incipient stages of glass production, for whatever reason, artisans may have found that small changes in the type or proportion of raw materials used in the glass batch would have caused the glass to be difficult to melt and work. For example, an increase in the proportion of silica in the batch could produce a glass that would melt at higher temperatures and, moreover, was workable for a shorter time (a so-called ‘short’ glass). Alternatively, a smaller proportion of reactive plant ash would have produced the same result (also potentially leading to a relatively high proportion of silica). In contrast, a drastic change in the batch recipe would probably have caused a nightmare of unpredictability in the ways in which the glass behaved when worked. Indeed, this was the reaction that a contemporary glass artist gave to the prospect of a new batch composition. Therefore, it is possible that early glass batch compositions may have been modified in a gradual way to be able to learn from, and react to, any changes. Constantly changing the conditions in which glass was produced would obviously have caused frequent and unpredictable results. Indeed, predictability is clearly important during each stage in the chaîne opératoire, leading to the control needed to produce the fully formed plant ash glass that was in use from c. 2500 to 1000 b.c. in the Middle East and used to make the first glass vessels. This is not to underestimate the excitement and perhaps the reverence that those involved had for the act of creating and experimenting with a ‘new’ material. As Vandiver and Kingery (Reference Vandiver, Kingery and Kingery1986, 32) have noted in the context of faience production, a period of innovation was followed by a phase when the technology became ‘a traditional method, difficult to change and suffering new innovations slowly and in small increments’. In other words, why innovate further if what you already have is satisfactory? Once the behaviour of hot liquid glass of a particular composition had been monitored, understood and ‘tamed’ to the extent that the required glass objects could be manufactured, there would have been little incentive to change raw materials or other aspects of the technology. This may well have been the case, but we should not exclude the possibility that the acts of experiment and innovation were viewed as essential parts of the ritual and economic fabric of society, and many products of such activities may not have survived.
The experimental phase would have been followed by a developmental phase. Although further experimentation with, for example, colourant materials, may well have occurred, the scientific proof for this phase could be provided by evidence of repeatedly combining raw materials to produce consistently similar results. In the experimental phase, the variation in the proportions of raw materials used might have been relatively wide; by the time the chaîne opératoire had become embedded, fewer decisions needed to be made and the procedures in glass production would have become more restricted and constrained in terms of choice. Therefore, the glass chemical compositions produced would also have become more tightly constrained. It is notable that during times of transition from one major glass technology to another, glass chemical compositions can become far more variable. This can be seen in, for example, the change from natron glass to wood ash glass in medieval Europe north of the Alps (see Chapter 4) when these two types of glass were mixed.
1.4 The First Glass: A Paradigm Shift?
Glass was the latest of the three main (inorganic) technologies – pottery, metal and glass – to appear. To assess whether the manufacture of the first glass can be regarded as a paradigm shift in the development of ancient technology, it is necessary to consider whether the processes involved in its production had a distinctiveness that sets the material apart from the technologies that already existed. The materials involved are those made from a vitreous component. These preceded glass and include glazed steatite and faience; these are focused on here. Like glass production, pottery and metal technologies frequently involved the construction of kilns and furnaces respectively. These structures are necessary so as to produce high temperatures and to be able to control heat and the gaseous atmosphere. Therefore, access to and preparation of ceramic materials or stone for constructing kilns and furnaces creates a link between all three technologies. Similarly, access to appropriate types of fuel would be necessary to achieve the temperatures and atmospheres.
1.4.1 Glazed Steatite, Egyptian Blue and Faience
Glazed steatite (or soapstone) was produced before the first faience. Steatite is mainly composed of talc with minor amounts of other minerals, such as chlorite; it is a hydrated magnesium silicate. Examples have been scientifically analysed by Vandiver (Reference Vandiver, Kaczmarczyk and Hedges1983a) and Tite and Bimson (1989). It has been found that the glazed surface of steatite contains high magnesia levels (c. 27%), indicating that the alkali forming the glaze and the copper must have reacted with the steatite. The key factor here is that the material on which glaze is formed is silica-rich.
