Our Earth imposes challenging seasonal obstacles for insect development, with few sites capable of continuously sustaining activity throughout the year. Low winter temperatures at temperate and polar latitudes are a conspicuous obstacle to continuous growth and reproduction. Not only is the cold a significant threat to insect survival, but the accompanying disappearance of plants, on which most insects depend for food, generates a seasonally depauperated environment not suitable for continuous development. Equally challenging are tropical dry seasons that lack critical food and water resources.
But, the advantages of diapause go well beyond avoidance of adverse seasons. A finely tuned diapause is critical for the precise timing of adult emergence in the spring. The fact that many insects are host specialists means that diapause is an essential element for coordinating the timing of adult emergence with the growth or flowering of favored host plants. By becoming dormant, insects also mitigate risks of predation, and periodic disappearance into diapause may help reduce biotic challenges from parasites and microbes.
Diapause occurring during the winter has the advantage of concomitant low temperatures that, for an ectotherm, help suppress metabolic rate and conserve energy reserves. Summer diapauses and diapauses in tropical insects lack this advantage and thus confront the extra challenge of conserving energy reserves in a warm environment. The ability to suppress metabolism without the aid of low temperature thus becomes a special challenge in select environments.
High latitudes pose yet another different challenge. Short growing seasons restrict development to narrow seasonal windows that may be inadequate for the completion of a single generation. Such conditions may require multiple years for completion of the life cycle, thus making it necessary for more than one developmental stage to enter diapause. In some cases, a developmental arrest may be repeatedly induced and broken to capitalize on brief favorable seasons.
Just as seasons have different profiles in different regions of the Earth, the role of diapause, the environmental regulators that are essential for coordinating the response, and the features of the diapause response can be expected to vary across the globe. Diapause thus emerges as a fascinating and plastic life-history trait whose evolution reflects adaptations essential for confronting challenges of life in a wide range of specific environments as well as life in a changing environment.
1.1 What Is Diapause?
1.1.1 Origin of the Term Diapause
The Greek word “diapause” means rest or interruption of work. The usage of this word has an interesting history (Lees Reference Lees1955). As first used by Wheeler (Reference Wheeler1893) in his work with the grasshopper Xiphidium ensiferum, diapause defined the brief halt in development and embryonic transition between anatrepsis (when the embryo of a hemimetabolous insect moves tail-first through the yolk, away from the pole) and katatrepsis (when the embryo then migrates to either the ventral or dorsal surface, depending on the species). Its meaning was expanded by Henneguy (Reference Hodková1904) to refer not to a stage of morphogenesis but the condition of arrested development, but Henneguy failed to make a distinction between the simple arrest prompted by cold and the type of arrest that is stage-specific and developmentally programmed. Shelford (Reference Shelford1929) recognized the need to make such a distinction and used the term quiescence for the interruption of growth due directly to an unfavorable condition, while he used the term diapause to refer to “spontaneous arrest,” an arrest that we now refer to as being developmentally programmed and stage-specific. This same sort of distinction was implied in the terms “diapause vrai” and “pseudodiapause” used by Roubaud (Reference Roubaud1930). Numerous and subtle distinctions can be made when arrested development is described for diverse species (Danks Reference Danks2002), and distinctions between species have led to rather complex classifications of insect dormancy (e.g., Müller Reference Müller1970, Mansingh Reference Mansingh1971, Ushatinskaya Reference Ushatinskaya1976a), resulting in the introduction of terms such as oligopause, parapause, pluvipause, and others to describe developmental arrests with slightly different features. My own bias is that the terms diapause and quiescence are useful distinctions, but I see no need for further subdivisions, assuming we recognize that some features of diapause and quiescence may vary among species.
The term diapause extends beyond the insect literature and includes numerous examples among other arthropods, especially ticks and mites (e.g., Belozerov Reference Belozerov2008, Lohmeyer et al. Reference Lohmeyer, Pound and George2009, Bryon et al. Reference Bryon, Kurlovs, van Leeuwen and Clark2017a). Diapause in Crustacea is well documented among copepods and brine shrimp (Marcus and Scheef Reference Marcus, Scheef, Nelson, Denlinger and Somers2010, Hand et al. Reference Hand, Denlinger, Podrabsky and Roy2016, Baumgartner and Tarrant Reference Baumgartner and Tarrant2017) and is prevalent in rotifers (Garcia-Rogers et al. Reference Garcia-Rogers, Lubzens, Fontaneto and Serra2019, Tarazona et al. Reference Tarazona, Lucas-Liedó, Carmona and García-Roger2020), as well as a few species of mollusks (Numata and Udaka Reference Numata, Udaka, Nelson, Denlinger and Somers2010). Tardigrades, invertebrates best known for their capacity to enter a cryptobiotic state, also include some species that diapause as embryos (Guidetti et al. Reference Guidetti, Altiero and Rebecchi2011). Diapause is also widely used to refer to delayed implantation of embryos in marsupials, polar bears, badgers, mink, anteaters, mice, gerbils, and over 130 other species of mammals (Renfree and Fenelon Reference Renfree and Fenelon2017) and is routinely used to refer to embryonic dormancies noted in non-mammalian taxa ranging from lizards to some of the bony fish species (Rafferty and Reina Reference Rafferty and Reina2012), sharks and rays (Waltrick et al. Reference Waltrick, Awruch and Simpfendorfer2012), and some of the bony fish species such as annual killifish (Martin and Podrabsky Reference Martin and Podrabsky2017, Hu et al. Reference Hu, Wang, Brind’Amour, Singh, Reeves, Lorincz, Alvarado and Brunet2020).
Although developmental arrest in nematodes is a similar form of dormancy, the arrest in nematodes is more commonly referred to as the dauer state (Riddle Reference Riddle, Riddle, Blumenthal, Meyer and Priess1997). Mammalian hibernation shares many features with insect diapause (Andrews Reference Andrews2019), but the unique physiological features of endotherms and ectotherms justify making a distinction between these two forms of dormancy. Though we may use different terms for these forms of animal dormancy, there is growing evidence that certain common themes operate across the animal kingdom and much can be gained from a quest for universal principles.
