Introduction
Biological control has been an important tactic in the management of Canadian forests over the past century, but one with varied success. Strictly speaking, the idea of using natural enemies or biological control to manage forest pests arose during the late 1800s with the earliest programmes directed against pests in both Canada and the United States of America. In the United States of America, Thanasimus formicarius Linnaeus (Coleoptera: Cleridae) was introduced from Germany to control an outbreak of native southern pine beetle, Dendroctonus frontalis (Zimmerman) (Coleoptera: Curculionidae) in 1892 (Furniss Reference Furniss2010), and in Canada the parasitoid Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae) was introduced near London, Ontario, Canada in 1882 for use against the imported currantworm, Nematus ribesii (Scopoli) (Hymenoptera: Tenthredinidae), a pest of woody plants in the genus Ribes Linnaeus (Grossulariaceae) (Beirne and Kelleher Reference Beirne and Kelleher1973). Unfortunately, neither of these early attempts was considered successful, perhaps foreshadowing some of the difficulties to be faced by Canadian biological control programmes over the coming years.
The resounding success of the introduction of the vedalia beetle, Rodolia cardinalis Mulsant (Coleoptera: Coccinellidae), into California, United States of America around 1888 to control the non-native cottony cushion scale, Icerya purchasi Maskell (Hemiptera: Monophlebidae), launched many of the early biological control research programmes across North America (Thompson Reference Thompson1939; Balch Reference Balch1960). Due to the Palaearctic origin of the pests, many of these projects were carried out in collaboration with international institutes such as the Commonwealth Agricultural Bureaux (now CABI) (McGugan and Coppel Reference McGugan and Coppel1962; Reeks and Cameron Reference Reeks and Cameron1971; Kelleher and Hulme Reference Kelleher and Hulme1984; Mason and Huber Reference Mason and Huber2002; Mason and Gillespie Reference Mason and Gillespie2013). In the following review, we define biological control as the manipulation of natural enemies (vertebrate, invertebrate, or microbial) to reduce pest populations below an acceptable level of damage (van Driesche et al. Reference van Driesche, Hoddle and Center2008), and focus solely on the use of arthropods and vertebrate animals. Microbes are dealt with in a related review (van Frankenhuyzen et al. Reference van Frankenhuyzen, Lucarotti and Lavallée2015).
Almost all biological control programmes have targeted what we now call invasive alien species; i.e., organisms not native to North America but with the potential to cause economic or ecological damage to important native plants or ecosystems. Here, we have elected to avoid the word “invasive”, and instead refer to species that have inadvertently or intentionally been introduced into North America since about 1500 as non-native (Langor et al. Reference Langor, Cameron, MacQuarrie, McBeath, McClay and Peter2014). As well, we have chosen to adopt the term “agent” throughout when referring to natural enemies used in biological control programmes, and “target” for organisms that cause damage or are subjects of suppression (sensu Eilenberg et al. Reference Eilenberg, Hajek and Lomer2001). “Target” is used in place of “pest” to avoid confusion because not all target species in programmes have been considered important pests, or even if they were in the past, may not be so today.
Canadian biological control programmes have used a range of tactics to exploit natural enemies and reduce pest damage in forests, including the three formally recognised as introduction, augmentation, and conservation biological control (van Driesche et al. Reference van Driesche, Hoddle and Center2008). All three tactics have been investigated in Canadian forests, but the most common has been introduction biological control. Classical biological control, or the intentional introduction of a non-native agent against a non-native target (Greathead Reference Greathead1994), is often the principal form of introduction, where a non-native biological control agent is collected from the same home range as that of the non-native target, and then released into the new habitat where the non-native target has become established, with the aim of re-establishing an “old host-natural enemy association”. A variation on this introduction approach is the attempt to create a “new host-natural enemy association”, either through the relocation of a non-native agent from outside of Canada against a native Canadian target, or through the relocation of a native agent against a non-native target, both within Canada. The basis for these two tactics rests on the expectation that such a new association will significantly reduce the target host population (Eilenberg et al. Reference Eilenberg, Hajek and Lomer2001). A final version of the introduction strategy is the simple translocation of an agent (non-native or native) from a site where it is well established to a new area where it is absent or in very low numbers, both within Canada. In this case, the goal is to exploit an already existing effective relationship between the agent and the target, which for some reason varies across the target’s geographic range. Regardless of the agent’s or target’s origin, all introduction strategies retain the underlying concept of introducing natural enemies to a new geographic location with the expectation of permanent establishment.