Egyptian blue was produced at high temperatures perhaps using repeated firings. It was made by heating together silica, copper alloy filings or crushed copper ore (such as malachite), calcium oxide, and a fluxing material, such as natron or trona (see Chapter 2) or, according to Lee and Quirk (Reference Lee and Quirke2000, 109), potash. It is a crystalline material and contains rectangular blue crystals of cuprorivaite, (Ca,Cu)Si4O10), unreacted quartz, and alkali-rich glass. Copper-bearing wollastonite is often present, and minor components include pyrite (FeS) and titanomagnetite (Fe3O4-Fe2TiO4), considered to have been introduced in desert sand (Jaksch Reference Jaksch, Seipel, Weiner and El Goresy1983, 533–5) and cassiterite (SnO2). Tite, Bimson and Cowell (1987) recognised three shades, dark blue, light blue and diluted light blue, the colour variation mainly being due to the size of cuprorivaite crystals and the proportion of alkaline glass. The shaping of objects made from it involves moulding, carving, cutting and grinding.
The earliest occurrence of Egyptian blue is the Fourth Dynasty (2575–2465 b.c.; Lucas 1962/1989, 342) with other examples dating to the Fifth Dynasty (2465–2323 b.c.; Blom-Böer Reference Blom-Böer1994). It was even used to decorate the Eighteenth Dynasty head of Queen Nefertiti (Wiedemann and Bayer Reference Wiedemann and Bayer1982). Studies of later Egyptian blue include that from Ugarit, Syria. The occurrence of it there, in late Bronze Age contexts, was associated with suitable raw materials and its scientific analysis using proton-induced X-ray emission and scanning electron microscopy suggest that it was made locally (Matoïan and Bouquillon Reference Matoïan2000, 990–3, fig. 8). Trace occurrences of strontium, chlorine, alumina and magnesia were found to be particularly discriminative.
The sight of another vitreous material, faience, would clearly have reminded its makers that the vitreous state existed: it was a hard, highly coloured, glazed material that often reflected the light. Before the first glass appeared, a vitreous layer would have been seen as decorative and/or as something functional that held together the sandy core of the object and also ‘held’ the colourant in place. In the production of faience, there must have been a deliberate control over the maximum temperatures achieved because over-firing would have produced greater fusion of silica and alkali and, at sufficiently high temperatures, the production of glass. Over-firing was therefore apparently to be avoided, although the kilns used (e.g. Nicholson Reference Nicholson2003) would have minimised the possibilities. Hodges (1992, 125) suggested that overheated faience might have provided the first sight of true glass. Tite et al. (2002) also have suggested that this was the case and that, in addition, poor compositional control might have played a part.
Hodges (1992, 62) has suggested that the production of faience from c. 4000 b.c. ‘can be looked upon as man's first real move into the world of synthesising the material he required’. Essentially the same raw materials, silica and plant ash, were used for making both faience and the first true glass, so the relationship between the technologies cannot be ignored. The Qom process of faience manufacture (discussed below), observed in Iran in the 1960s, involved heating these raw materials together at temperatures up to c. 950°C. However, to produce properly fused glass, at least 200°C. higher temperatures are necessary, involving more and/or different kinds of fuels. Nevertheless, by using the same principle of synthesising raw materials to produce a different material, the first glass was produced. Although the chaîne opératoire would have involved similar initial preparatory steps as in faience production, the crushing of quartz pebbles and ashing of plants, there would have been a number of differences. The higher temperatures involved in making glass would have necessitated a furnace rather than a small kiln. A second difference is that faience objects resulted directly from the single process of firing, whereas the production of raw glass was a starting point: to make objects, the raw glass needed to be reheated and worked.