In most cases diapause is one of two developmental alternatives produced by the same genotype, thus it fits the classic definition of polyphenism, coined by Ernst Mayr (Reference Mayr1963). Insects of the same genotype can either enter diapause or develop without diapause, a developmental decision that depends on the seasonal environmental cues received. The term polyphenism is thus distinct from the common usage of polymorphism, a term usually restricted to differences that have a genetic basis. The features of diapause, in which the environment dictates the phenotype (diapause or nondiapause), conform to the classic definition of phenotypic plasticity or a plastic response to environmental conditions.
1.1.2 Diapause Is a Programmed Event
Diapause is a programmed, stage-specific arrest or retardation of development commonly used to circumvent an adverse season. Upon entry into diapause, insects remain in this arrested state for some time, even if prevailing environmental conditions are favorable for development.
“Programmed” implies that it is not an immediate response to environmental adversity but a response to environmental signals that have been received in advance of the actual onset of diapause or, alternatively, is hard-wired genetically to occur at a specific stage. The distinction between an environmentally programmed diapause and one that is hard-wired genetically is captured in the terms “facultative” and “obligate” diapause. Facultative diapause implies a plastic response dependent upon receipt of specific environmental cues commonly received well in advance of diapause onset. By contrast, obligate diapause refers to a genetically programmed diapause that occurs at a specific stage regardless of the prevailing environmental input. While an insect with an obligate diapause is likely to complete only one generation a year, an insect with a facultative diapause has the flexibility to complete multiple generations, commonly several nondiapausing generations during the summer, followed by an overwintering generation that enters diapause.
Both obligate and facultative diapauses can sometimes be seen within the same family, such as the stink bugs Pentatomidae (Musolin and Saulich Reference Musolin, Saulich and McPherson2018). Certain trends exist within taxa such as hymenopteran parasitoids: parasitoids that attack univoltine (one generation/year) hosts tend to have an obligate diapause, while those attacking polyvoltine (multiple generations/year) hosts usually have a facultative diapause (Polgar and Hardie Reference Polgar and Hardie2000). Although rare, one species may exhibit both an obligate and facultative diapause: certain populations of the European spruce bark beetle Ips typographus harbor both of these diapause phenotypes (Schebeck et al. Reference Schebeck, Hansen, Schopf, Ragland, Stauffer and Benz2017). Some species that appear to have an obligate diapause may, under close scrutiny, avert diapause under specific circumstances. For example, the saturniid moth Hyalophora cecropia, a species that always enters pupal diapause when reared outside, can actually be enticed to develop without entering diapause when exposed to artificially long daylengths (Waldbauer Reference Waldbauer1996); hence this species, long thought to be an iconic example of a species with an obligate diapause, is more correctly recognized as a species with a facultative diapause, albeit a species that normally completes only a single generation each year and appears to seldom exploit its potential to skip diapause.
The beauty of the programming aspect of diapause is that it provides a preparatory phase that allows sequestration of additional energy reserves, augmentation of cuticular waterproofing, changes in color to match the winter or dry season habitat, migration, and selection of a well-protected hibernaculum (overwintering refuge).
1.1.3 Making a Distinction between Diapause and Quiescence
The programmed feature of diapause is in contrast to quiescence, a form of dormancy that is an immediate response to adversity and includes no preparatory phase. Unlike diapause, quiescence is broken immediately when favorable conditions return. Quiescence offers the capacity to quickly stop and restart development multiple times and at any stage in response to certain environmental challenges. For example, an insect placed in a refrigerator will halt development and become inactive but will resume development almost immediately when retrieved from the cold environment. Similarly, an insect denied food may enter an arrest that is terminated immediately when food again becomes available. This sort of rapid entry into and recovery from a dormant state distinguishes quiescence from diapause. Distinct endocrine signatures underlie these two forms of developmental arrest, as noted in adult females of the linden bug Pyrrhocoris apterus (Hodková and Okuda Reference Hodková and Okuda2019). The corpora allata (CA) cease producing the juvenile hormone needed for vitellogenesis in both starvation-induced quiescence and diapause, and when food again becomes available the CA is immediately activated in starved bugs but not in diapausing bugs.
One of the interesting current debates is whether the well-studied adult dormancy in Drosophila melanogaster is a programmed arrest (i.e., a diapause) or a simple quiescence that is temperature-induced. Although the term diapause is frequently used in this context (e.g., Zonato et al. Reference Zonato, Collins, Pegoraro, Tauber and Kyriacou2017), strong arguments counter that quiescence is a more appropriate term for the dormancy of D. melanogaster (e.g., Emerson et al. Reference Emerson, Bradshaw and Holzapfel2009b,Reference Emerson, Uyemura, McDaniel, Schmidt, Bradshaw and Holzapfelc, Saunders Reference Saunders2020b) due to the immediacy of the response. One strong argument that the dormancy of D. melanogaster should not be regarded as diapause is the fact that egg chambers of the female initiate yolk uptake but then, in response to the low temperatures or other stresses that evoke the dormancy response, degenerate, an energy-consuming event not normally observed in a diapausing insect (Lirakis et al. Reference Lirakis, Dolezal and Schlötterer2018). The fact that this same response can be evoked in tropical populations of D. melanogaster has been used to suggest that the dormancy is not a diapause, but many tropical species do indeed have a diapause (see Section 2.3), thus that argument is not compelling. That egg chambers degenerate, that other stresses such as starvation elicit an identical response, and that the response is so volatile support the argument that dormancy noted in D. melanogaster represents a general stress response in this species, rather than a strictly binary trait. Yet, an examination of latitudinal clines suggests that certain populations of D. melanogaster do indeed possess a photoperiodically programmed diapause while others have features more akin to quiescence (Tatar et al. Reference Tatar and Yin2001, Anduaga et al. Reference Anduaga, Nagy, Costa and Kyriaou2018).
A distinction between diapause and quiescence can sometimes be challenging to discern in other species as well. The invasive species Drosophila suzukii seems to show a slight photoperiodic response but, like D. melanogaster, its winter dormancy is more akin to quiescence (Everman et al. Reference Everman, Freda, Brown, Schieferecke, Ragland and Morgan2018). The mountain pine beetle Dendroctonus ponderosae was initially reported to overwinter in quiescence, but more recent experimental evidence shows a period of developmental latency, suggesting that the overwintering stage of the larvae can more accurately be described as diapause (Bentz and Hansen Reference Bentz and Hansen2017). It is still unclear whether the midsummer disappearance of the yellow dung fly Scathophaga stercoraria in Central Europe can be attributed to diapause or quiescence (Blanckenhorn et al. Reference Blanckenhorn, Henseler, Burkhard and Briegel2001). Although less common, quiescence is also a viable option for overwintering, as noted in the diamondback moth Plutella xylostella, a species found in various life stages during winter in eastern North America (Dancau et al. Reference Dancau, Mason and Cappuccino2018). Perhaps as a bet-hedging strategy, the European water strider Velia caprai can overwinter both as an adult in quiescence and as an embryo in diapause (Ditrich and Koštál Reference Ditrich and Koštál2011).