Conservation biological control has the goal of retaining or sustaining natural agent populations in an area so that pest outbreaks will be prevented or shortened and their impacts lessened. This strategy can include managing for diverse vegetation types, forest stand structures, and age class distributions, as well as reducing intensive forest management practices such as pesticide applications (van Driesche et al. Reference van Driesche, Hoddle and Center2008). Although the vast expanses of naturally forested regions in Canada seem to provide a prime target for this approach in biological control, few programmes have been conducted to date.
Canada has been a leader in developing methods for augmentation biological control. This strategy focusses on increasing agent populations that are already present, through mass rearing and release, either with the intent for the build-up of agents (inoculative release) or for their repeated application (inundative release). In inoculative releases, agents are meant to suppress the target and persist over one season, whereas in inundative releases, agents are intended to suppress a target and not persist (van Driesche et al. Reference van Driesche, Hoddle and Center2008). While most of the introduction programmes in Canada have made use of inoculative releases during their early stages (because it has always been necessary to rear agents in the laboratory in order to obtain sufficient numbers for release and establishment), repeated, inoculative biological control releases for seasonal augmentation have had limited scope in Canadian forests. In contrast, inundative releases have shown great potential for managing at least one native forest target in Canada.
Interpreting success in the context of Canadian biological control programmes has always been a challenge. Too often, success stories have been the only ones reported, and, in many cases, projects originally deemed successful, have later been labelled as unsuccessful (e.g., Hall et al. Reference Hall, Ehler and Bisabri-Eshadi1980; Hopper and Roush Reference Hopper and Roush1993; Gurr and Wratten Reference Gurr and Wratten2000). Assessment is also made difficult when one considers that potentially effective techniques developed and tested during research, have often never been made operational (e.g., Mills Reference Mills1983; Smith et al. Reference Smith, Wallace, Howse and Meating1990). This, combined with the fact that non-target impacts have rarely been assessed in Canada (even though they may have been shown in the United States of America) (e.g., Timms et al. Reference Timms, Walker and Smith2011), suggests that criteria for measuring success in biological control lack consistency. In this review, we have divided the last 130 years into four general eras and taken a chronological approach to examine biological control programmes in Canadian forests. The success in each case is interpreted with respect to the activities and goals of the era. In the final section, we discuss the broader implications in terms of approach, implementation, success, and relationship to the underlying science.
Summary of agents released in Canadian forests
Since 1882, 41 forest insect species have been the target of biological control programmes in Canadian forests (Table 1). The native oak looper, Lambdina fiscellaria somniaria (Hulst) (Lepidoptera: Geometridae), was originally identified as a separate target, but because this subspecies is now considered a polymorphic form of the native hemlock looper, L. fiscellaria (Guenée) (Sperling et al. Reference Sperling, Raske and Otvos1999), these two species have been combined here. Of the 41 targets, slightly more than half were non-native (n=20) versus native (n=18). The origin of the remaining three targets (Holarctic versus adventive) is difficult to assign (see The early era: 1882–1945). Of the 20 non-native targets, all but two were of European origin (Table 1), the exceptions being: the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), native to Asia (Keever et al. Reference Keever, Nieman, Ramsay, Ritland, Bauer, Lyons and Cory2012); and the satin moth, Leucoma salicis (Linnaeus) (Lepidoptera: Lymantriidae), which has an unknown distribution (but suspected to be Palaearctic).