The first (sixteenth–fifteenth century b.c.) glass vessels echo the shape of faience vessels (Peltenburg Reference Peltenburg, Bimson and Freestone1987). It is therefore likely that certain aspects of the production processes of faience contributed to a chaîne opératoire, leading to the production of the first deliberately made raw glass and the first glass vessels. Some of the earliest glass vessels have been found at Alalakh, in modern-day Turkey (see Chapter 5). They are stratified with faience vessels, so in theory it ought to be possible to see whether the faience industry there reacted to the introduction of glass (Peltenburg Reference Peltenburg, Bimson and Freestone1987, 14). Although the number of artefacts is small there is no clear technological 'anticipation' in 16th century b.c. faience technology and the emergence of the first glass vessels. As Peltenburg (Reference Peltenburg, Bimson and Freestone1987, 18) has noted, 'Faience-working therefore provided but a general background rather than a direct impetus for glass production, which, as has often been stated, appears suddenly.' If anything, the closest link, in terms of vessel shape, is between glass and glazed pottery (Peltenburg Reference Peltenburg, Bimson and Freestone1987, fig. 3).The raw materials for faience production are crushed quartz, plant ash and copper (oxide). Using laboratory replication and microstructural examination of polished sections, Tite and Bimson (Reference Tite and Bimson1986) defined three techniques of faience production. Moreover, Vandiver (Reference Vandiver, Kaczmarczyk and Hedges1983, 1998) emphasised the importance of observing drips of glaze, drying or firing marks and variations in glaze thickness as ways of suggesting the methods of glazing used. As with the production of glazed steatite, fluxing of the silica by an alkali-rich plant ash created the lustrous glassy surface, and the vitreous network in some cases also provided strength to the faience object. The efflorescence technique involved mixing the raw materials with water to create a mouldable paste. The water in which the alkali was dissolved was drawn to the surface by evaporation, resulting in a concentration of the alkali in the surface layers. When the objects were fired, they hardened as a result of the alkali fusing with the quartz and the copper. The Qom technique, referred to earlier, involved cementation, first by making the moulded faience object, and then submerging it in a glaze mixture and firing it. The third technique of faience production speaks for itself: it involves the direct application of a glaze slurry to the moulded object followed by firing. However, Vandiver (Reference Vandiver, Colinart and Menu1998) has pointed out that it can be difficult to prove with certainty which production technique was used to make faience based on microstructural characteristics. Henderson has noted metallic inclusions, such as tin and antimony in Bronze Age Swiss and early Iron Age Balearic faience (Henderson Reference Henderson1988b, 438, figs. 1a and 1b; Henderson Reference Henderson, Lull, Micó, Herrada and Risch1999). Their occurrence is a means of characterising the copper minerals used to produce a turquoise colour in the vitreous phases of faience. A ‘transitional’ material between glass and faience, ‘glassy faience’, exists (Lucas and Harris Reference Lucas and Harris1989, 164–5; Shortland and Tite 1998; Nicholson 2000; Santopadre and Verità Reference Santopadre and Verità2000; Shortland 2000) in which there is a high proportion of glass in the quartz-rich object. Some glassy faience predates glass.
Moulded faience objects were made in a single process, which, unlike moulded glass, did not require subsequent working. Faience was evidently manufactured at Kerma in the Sudan c. 2000 b.c. Amongst the evidence were faience moulds, an indication of primary faience production. On the basis of the distribution of faience, its production debris and the historical evidence for its production, Shortland, Nicholson and Jackson (2001, 154–6) noted that it is likely that temples controlled some faience workers (in Egypt) but that there was also an independent private sector that produced faience found/used in a wide range of contexts, including those of a low social status. Despite the fact that the first faience was made much earlier than glass and that opaque moulded glass is quite similar in appearance to faience, faience continued to be made well after glass was introduced. Tite et al. (2007) emphasised the conservatism in the production of plant ash faience glazes with distinctively low calcium and high potassium oxide contents from the Egyptian Middle Kingdom until at least the Eighteenth Dynasty, presumably a reflection of a repeated series of technological processes using similar raw materials from similar geological sources. Relatively recent discoveries (Nicholson 2002, 2003) confirm that faience vessels were still being made as late as the Ptolemaic–early Roman periods at Kom Helul, Memphis, in Egypt. The comprehensive evidence consisted of saggars, three-pointed stands and furnaces, some of which were preserved to at least 3 metres in depth. At Kom Helul it is thought that a stack of saggars containing the faience vessels was placed inside the furnaces. The continuing ‘late’ manufacture of faience must indicate that the tradition of making it was firmly established in Egypt and that it filled a ritual niche that the production and use of, for example, faience ushabti figurines, that could not be replaced by glass (see Fig. 1.2).