Though diapause, with its opportunity for preparation, would appear to be the ideal option for bridging unfavorable seasons, there are certain advantages to quiescence. Not only is it an option available to any developmental stage, but it also offers flexibility for rapidly responding to environmental conditions. For example, when temporary rock pools inhabited by the midge Polypedilum vanderplanki dry up in Nigeria, midge larvae enter a cryptobiotic state, a form of quiescence that is quickly broken when the rains return (Hinton Reference Hinton1951, Cornette and Kikawada Reference Cornette and Kikawada2011). This form of dormancy offers extreme flexibility, for both time of entry as well as exit from the dormant state. Quiescence may also be the preferable state for aquatic insects residing in cool, high-latitude streams (Danks Reference Danks1987). Unlike the habitat of the African midge, cool streams represent a stable habitat, and quiescence enables swift transition between an active and inactive lifestyle, making it possible to maximally exploit periods when temperatures exceed a certain threshold.
1.1.4 Stage Specificity
The adjective “stage-specific” implies that diapause capacity is restricted to a single stage of development for each species. Although diapause can occur in embryos, larvae, pupae, or adults, for any one species the capacity for diapause is usually restricted to a single stage. There are, however, good examples of species, especially those confronting short growing seasons at high latitudes, that have the capacity for diapause at two stages of development. Northern populations of the spruce budworm Choristoneura biennis first overwinter in a second-instar larval diapause and spend the second winter in a final larval-instar diapause (Nealis Reference Nealis2005), northern populations of the blow fly Calliphora vicina diapause both as third-instar larvae and as adults (Vinogradova and Reznik Reference Vinogradova and Reznik2013), and the bruchid beetle Bruchidius dorsalis diapauses in cooler areas as a final-instar larva (Kurota and Shimada Reference Kurota and Shimada2003a) and as an adult in warmer regions (Kurota and Shimada Reference Kurota and Shimada2003b). Larval diapause in the longicorn beetle Psacothea hilaris is unusual in that it can occur across a wide developmental range from the fifth to ninth instar, although it most commonly occurs in the sixth or seventh instar (Asano et al. Reference Asano, Munyiri, Shintani and Ishikawa2004). In central Sweden, the butterfly Parage aegeria diapauses as a pupa, but both larval and pupal diapause are common in southern Sweden (van Dyck and Wiklund Reference van Dyck and Wiklund2002). Lycaena hippothoe, a butterfly with a wide Eurasian range, is capable of entering larval diapause as either a third or fourth instar (Fischer and Fiedler Reference Fischer and Fiedler2002). However, in the majority of insect species, the capacity for diapause resides in only one stage. This sort of stage specificity is not true for quiescence. Insects can become quiescent in any developmental stage, although the threshold for entering quiescence may differ among stages.
1.1.5 Diapause Development
In the strictest sense, development is not completely arrested during diapause. A slow progression of developmental processes occurs during diapause, culminating eventually in termination of diapause, a progression that Andrewartha (Reference Andrewartha1952) referred to as diapause development. Diapause is best viewed not as a “stop/start” event but as a dynamic developmental trajectory operating on a much slower time scale than the developmental events observed in nondiapausing insects. Although covert molecular events are occurring as diapause progresses in all species, in most cases development appears at a standstill. In a few cases, however, overt changes can be noted during diapause, generating a response more akin to a slowing of the developmental rate rather than a complete developmental arrest. For example, during adult diapause in females of the mosquito Culex pipiens, ovarian follicles progressively enlarge, but it takes more than 20 weeks for the follicles to attain the same size attained by a nondiapausing female in 3 days (Readio et al. Reference Readio, Chen and Meola1999). Similarly, the sexually produced embryos of the pea aphid Acyrthosiphon pisum overwinter in a diapause that can best be described as extremely slow, albeit progressive development during the interval between anatrepsis and katatrepsis (Shingleton et al. Reference Shingleton, Sisk and Stern2003). Legs and body organs continue to grow but at a rate that is largely temperature-independent, a contrast to the temperature-dependent growth rate observed after katatrepsis. A similar slow progression of development is noted for diapausing embryos of aphids Cinara cupressi and Cinara juniperi (Durak et al. Reference Durak, Dampc, Dampc, Bartoszewski and Michalik2020): Mitotic cell division continues at a slow pace, resulting in a doubling of body size from day 16 to day 70 after oviposition. In some Orthoptera, for example the cricket Modicogryllus siamensis, diapause is manifested not as a complete halt in development but as a much slower rate of development that incorporates additional nymphal molts (Miki et al. Reference Miki, Shinohara, Chafino, Noji and Tomioka2020).
1.2 Who Does It and in What Stage?
Diapause has evolved numerous times, as discussed in Chapter 11. In some taxa, diapause characteristically occurs at one certain stage, but that is not universally true. In some families and genera, the stage of diapause is not highly conserved. Among the many species of flesh flies (family Sarcophagidae) that have been examined from around the world, those from South America appear to lack the capacity for diapause (Denlinger et al. Reference Denlinger, Chen and Tanaka1988a), but all species examined from North America, Europe, Asia, and Africa have a pupal diapause. Ladybird beetles (family Coccinellidae), due to their importance as biological control agents, have been examined extensively, and reports on species from Europe, North America, and Asia consistently reveal the capacity for an adult diapause (Hodek Reference Hodek, Hodek, van Emden and Honek2012).