Table 1 Native and non-native forest insect species targeted for biological control in Canadian forests between 1880 and 2014, and the agents released against each species. Species names follow current taxonomy. Unless indicated, where only the genus name is given denotes that an unidentified species was released.

References: McLeod et al. (Reference McLeod, McGugan and Coppel1962), Reeks and Cameron (Reference Reeks and Cameron1971), Beirne and Kelleher (Reference Beirne and Kelleher1973), Kelleher and Hulme (Reference Kelleher and Hulme1984), Mason and Huber (Reference Mason and Huber2002), Mason and Gillespie (Reference Mason and Gillespie2013), Roscoe et al. (Reference Roscoe, Lyons, Ryall and Smith2015).
* =Neocnemodon Goffe, likely two species released: Cnemondon latitarsus Egger and C. pubescens Delucchi and Pschorn-Walcher
† =Pullus Mulsant
‡ D.B.L., personal observation
§ Given the host, the target was likely juniper scale C. juniperi (Bouché) not C. visci.
|| Two species were released, one subsequently identified as Drino gilva (Hartig)
¶ Later determined to be a species complex, all agents released under this name.
** Listed as species “Number 1” in McLeod et al. (Reference McLeod, McGugan and Coppel1962)
†† Listed as species “Number 72” in McLeod et al. (Reference McLeod, McGugan and Coppel1962)
‡‡ Formerly recognised as two subspecies L. f. fiscellaria (hemlock looper) and L. f. somniaria (Hulst) (oak looper) (Sperling et al. Reference Sperling, Raske and Otvos1999), C. sycophanta was released against L. fiscellaria feeding on oak, see text for discussion.
§§ Early releases were likely made against native North American populations, later releases likely targeted introduced European populations, see text for discussion.
A total of 158 agents have been released in biological control programmes against forest insects in Canada (Table 1). Of these, 28 species were released against multiple targets, the most frequent being: Drino bohemica Mesnil (Diptera: Tachinidae) against 12 targets; Dahlbominus fuscipennis (Zetterstedt) (Hymenoptera: Eulophidae) and Pleolophus basizonus (Gravenhorst) (Hymenoptera: Ichneumonidae) both released against seven targets; and Exenterus abruptorius (Thunberg) (Hymenoptera: Ichneumonidae), E. amictorius (Panzer), and T. minutum, each released against five targets (Table 1; Fig. 3).
The release of biological control agents in Canada began during the late 1880s, peaked in the 1940s, declined dramatically during the 1960s, and remained low until the last decade when it fell again (Fig. 1). A similar trend is seen for both number of species targeted (Fig. 1) and number of agents released (Fig. 2). Parasitoids (i.e., insects of which the immatures feed on other arthropods, eventually killing them; after Godfray Reference Godfray1994) dominated those agents released in Canadian forests over all decades, while predators were somewhat important only during the 1950s and 1960s (Fig. 2). The species of parasitoids and predators released span seven taxonomic orders, but are dominated by the order Hymenoptera (Table 2). Only two agents have not been insects; a predatory mite, a species of Balaustium Heyden (Arachnida: Trombidiformes: Erythraeidae), introduced to Québec, Canada from Pakistan in 1967 against the non-native balsam woolly adelgid, Adelges piceae (Ratzeburg) (Hemiptera: Adelgidae) (Clark et al. Reference Clark, Greenbank, Bryant and Harris1971), and the masked shrew, Sorex cinereus Kerr (Mammalia: Soricidae), introduced into Newfoundland from New Brunswick, Canada in 1958 as a predator of the larch sawfly, Pristiphora erichsonii (Hartig) (Hymenoptera: Tenthredinidae) (Buckner Reference Buckner1959).