Another material, which appeared about the same time as the first glass vessels (c. sixteenth and fifteenth centuries b.c.), were glazed ceramics. The glaze is often not particularly durable, so it is difficult to analyse it scientifically. Scientific analysis has been carried out on pottery glazes from Tell ‘Atshana (Peltenburg Reference Peltenburg1969), Ras-Shamra-Ugarit, Meskene, Kition (Matoïan and Bouquillon Reference Matoïan and Bouquillon1999), Kish (Hedges and Moorey Reference Hedges and Moorey1975), Failaka (Pollard and Højlund Reference Pollard and Højlund1983), Tell al-Rimah (Pollard and Moorey Reference Pollard and Moorey1982), Tell Brak (Henderson Reference Henderson, Lull, Micó, Herrada and Risch1999b) and (of a somewhat later date) Kish, Nineveh and Nippur (Hedges Reference Hedges1976, 1982). For the glazes that are not too weathered, it is clear that, in general, they are of the expected plant ash composition with elevated potassium and magnesium oxide contents (Tite, Shortland and Paynter Reference Tite, Shortland and Paynter2002). However, the glazes from Failaka, analysed using atomic absorption spectroscopy, have anomalously high magnesia and low potassium levels. Pollard and Højlund (Reference Pollard and Højlund1983, 199) have suggested that this was probably due to the use of a high magnesia raw material. Unexpectedly, an example from Tell Brak indicates that natron may well have been used as the alkali source (Henderson Reference Henderson1997, table 6b, sample Br16). Matoïan and Bouquillon (Reference Matoïan and Bouquillon1999, 74 and 2003) are of the opinion that there was a specialised production of glazed pottery at Ugarit. This is partly based on its rarity in the southern Levant and partly because it was unknown in Egypt. Similar vessels have been found on sites on the southern and south-eastern coasts of Cyprus. Matoïan and Bouquillon (2003, 344) note that the mineralogical characteristics of the pottery bodies are likely to be those of the environment of Ras Shamra-Ugarit.
Tite et al. (2002) have pointed out that glass and metal working would have involved hot viscous fluids and therefore that metal workers may have played a part in the emergence of glass working. A further important process that the metal and glass technologies have in common is annealing, and this may have constituted another input from metal technology. A suggested trigger for technological change/advancement, which may have led to the sharing of technological knowledge, was put forward by Tite et al. (2002, 588): ‘it can be argued that the discovery of techniques necessary for hot-working glass was the result of political upheavals that occurred in Egypt and the Near East leading to changing controls over artisan organisation.’ This may well be part of the explanation, although given that the manufacture of the earliest glasses in Bronze Age Mesopotamia and Egypt was very much an elite pursuit, whereas metal and faience production were not necessarily, and that glass production was described in great detail in cuneiform texts, unlike metal and faience production, the explanation is probably more complex than this. In conclusion, between c. 4500 and 2500 b.c., any glass produced formed part of something else and was not a separable entity in its own right. This is important, because until there was a realisation that glass could be manufactured and used to make separate objects, no progress would be made in the manufacture of glass objects. Once it was realised that raw (unworked) glass could be manufactured, it would have acted as a technological trigger to further developments; it may simply have been the ‘first’ sight of a translucent block of coloured glass that triggered further developments. Whether it would initially have been perceived as a man-made coloured form of rock (crystal), or something entirely new, because it was formed in an entirely different way and had a number of different properties, it is difficult, if not impossible, to assess. The corollary of this is that if a glass melt was allowed to cool too slowly past the transition temperature (Tg), it would have produced what might have been regarded as a kind of coloured stone–coloured crystalline silica. Indeed it is inevitable that the silica-rich crystals resulting from devitrification would have been produced during the early stages of (unsuccessful) glass production. Subsequently devitrified glass may have provided a visual link between semi-precious ‘stones’ and true glass; it is no coincidence that early glass was referred to as a ‘stone’ (see Chapter 5).
Thus, there are several aspects to the production of the first glass that can be claimed as forming part of a paradigm shift. First, to deliberately produce a block of brilliant translucent glass that reflected and refracted light was clearly a break from what had gone before technologically. The addition of trace levels of colourants to the glass, such as cobalt and copper, was a highly controlled new technological process. Moreover, the creation of an opaque glass that involved the addition of crystalline materials to the glass melt which were retained at high temperatures, or the development of crystals out of solution by heat treatment (and its link to annealing), were also new processes (such as the copper droplets in Fig. 1.4). They were especially important because, together with, or instead of, the addition of trace levels of transition metals that dissolved in the glass, the crystals coloured the glass. The social and ritual significance of (glass) colour in ancient societies cannot be underestimated. Glass coloration can be regarded as a primary driving force behind the high levels of innovation in making the first glass (Duckworth Reference Duckworth2011). Ancient opaque glasses are what today would be labelled as glass ceramics: the first opaque glasses were therefore the earliest glass ceramics. However, over and above all of these innovative processes, which by themselves can be regarded as major variations and innovations from existing technologies, was the fusion of glass from its primary raw materials. When compared with metal and pottery manufacture, there is little resemblance between glass raw materials (plant ash and silica) and glass. In every respect the manufacture of the first glass can therefore be regarded as a paradigm shift. In the next section, the difficulties of interpreting evidence for primary and secondary glass production are discussed.