At the other extreme, the genus Drosophila contains species that diapause as larvae (D. deflexa), pupae (D. obscura), or adults (e.g., D. virilis and D. obscura groups) (Lumme Reference Lumme and Dingle1978). The diapause stage differs in two closely related Swiss dung flies that occupy a similar niche: Scathophaga stercoraria diapauses as a pupa, while Sepsis cynipsea diapauses as an adult (Blanckenhorn Reference Blanckenhorn1998a). Scandinavian butterflies within the Nymphalini tribe show remarkably different overwintering strategies (Wiklund et al. Reference Wiklund, Lehmann and Friberg2019). One of the nymphalids enters pupal diapause, two migrate south for the winter, four are univoltine and have an obligate adult diapause, and three have a partial second generation (two species have a facultative adult diapause determined by larval daylength, and one species shunts half the population into an obligate adult diapause while the other half enters a facultative adult diapause dependent on larval daylength). Stink bugs, family Pentatomidae, are known to diapause as embryos, nymphs, and adults (Musolin and Saulich Reference Musolin, Saulich and McPherson2018). Most bruchid beetles diapause as adults but at least two temperate species, Kytorhinus sharpianus and Callosobruchus ademptus, diapause as larvae (Kurota and Shimada Reference Kurota and Shimada2001). Two closely related weevils differ considerably in their diapause attributes: Exapion ulicis diapauses as an adult and lays eggs in the spring, while Exapion lemovicinum lays eggs in the autumn and diapauses overwinter as a larva, responses driven largely by different fruiting times of their host plants (Barat et al. Reference Barat, Vernon, Tarayre and Atlan2010). Among mosquitoes, certain trends emerge (Denlinger and Armbruster Reference Denlinger, Armbruster and Raikhel2016, Diniz et al. Reference Diniz, de Albuquerque, Oliva, de Melo-Santos and Ayres2017), as shown in Figure 1.1. While embryonic and larval stages predominate as the diapause stage in the genus Aedes, most mosquitoes in the genus Culex diapause as adults. In other genera, such as Anopheles, diapause is reported in embryonic, larval, and adult stages. No mosquitoes appear to use the pupal stage for diapause. Among crickets, embryonic diapause is the most prevalent stage of diapause, represented by over 80% of the species in Japan and the eastern United States (Masaki and Walker Reference Masaki and Walker1987). Among 200 species of mirid bugs from the Netherlands, 85% diapause as embryos (Cobben Reference Cobben1968), and among 14 species representing 5 genera of plant bugs in the subfamily Mirinae, 4 genera consistently diapause as embryos and members of another genus, Lygus, consistently diapause as adults (Saulich and Musolin Reference Saulich and Musolin2020). So, some trends are noted within taxonomic groups, but many exceptions are evident. Diapause is a highly variable trait, not only among closely related species but also among populations, as further discussed in Chapter 3.
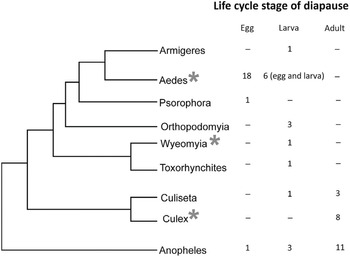
Figure 1.1 Phylogenetic distribution of life cycle stages of diapause in nine mosquito genera. * indicates genera containing at least one species for which considerable information exists on the transcriptional basis for diapause.
1.2.1 Embryonic Diapause
Embryonic diapause is especially well documented in Orthoptera (crickets, grasshoppers, walking sticks), true bugs in the order Heteroptera, and butterflies and moths in the order Lepidoptera, and among the lower Diptera, such as mosquitoes and midges. It occurs in some Coleoptera but appears to be less common in that order. Diapause is not restricted to a specific stage of embryonic development but has been noted across a wide range of embryonic stages. During embryonic development, the zygote divides (cleavage), forms a layer of cells surrounding the yolk (blastoderm formation), and in hemimetabolous insects, the blastoderm then invaginates slightly and migrates (blastokinesis). The embryo first migrates posteriorly (anatrepsis) and then dorsally (katatrepsis), movements that are somewhat simplified in holometabolous insects (Panfilio Reference Panfilio2008). Diapause can apparently intercede at almost any point during embryogenesis, from the early stages of blastoderm formation through to the completion of pharate larval development (at which point the first-instar larva is completely formed but has not yet hatched). A study on the crane fly Tipula simplex suggests that diapause in this species occurs prior to cleavage (Hartman and Hynes Reference Hartman and Hynes1980), although additional studies at the electron microscope level would be helpful in validating this report. One particularly well-documented case of a species with an early-stage diapause is the band-legged ground cricket Dianemobius nigrofasciatus (Tanigawa et al. Reference Tanigawa, Matsumoto, Yasuyama, Numata and Shiga2009). This diapause occurs prior to germ band formation when the embryo is merely a cellular blastoderm, a continuous layer of single cells. Diverse stages of embryonic diapauses have been documented for other species. Diapause occurs in a pre-anatrepsis stage in the Australian plague locust Chortoicetes terminifera (Deveson and Woodman Reference Deveson and Woodman2014) and balsam twig aphid Mindarus abietinus (Doherty et al. Reference Doherty, Guay and Cloutier2018). The well-studied commercial silk moth Bombyx mori also enters diapause quite early, when the blastoderm is in the form of an unsegmented dumbbell (Yamashita and Hasegawa Reference Yamashita, Hasegawa, Kerkut and Gilbert1985). Embryonic diapause in the pea aphid A. pisum occurs at a slightly later stage when the embryo is fully segmented and the limb buds are clearly defined (Shingleton et al. Reference Shingleton, Sisk and Stern2003). Embryonic development in a Tibetan population of Locusta migratoria is classified into 27 stages, with diapause interceding at stage 19, a stage prior to katatrepsis, occurring 9 days after oviposition at 30°C (Su et al. Reference Su, Wei, Tan, Li, Li, Fu, Wang, Kang and Yao2019, Wang et al. Reference Wang, Fan, Zhou, Gao, Wei and Kang2021). Two Japanese cicadas diapause at distinctly different developmental stages prior to katatrepsis: Cryptotympana facialis diapauses upon completion of body segmentation when the antennal buds are segregated from the cephalic lobes, while Graptopsaltria nigrofuscata diapauses at a slightly later stage when the antennae, maxillary buds, and thoracic appendages have already differentiated (Moriyama and Numata Reference Moriyama and Numata2008). At the other extreme, the katydid Eobiana engelhardti subtropica enters diapause as an almost fully developed embryo (Higaki and Ando Reference Higaki and Ando2005), and many Lepidoptera, such as the gypsy moth Lymantria dispar, enter diapause at the completion of embryogenesis when the embryo is more appropriately termed a pharate first instar (Leonard Reference Leonard1968). When diapause in the gypsy moth is broken, the fully developed pharate first-instar larva consumes the final remaining yolk, breaks through the chorion, and begins its life as a first-instar larva.