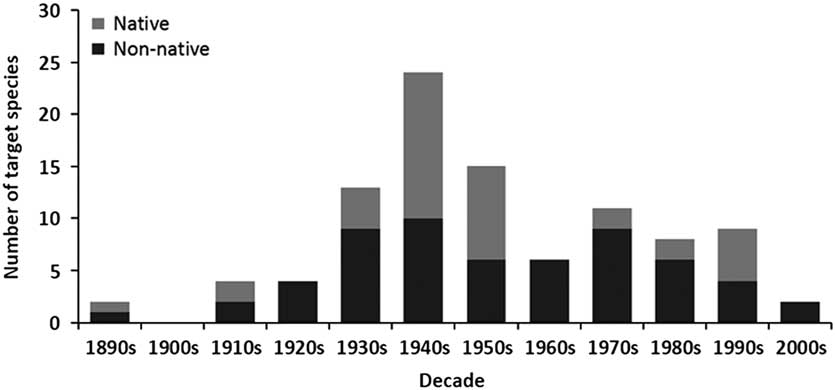
Fig. 1 The number of native and non-native forest insect species targeted for biological control in Canada between the 1890s and 2000s.
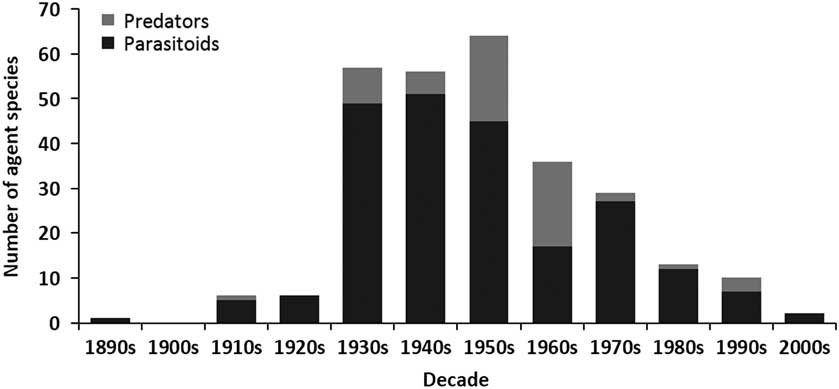
Fig. 2 The number of parasitoid and predator agents released in biological control programmes in Canadian forests between the 1890s and 2000s.
Table 2 Number of native and non-native forest insect species in each major order targeted for biological control, and the number of native and non-native insect parasitoids and predators released in Canadian forests.

By far the majority of agents used for biological control in Canadian forests have been non-native species (Table 2; Fig. 3), dominating every decade except the 1910s, 1940s, and 1950s when they represented about half (Fig. 1). Like the species they target, most agents (n=104) have originated from Europe, followed by North America (n=32), and then Asia (n=23) (Table 1; Fig. 3). While agents have also come from Australia (n=1) and South America (n=1), none of these have established (Kelleher and Hulme Reference Kelleher and Hulme1984). The dominance of European agents in this timeline reflects their use in classical biological control programmes, those aimed at non-native European targets in Canada (Fig. 3). Indeed, over 90% (149/161) of the Canadian biological control programmes in forests fit this definition of introduction biological control, and more specifically classical biological control (130/161). The few exceptions include; the non-native ambermarked birch leafminer, Profenusa thomsoni (Konow) (Hymenoptera: Tenthredinidae), controlled by the native parasitoid, Lathrolestes thomsoni Reschikov (Hymenoptera: Ichneumonidae) (MacQuarrie et al. Reference MacQuarrie, Langor, Digweed and Spence2013a), and the native Swaine jack pine sawfly, Neodiprion swainei Middleton (Hymenoptera: Diprionidae), suppressed by the non-native parasitoid, P. basizonus (Price and Tripp Reference Price and Tripp1972). The only instance of a native agent targeting a native species is through inundative release and augmentation of the native egg parasitoid, T. minutum against the native spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae), and the native spruce bud moth, Zeiraphera canadensis Mutuura and Freeman (Lepidoptera: Tortricidae) (Smith et al. Reference Smith, Wallace, Howse and Meating1990; West et al. Reference West, Kenis, Bouchier, Smith and Butt2002b).