1.5 Evidence of Production Sites
The discovery and excavation of glass furnace sites might be expected to provide ‘fixed points’ in the investigation of glass production of all ages. However, the interpretation of archaeological excavations is rarely straightforward. The evidence for glass making and working can be variable and inevitably incomplete. This is hardly unexpected, given that this is true of any archaeological excavation. A first crucial question to be addressed is whether primary or secondary glass production has occurred. Evidence for primary glass production can consist of evidence of fritting, including overheated frit and fritting ovens (see Chapter 11); the structure and scale of glass furnaces can also provide an indication (Henderson 2000, 39–40). If a fritting oven is discovered, then this is clear evidence for primary production. Even though frit could potentially also be imported, this does not detract from the evidence of primary production. The recent identification of the evidence for fritting in thirteenth century b.c. Qantir (Rehren and Pusch Reference Rehren and Pusch2005) and fourteenth century b.c. Tell el-Amarna (Smirniou and Rehren Reference Smirniou and Rehren2011) are rare examples. Tank furnaces that can be up to 6 metres long (see Chapter 7) can potentially be used for reheating glass or for fusing primary raw materials. Scientific analysis, especially involving the use of isotopic analysis in an environmental context can establish whether glass was fused using local raw materials and whether mixing has occurred. There is also the possibility that a tank furnace was used for both primary and secondary glass production.
The evidence for glass working, including blowing, moulding, shaping, manipulating and decorating, leaves a range of evidence, including glass furnaces incorporating annealing chambers, separate annealing ovens, relic deposits of fuel, moulds, crucibles, drops, dribbles and pulls of glass. Pulls of glass were extracted so as to test the working properties of the glass as it was heated up and to observe its colour. In an ideal world, tools for working glass, such as blowing irons and pontil rods, would be found. Given that the floors of glass workshops are likely to be clean, it is unlikely that when deserted, a range of useful evidence for past activities would remain. If glass is found, unless fused to the furnace or nearby surfaces, it is most likely that it would be redeposited and may even derive from other glass workshops or nearby furnaces. There may be associated layers of material that can be shown to result from the glass workshop that provide evidence for the colour and chemical composition of the glass being produced and (just possibly) the kinds of glass vessels made in the workshop. Nevertheless, there is always the possibility that even the archaeological layers connected to glass production contain imported scrap glass (for recycling), including vessel fragments. So it would be easy to make an assumption that glass vessel fragments found in or around glass-working workshops or furnaces were made there, but this assumption needs to be scrutinised very carefully.
Scientific analysis set in an environmental/ecological context can provide evidence that the glass was fused using local raw materials. However, glass objects may well have been made from imported raw glass (see Chapter 11). Experimental archaeology combined with scientific analysis can help to provide evidence for the kinds of by-products that can result from glass making, the extent to which they are likely to survive unaltered and how easily they can be connected to the glass being made at the site (Paynter Reference Paynter2008).