Different stages of embryonic diapause may exist in closely related species. The Heteroptera present a fascinating array of diapausing embryonic stages (Cobben Reference Cobben1968). Nearly all conceivable stages of embryonic development, ranging from the early blastoderm through to pharate first-instar nymph, are used by this taxon as the diapausing stage (Figure 1.2). This observation underscores the idea that natural selection can capture different embryonic stages among closely related species. One caveat is that the array of diapause stages depicted in Figure 1.2 is based on single snapshots in time, thus any sort of progression as noted in pea aphid embryos (Shingleton et al. Reference Shingleton, Sisk and Stern2003) would be obscured.

Figure 1.2 Stages of embryonic diapause in different species of Heteroptera. A, Plagiognathus arbustorum; B, Megalocoleus molliculus; C, Pantilius tunicatus; D, Loricula elegantula; E, Leptopterna ferrugata; F, Coranus subapterus; G, Picromerus bidens; H, Himacerus apterus; I, Myrmus miriformis; J, Chiloxanthus pilosus; K, Notonecta lutea; L, Notonecta maculata; M, Chiloxanthus pilosus; N, Salda littoralis; O, Notonecta reuteri; P, Nysius thymi.
Early studies forming the foundation for embryonic diapause include extensive work on B. mori, initiated by Fukuda (Reference Fukuda1951) and Hasegawa (Reference Hasegawa1951), followed by beautiful studies from Yamashita (Reference Yamashita1996) and his colleagues, experiments on the gypsy moth L. dispar (Leonard Reference Leonard1968, Hoy Reference Hoy1977), detailed series of experiments in Japan on diapause in several species of crickets by Masaki (Reference Masaki1967) and his students, and experiments with French populations of L. migratoria (Le Berre Reference Le Berre1953). More recently, embryonic diapause of the mosquito Aedes albopictus is attracting considerable attention as it invades new territories in Europe and North America (Armbruster Reference Armbruster2016).
1.2.2 Larval Diapause
Larval (or nymphal) diapause is common among Lepidoptera, certain groups of Diptera, Hymenoptera, Coleoptera, Neuroptera, Odonata, Orthoptera, Hemiptera, and Plecoptera. Among the Lepidoptera, some species diapause in early larval instars, such as the first overwintering generation of the northern population of the spruce budworm Choristonerura biennis (Nealis Reference Nealis2005). But, more commonly diapause is entered at the end of the final larval instar when larvae have attained their full size and have ceased feeding. Although less common, a few Lepidoptera diapause midway through larval development. For example, the copper butterfly Lycaena tityrus diapauses as a third-instar larva before molting in the spring to a final, fourth-instar larval stage (Fischer and Fiedler 2001). L. hippothoe, a species with five larval instars, overwinters in either a third or fourth larval instar (Fischer and Fiedler Reference Fischer and Fiedler2002), and the large copper butterfly L. dispar batavus, diapauses as a second instar (Nicholls and Pullin Reference Nicholls and Pullin2003). The European corn borer Ostrinia nubilalis (Beck and Hanec Reference Beck and Hanec1960) and the southwestern corn borer Diatraea grandiosella (Chippendale and Reddy Reference Chippendale and Reddy1972) are among the best-studied examples providing early insights into larval diapause in Lepidoptera.
Among the lower Diptera, the pitcher plant mosquito Wyeomyia smithii offers the most robust and extensive body of literature on larval diapause, thanks mainly to the dedicated work from the Bradshaw-Holzapfel Laboratory and that of their colleagues (e.g., Bradshaw and Lounibos Reference Bradshaw and Lounibos1972). Larval diapause of W. smithii can intercede during either the third or fourth instar in certain populations (Lounibos and Bradshaw Reference Lounibos and Bradshaw1975), but a third-instar diapause is most prevalent. The mosquito Anopheles barberi most commonly enters diapause in the second instar, but some individuals diapause in the third instar as well (Copeland and Craig Reference Copeland and Craig1989).
Among the higher Diptera, I am not aware of larval diapause occurring in stages earlier than the fully grown, post-feeding third (final) larval instar, also known as the wandering stage. A well-studied example of such a third-instar fly diapause is the blow fly C. vicina (Vinogradova and Zinovjeva Reference Vinogradova and Zinovjeva1972, Saunders Reference Saunders2000). More recently, the drosophilid Chymomyza costata (Shimada and Riihimaa Reference Shimada and Riihimaa1990, Koštál et al. Reference Koštál2011) joins C. vicina as a powerful model for larval diapause in Diptera. The final larval instar also dominates as the diapausing stage for larval diapause in the Hymenoptera. Diapause in the ectoparasitoid Nasonia vitripennis is perhaps the most thoroughly studied larval diapause among the Hymenoptera, prompted by several key early papers (Schneiderman and Horwitz Reference Schneiderman and Horwitz1958, Saunders Reference Saunders1965). More recent studies on the fifth-instar larval diapause of the alfalfa leafcutting bee Megachile rotundata adds this economically important species to the list of well-studied examples of larval diapause among the Hymenoptera (Yocum et al. Reference Yocum, Kemp, Bosch and Knoblett2006).
1.2.3 Pupal Diapause
Pupal diapause, best known for Lepidoptera and Diptera, is also noted in a few Hymenoptera but appears to be completely absent in Coleoptera, the largest insect order. The arrest usually occurs in the true pupal stage, that is, before the onset of adult differentiation. However, in a few species of moths (Sahota et al. Reference Sahota, Ruth, Ibaraki, Farris and Peet1982, Reference Sahota, Ibaraki and Farris1985, Monro Reference Monro, Novak and Sláma1972), diapause occurs in pharate adults, that is, when adult development has been completed but the insect is still within the pupal cuticle. Thus, development can be halted either before differentiation begins, the most common stopping point, or after it is completed, but not at some midpoint between these two extremes. This is in marked contrast to embryonic diapauses that can intercede at nearly any point during the course of embryonic development.
Pioneering studies on the saturniid moth H. cecropia (Williams Reference Williams1946), the tobacco hornworm Manduca sexta (Rabb Reference Rabb1966), the flesh fly Sarcophaga argyrostoma (Fraenkel and Hsiao Reference Fraenkel and Hsiao1968), and fruit flies in the genus Rhagoletis (Bush Reference Bush1969) provide the backdrop for much recent work on pupal diapause and for understanding seasonal patterns of distribution. Experiments using species from the agriculturally important Heliothis/Helicoverpa complex of moths add a more recent model for understanding pupal diapause (Meola and Adkisson Reference Meola and Adkisson1977, Xu and Denlinger Reference Xu and Denlinger2003).
1.2.4 Adult Diapause
Adult diapause is characterized by the arrested development of the ovaries, testes, accessory glands and related reproductive structures, suppressed feeding, locomotor and mating behavior, and in some cases, degeneration of the flight muscles. Adult diapause is common among Coleoptera, Lepidoptera, Diptera, Hemiptera, Orthoptera, Neuroptera, Trichoptera, Thysanoptera, as well as the Acarina. It is most frequently manifested in newly emerged adults that are not yet reproductively active, and characteristically the adults remain reproductively inactive until diapause is terminated. But, there are exceptions where diapause is entered following a bout of reproductive activity. For example, the leaf beetle Diorhabda elongata when transferred from long days to short days, can switch to diapause after a period of egg-laying (Bean et al. Reference Bean, Dudley and Keller2007a), and before entering summer diapause, females of the Colorado potato beetle Leptinotarsa decemlineata may lay a few eggs (Tauber et al. Reference Tauber, Tauber, Obrycki, Gollands and Wright1988). As discussed in Section 3.2, a few species exit and enter adult diapause multiple times.
A short period of feeding by newly emerged adults is sometimes essential for entry into diapause. Without feeding the twospotted spider mite Tetranychus urticae fails to assume the bright orange coloration of diapause and fewer individuals enter diapause (Kawaguchi et al. Reference Kawaguchi, Manabe, Sugawara and Osakabe2016). Although feeding may continue in some diapausing adults, feeding intensity is greatly suppressed, as reported for several species of Drosophila (Matsunaga et al. Reference Matsunaga, Takahashi, Yoshida and Kimura1995), the blow fly Phormia regina (Stoffolano Reference Stoffolano1975), and the ladybird beetle Harmonia axyridis (Gao et al. Reference Gao, Wei, Liu, Wang, Zhou and Wang2019), among others. In P. regina, like other flies, the proboscis is extended when tarsi of the adult detect a food source. Interestingly, the threshold for this tarsal response differs little between diapausing and nondiapausing flies, suggesting that the feeding inhibition during diapause is controlled by the central nervous system rather than the peripheral system (Stoffolano Reference Stoffolano1975). Distinctions may also occur in the food source used by diapausing and nondiapausing adults. This is especially evident in the northern house mosquito Culex pipiens. While nondiapausing females are avid blood-feeders, females programmed for diapause feed exclusively on nectar (Bowen Reference Bowen1992). Only at the completion of diapause do females regain an interest in seeking a blood meal from their avian hosts (Faraji and Gaugler Reference Faraji and Gaugler2015).
Diapause in males has been examined less extensively than in females (Pener Reference Pener1992), but in many cases, the same sort of reproductive shut-down is seen in both sexes (Kubrak et al. Reference Kubrak, Kučerová, Theopold, Nylin and Nässel2016, Urbanová et al. Reference Urbanová, Bazalová, Vanečková and Doležel2016, Ala-Honkola et al. Reference Ala-Honkola, Kauranen, Tyukmaeva, Boetzl, Hoikkala and Schmitt2018). Mating behavior is curtailed. Accessory glands remain undeveloped. Spermatogenesis ceases and most undifferentiated sperm degenerate. The only cells within the testes that appear to be affected by diapause in larvae of the waxworm Galleria mellonella are spermatocytes in proximal regions of the testicular follicles, cells that undergo apoptotic degeneration (Bebas et al. Reference Bebas, Cymborowski, Kazek and Polanska2018). In diapausing males of the leaf beetle Gastrophysa atrocyanea, the testes slowly increase in size during the first two to three months of diapause and then regress and remain small for the remaining three to four months of diapause (Ojima et al. Reference Ojima, Ishiguro, An, Kadosawa and Suzuki2015). During diapause in the nymphalid butterfly Polygonia c-aureum, and presumably in other Lepidoptera that characteristically produce both nucleated eupyrene sperm (fertile) and anucleated apyrene sperm (infertile), production of both types of sperm is synchronously reinitiated at diapause termination (Hiroyoshi et al. Reference Hiroyoshi, Reddy and Mitsuhashi2017).
Diapausing females are characteristically not attractive to either diapausing or nondiapausing males, and diapausing males usually will not attempt to mate with sexually attractive females. Males of Drosophila montana that are reproductively active will court reproducing, post-diapause females but completely ignore diapausing females (Ala-Honkola et al. Reference Ala-Honkola, Kauranen, Tyukmaeva, Boetzl, Hoikkala and Schmitt2018). The same is true for D. melanogaster (Kubrak et al. Reference Kubrak, Kučerová, Theopold, Nylin and Nässel2016). Diapausing males of the grasshopper Anacridium aegyptium fail to display mating behavior until several months after reaching the adult stage (Greenfield and Pener Reference Greenfield and Pener1992). Late in diapause, males of the fungus beetle Stenotarsus rotundus will attempt to mate if they are physically handled, but mating is not normally observed until the environmental signal for diapause termination has been received (Tanaka et al. Reference Tanaka, Wolda and Denlinger1987b).
Foundational experiments on the Colorado potato beetle L. decemlineata (de Wilde and Bonga Reference de Wilde and Bonga1958), the grasshopper Oedipoda miniata (Pener and Broza Reference Pener and Broza1971), linden bug P. apterus (Hodek Reference Hodek1968), lacewing Chrysopa carnea (Tauber and Tauber Reference Tauber and Tauber1970a,Reference Tauber and Tauberb), and mosquito Culex pipiens (Spielman and Wong Reference Spielman and Wong1973) offer a rich launching point for more recent studies on adult diapause. Though D. melanogaster has a rather weak adult dormancy (Saunders et al. Reference Saunders, Henrich and Gilbert1989), probably most correctly termed a quiescence (Section 1.1.3), the powerful genetic tools available for this model species have prompted a surge of recent interest in this species, as well as in other members of the genus.