1.6 Conclusions
The manufacture of glass represents a significant development in terms of material transformations beyond the manufacture of a vitreous component in faience. It involved the full solution of colourants in the glass, producing a translucent highly coloured, smooth, refractive material quite unlike anything else that had been made before. Its first appearance, and subsequent uses, can be regarded as a paradigm shift. Furthermore, heat treatment of glass to promote the growth of calcium antimonate crystals and opacity in coloured glass produced a material that was very close in appearance to semi-precious stones. In modern terms, it would be classed as a glass-ceramic, and the mid-second millennium b.c. saw the first examples in the world being made. The heat treatment of glass to produce opacity was a new departure in technology. It may possibly have been ‘borrowed’ from a similar procedure involving heat treatment, metal annealing, although the latter did not produce the same obvious visual results. The idea of heat treating the glass, by reheating it to produce opaque glasses, probably formed part of an evolution in the understanding of how molten glass behaved at various temperatures, including the cooling rate of the melt at the appropriate rate past the transition temperature (Tg) so as to produce glass rather than silica crystals. In the initial stages of making the first glass, it is inevitable that glassmakers cooled the glass melt too slowly and that coloured crystalline silicates instead of glass were produced. The reason why this occurred was that the rate at which the melt was cooled led to a solid (crystals) rather than a (super-cooled) amorphous liquid-glass forming. In scientific terms, the properties of glass and crystalline silicates are quite different, as measured by volume per unit mass, the coefficient of expansion and the specific heat capacity (Guinier Reference Guinier1984, 168–9, fig. 8.7). Although the creation of glass was still possible by remelting the silicate crystals produced, their creation must have contributed to the description of glass as a stone, the crystals having greater similarity to minerals, with internal refractivity, than to amorphous glass. The transformation of these silicates into glass must have provided a great sense of control in the transformation of natural ‘rocks’ into glass (synthetic stones). A third new process involving heat treatment was the annealing of fully formed glass objects at relatively low temperatures, leading to durable and longer-lasting glass vessels and other objects.
The synthesis of opaque glass to imitate semiprecious stones was accompanied by a range of other groundbreaking innovations. These included the production of the first core-formed vessels, the first mosaic glass vessels using sections of opaque glass assembled in a mould and the first mass production of core-formed vessels (by the fourteenth century in Egypt); see Chapter 5. The fifteenth and fourteenth centuries b.c. therefore saw several contemporary seminal innovations in glass technology. From at least c. 1370 b.c., larger furnaces and associated structures must have been built in Egypt, not only to accommodate a sufficient volume of glass to make larger numbers of objects but also to have afforded sufficient control of the heat source to be able to fully melt raw glass and to be able to provide a consistently high temperature of c. 1100°C and above so that plant ash glass would remain fluid enough to gather it on a core. To wind a decorative glass filament or cable around the vessel and to marver the decoration into the vessel surface (by rolling it across a flat metal surface) would have involved reheating it several times. Finally, an annealing oven or chamber allowing plant ash glass vessels to cool slowly through the critical annealing temperature of 529°C and an annealing range of between about 500 to 575°C for a soda-lime glass (Brill Reference Brill1988, 279–81) would have been necessary from an early stage. A change in glass viscosity (measured in units of poise) occurs between the temperature at which the glass is melted (about 2–3) and the annealing point (13). All of these high temperature operations would have required a clear understanding of the calorific values produced by combusting a range of fuel types.
A range of characteristic smells would have been produced by burning different fuel types at different stages of the production process and also during the process of glass melting. For example, during the fritting process, if sufficient sulphates were present in the raw materials, the evolution of sulphur dioxide gas would have produced a characteristic smell of rotten eggs for that stage of the process! The colour of the glass melt and of the flame produced would similarly have provided ways of assessing the stage that the chemical reactions had reached. For example, the cuneiform texts mention the production of Dusu-coloured glass (Oppenheim et al. 1970, 47–48): once a mixture of three raw materials begins to glow green, it is taken out of the kiln and ground finely (this is presumably frit, a sintered combination of glass raw materials; see Fig. 1.5). It is then placed in the kiln again and heated until it glowed yellow at which point glass is allowed to form. Observed colour changes in the glass batch are also described in later (medieval) texts (e.g. Theophilus presbiter).
The ritual associated with the use of specific raw materials at particular times of year would have provided a driving force for each link in the chaîne opératoire in glass production. Therefore, a combination of natural raw material properties and man-induced forces led to the production of the first true synthetic material.

1.1. Australian aboriginal arrowheads knapped from bottle glass (photo: J. Henderson; reproduced with kind permission of Håken Wahlquist and the Ethnographic Museum, Stockholm).

1.2. Two faience ushabti figures from Deir el Bahr (c. 1000–900 b.c.; photo: J. Henderson; produced with kind permission of the Museum of Mediterranean and Near Eastern Antiquities, Stockholm).