In some cases, only adult females enter diapause. Males of the northern house mosquito Culex pipiens inseminate females in the autumn and then die without entering diapause, leaving only inseminated, diapausing females, to survive the winter (Spielman Reference Spielman1964). Similarly, only females of the twospotted spider mite Tetranychus urticae (Veerman Reference Veerman, Helle and Sabelis1985, Suzuki and Takeda Reference Suzuki and Takeda2009), anthocorid bugs in the genus Orius (Musolin and Ito Reference Musolin and Ito2008), eastern yellow-jacket Vespula maculifrons (Kovacs and Goodisman Reference Kovacs and Goodisman2012), bumble bees Bombus impatiens (Amsalem et al. Reference Amsalem, Galbraith, Cnaani, Teal and Grozinger2015), and B. terrestris (Colgan et al. Reference Colgan, Finlay, Brown and Carolan2019), among others, overwinter in diapause, after mating in the autumn. In the autumn, overwintering females of the hover fly Episyrphus balteatus accumulate impressive fat reserves, while males fail to do so and all males succumb by the end of December, leaving only the inseminated females to survive until spring in northern Germany (Hondelmann and Poehling Reference Hondelmann and Poehling2007). In tropical Australia, only females of the nymphalid butterfly Hypolimnas bolina appear to use diapause to bridge the dry season (Pieloor and Seymour Reference Pieloor and Seymour2001). Males of this species survive the dry season and have sperm present throughout the year, suggesting they are consistently able to take advantage of unpredictable female activity.
In other species such as the ladybird beetle Coccinella septempunctata (Hodek Reference Hodek, Hodek, van Emden and Honek2012), the Colorado potato beetle L. decemlineata (de Wilde et al. Reference de Wilde, Duintjer and Mook1959), and the handsome fungus beetle S. rotundus (Wolda and Denlinger Reference Gnagey and Denlinger1984) both sexes diapause and mating occurs only when diapause is terminated. But, there are exceptions. A high proportion (40–60%) of central European populations of C. septempunctata mate prior to entering diapause, while Ceratomegilla undecimnotata, a ladybird beetle that hibernates in the same area, delays mating until spring (Hodek and Ceryngier Reference Hodek and Ceryngier2000). Most females of the linden bug P. apterus mate in the spring after diapause has been completed, but a few females (up to 7% in South Bohemia) mate prior to overwintering and retain viable sperm throughout the winter (Socha Reference Socha2010). Similarly, both sexes of the kudzu bug Megacopta cribraria overwinter in adult diapause, and most females delay mating until spring, but approximately 15% of overwintering females mate before the onset of diapause and thus store sperm for up to seven months (Golec and Hu Reference Golec and Hu2015). Female dung flies (family Sepsidae) store sperm over winter, but fertility increases when they also have an opportunity to mate in the spring (Zeender et al. Reference Zeender, Roy, Wegmann, Schafer, Gourgoulianni, Blanckenhorn and Rohner2019). It seems unlikely there are cases in which only adult males diapause.
The fate and survival of stored sperm within diapausing females have received little attention in insects. However, in the marine copepod Neocalanus flemingeri, sperm within fertilized females remain quiescent during diapause and complete maturation inside the female, as indicated by upregulation of spermatogenesis toward the end of diapause (Roncalli et al. Reference Roncalli, Sommer, Cieslak, Clarke, Hopcroft and Lenz2018). Similar scenarios may operate in insects, but such results have not been documented.
Mating prior to diapause sometimes alters the overwintering female’s physiology through unexpected mechanisms. Pre-diapause mating boosts the abundance of antimicrobial hemolymph peptides in the buff-tailed bumble bee B. terrestris, an effect that is sustained throughout diapause and likely enhances survival of the diapausing queen (Colgan et al. Reference Colgan, Finlay, Brown and Carolan2019).
Little is known about the physiology of males that die rather than entering diapause. Do the environmental factors that trigger diapause in females also impact male physiology? Sperm produced by the male are transferred to the female’s spermatheca in the autumn and remained stored there until the following spring. Do they have special properties or are they indistinguishable from sperm produced by their nondiapausing male counterparts that mature under spring or summer conditions?
1.3 Do Colonies of Social Insects Diapause?
Overwintering survival of many social insects is vested in a queen that overwinters and restarts the colony again in the spring, but many social insects including honey bees, termites, and ants maintain their colony structure throughout the winter. Are these overwintering colonies in diapause? The literature usually avoids this terminology, and instead opts for terms such as summer bees and winter bees. Yet, when viewed as a superorganism, the winter phases of colony life share many attributes of diapause.
Ant colonies that Kipyatkov and his colleagues (e.g., Kipyatkov and Lopatina Reference Kipyatkov and Lopatina1999, Kipyatkov Reference Kipyatkov and Kipyatkov2006) have studied in northern Russia are considered to be in diapause during the northern winter. Like most diapausing insects, ants in several genera have been documented to respond to short daylength by entering a nonreproductive phase. In some species, only adults and workers are capable of entering diapause (tribe Formicini), but in several other species, larvae also have the capacity for diapause. Which larval stage is used for diapause varies with genus: Diapause occurs in early instars (1–3) in Lepisiota, Plagiolepis, Tapinoma, and some Camponotus, in middle instars (2–4) in some Camponotus, in late instars (3–4) in Harpagoxenus, Leptothorax, Temnothorax and Messor, and in the final larval instar in Manica, Diplorhoptrum, Leptanilla, Monomorium, Myrmica and Tetramorium, and in all six instars in Aphaenogaster, Crematogaster, Lasius, Paratrechina, and some species of Camponotus. In southern Russia, larvae overwinter only once, but as the growing season shortens in the north, more and more larvae overwinter twice or more. In many of these taxa, overwintering larvae give rise to alates (winged adults that are reproductively active) that emerge in the spring to establish new colonies. Few physiological properties have been evaluated in these overwintering colonies, but the cessation of reproduction, the response to daylength, and the stage specificity of the overwintering stage share many of the attributes one expects to see in diapause. In the fire ant, Solenopsis invicta, a species recently introduced into the southern portion of the United States, clear behavioral and physiological attributes distinguish summer and winter colonies (Tschinkel Reference Tschinkel1993, Cook et al. Reference Cook, Eubanks, Gold and Behmer2016): Summer colonies have faster turnover rates, higher rates of metabolism, and nutrient utilization patterns that are distinct from patterns noted in winter colonies. What prompts these seasonal shifts, however, remains undefined and can be challenging to uncover in this and other social species.
In honey bees (Apis mellifera), winter workers tend the queen and continue to regulate hive temperatures, but they remain rather idle, they no longer forage and have reduced duties in brood rearing. There is a striking difference in their longevity compared to summer bees. Although summer bees live for 15–38 days, the lifespans of winter bees average 140 days, a tenfold increase over summer bees (Winston Reference Winston1987). In addition, winter bees have a more extensively developed fat body than their summer counterparts and display a more robust immune response (Dostálková et al. Reference Dostálková, Dobeš, Kunc, Hurychová, Škrabišová, Petrivalsky, Titera, Havlík, Hyršl and Danihlík2021). Brood-rearing activity declines in response to short days and is accelerated by long days (Kefuss Reference Kefuss1978). Thus, the winter bee is in many ways similar to other insects in diapause. The transcriptional profile of winter bees, based on 14 candidate genes, differs from that of summer bees: Abdominal tissues resemble profiles of nurse bees and thoracic tissues resemble foragers (Bresnahan et al. Reference Bresnahan, Döke, Giray and Grozinger2021), thus the winter bee appears to be a distinct phenotype. Thermoregulatory activities that invoke the use of thoracic flight muscles in winter bees are presumably the functional link with summer-active forager bees. A full transcriptomic analysis will be useful in searching more broadly for expression patterns that may be shared with other insects in diapause, especially bumble bee queens and other Hymenoptera that overwinter in diapause. It is unclear which seasonal environmental features are responsible for the switch to the production of winter bees. Whether the winter bee should be considered to be in diapause is a semantic issue. Although it is reasonable to view winter bees as being in diapause, the more important issue is to at least recognize that winter bees and diapausing insects do share some common attributes.
The same can be said for many species of termites. Workers of the eastern subterranean termite Reticulitermes flavipes survive low temperatures better when subjected to a decreasing thermoperiod and photoperiod prior to the onset of winter, a response that appears to drive the workers to a greater depth where temperatures are not as severe (Cabrera and Kamble Reference Cabrera and Kamble2001). Activity levels remain low during winter, but workers do not appear to sequester additional fat reserves. During the dry season in the African savanna, termite densities are low and activity is suppressed (Davies et al. Reference Davies, Eggleton, van Rensburg and Parr2015), but when the rains arrive, the next generation of reproductives, the alates, emerge from the ground in enormous numbers to initiate the next cycle of nest founding and reproduction. The pause in development that precedes the emergence of reproductive adults is in many ways akin to post-diapause quiescence. The alates are fully capable of progressing to the next phase of their life cycle, but they fail to do so until the proper environmental stimulus (rain) arrives. Again, termite researchers do not refer to this waiting period as diapause and post-diapause quiescence, but this life cycle does indeed share features in common with diapause.
1.4 Phases of Diapause
The impact of diapause on the insect life cycle is profound, extending from the perception of environmental signals used to program diapause, through diapause itself, to alteration of traits manifested after diapause has ended. In some cases the influence is also transgenerational, initiated by the parents and extending to subsequent generations. It is this pervasive impact of diapause across a broad temporal and developmental scale that justifies identifying diapause as an alternative life cycle, a syndrome that encompasses more than just the diapausing stage.
Although numerous terms have been used to define different phases of diapause in the older literature, the terminology championed by Koštál (Reference Koštál2006) and articulated also by others (e.g., Tauber and Tauber Reference Tauber and Tauber1976, Tauber et al. Reference Tauber, Tauber and Masaki1986, Danks Reference Danks1987, Denlinger Reference Denlinger2002) broadly divides diapause into three phases: pre-diapause, diapause, and post-diapause (Figure 1.3).

Figure 1.3 Schematic depiction of three major phases of diapause (pre-diapause, diapause, post-diapause) and finer subphases including environmental induction, preparation, initiation, maintenance, termination, and post-diapause quiescence. Changes in diapause intensity are represented by dotted lines (a and b) under two hypothetical constant conditions, while the solid line (c) indicates a change in environmental conditions that favors diapause termination.
Pre-diapause includes the environmental programming events (also known as diapause induction) as well as the preparative steps that the insect undergoes prior to diapause. These two phases of pre-diapause are usually separate in time but may overlap, depending on the species. Diapause itself encompasses the total period when the insect is refractory to the progression of development, even though favorable environmental conditions are present. Diapause can be divided into three distinct phases, including diapause initiation (or onset), maintenance, and termination. It is during the initiation phase that development ceases or dramatically slows, and the metabolic rate usually drops, although some feeding and accumulation of energy stores may continue. The intensity of diapause usually increases during this period, that is, it becomes increasingly more difficult to reverse the diapause decision by external stimuli.
The period of diapause maintenance is the longest, extending from diapause initiation to diapause termination. It is during this period that the insect gradually acquires the capacity to bring diapause to an end. Diapause termination marks the time when the actual diapause period is completed and the insect can resume development if the correct conditions are present. This point marks a rapid developmental transition defining the end of diapause. Diapause termination usually occurs long before the insect actually resumes development.
Post-diapause includes all developmental events that ensue following diapause termination. Post-diapause quiescence refers to the period between diapause termination and the actual resumption of development, an interval that frequently encompasses many months in temperate latitudes. For example, in northern latitudes, diapause may be terminated in early winter, but development may not be reinitiated until the arrival of warm days in spring because cold conditions in late winter are not permissive for development. The distinction between diapause and post-diapause quiescence is frequently overlooked because the two phases are morphologically indistinguishable. The only obvious distinction is whether the insect is capable of reinitiating development if favorable conditions are present. However, from a developmental perspective and for projecting the timing of spring emergence, the distinction between diapause and post-diapause quiescence is extremely meaningful. Following a period of post-diapause quiescence, development resumes when favorable conditions are present, and the ensuing development follows a developmental trajectory that usually closely matches that of its nondiapausing counterparts. It is not uncommon for post-diapausing insects to exhibit fitness consequences that result from having experienced diapause.
In subsequent chapters, I use this framework to discuss more fully the environmental programming events involved in diapause induction (Chapter 5), preparation (Chapter 6), diapause (Chapter 7), termination of diapause, and resumption of development (Chapter 8), as well as consequences of diapause (Chapter 4).