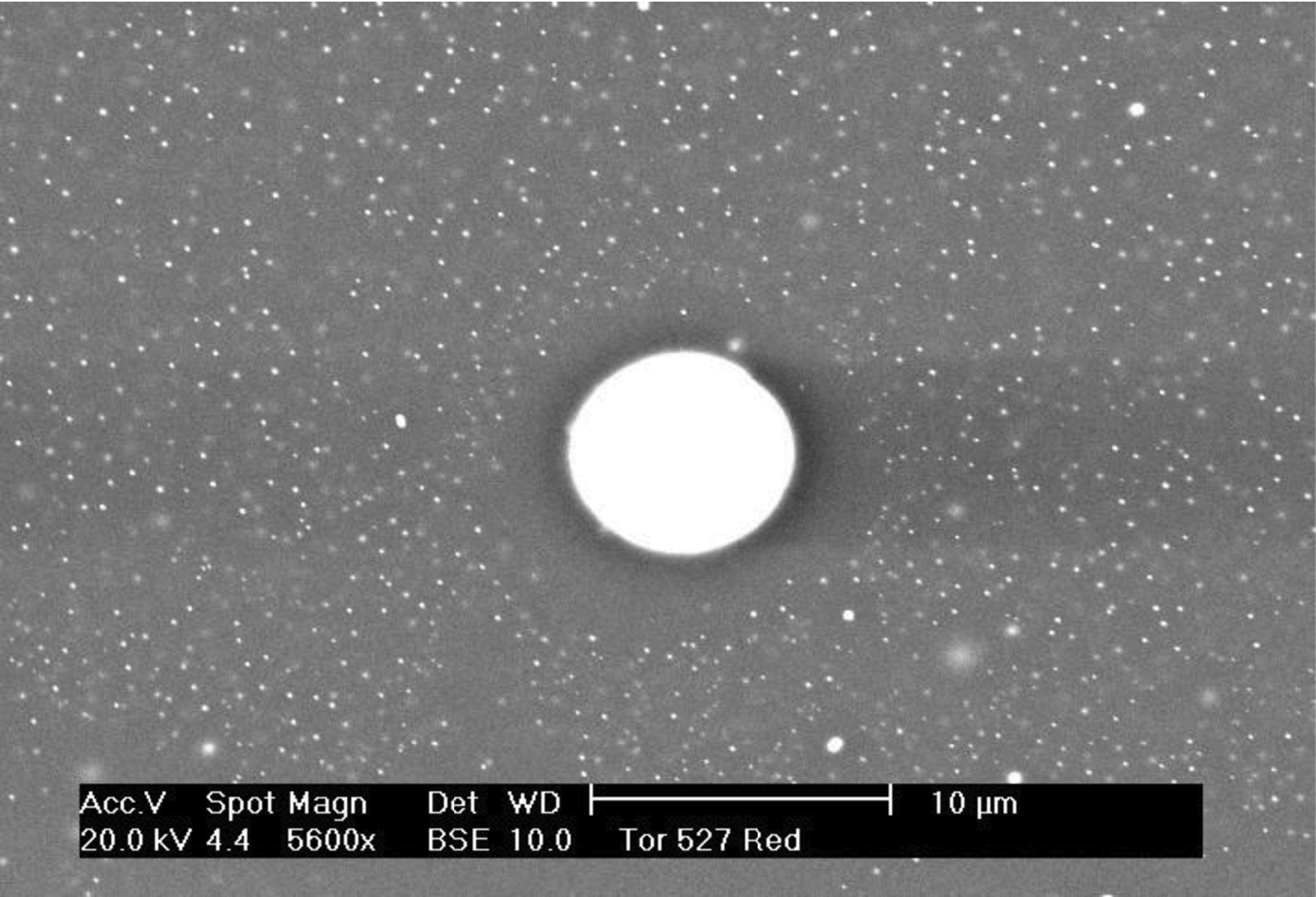
1.3a. A magnified (backscattered electron) image of a faience specimen from Hauterive Champrevèyres, Lake Village, Switzerland c. 1100 b.c. section (pale grey = glassy copper-rich glass matrix, dark grey crystals = silica; white crystals = tin oxide). 1.3b Diagram of 1.3a: hatched crystals = silica, broken lines and stipled areas = pores in sample.

1.5. Two small pieces of overheated frit found at the ninth-century glassmaking site of al-Raqqa, Syria (photo: J. Henderson). The left hand piece is 2.5cm wide.