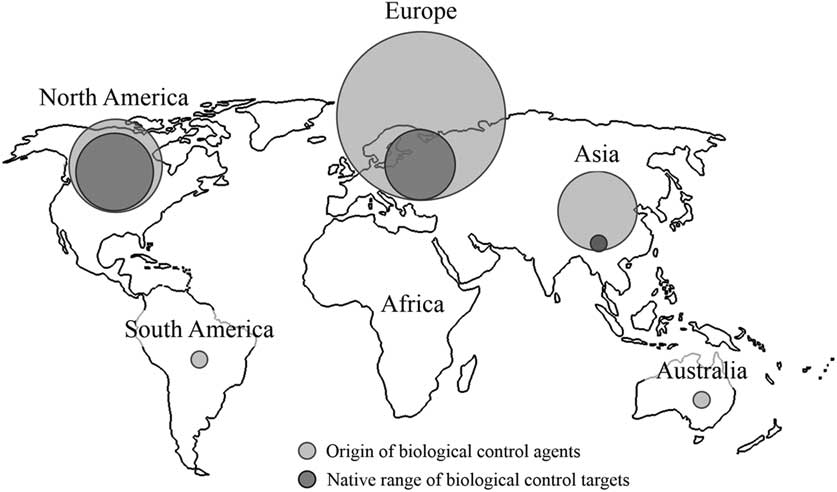
Fig. 3 Geographic origin of biological control targets and agents in Canadian forests. Circles are scaled to relative abundance of species.
The use of native agents in biological control programmes across Canadian forests merits some interpretation. Twelve of the 32 of the native agents used have been for augmentation biological control (either intentional or unintentional), and were already present in the area where they were released. For instance, two parasitoids of larch sawfly, Bessa harveyi (Townsend) (Diptera: Tachinidae) and Tritneptis klugii (Ratzenburg) (Hymenoptera: Pteromalidae), were both widely redistributed within Canada before they were found to be native North American species (McGugan and Coppel Reference McGugan and Coppel1962). Of the other native agents, 19 were simply relocated within North America to locations where the target was present but the agent was absent.
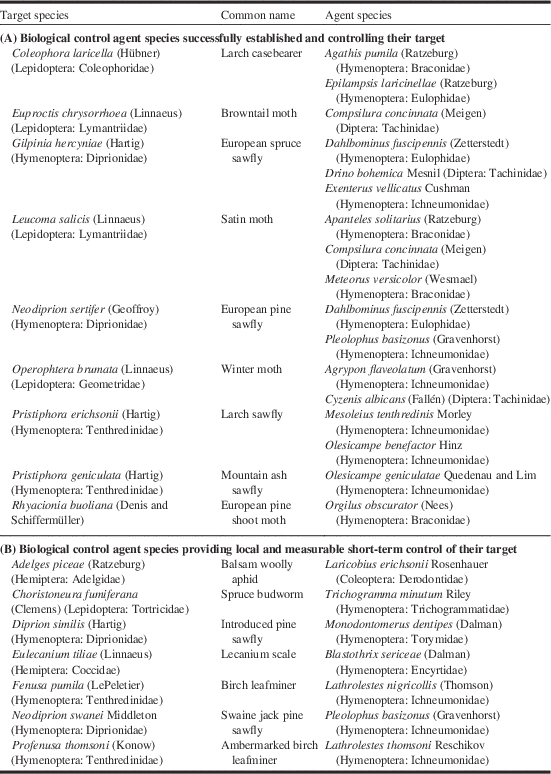
The number of successful biological control programmes in Canada has never been established conclusively, as “success” often depends on the goal and strategy used (e.g., introduction, augmentation, or conservation biological control). Previous estimates of success, based primarily on introduction or inoculative augmentation programmes, have tallied successful control against either five (Beirne Reference Beirne1975), seven (Munroe Reference Munroe1971; Hulme Reference Hulme1988), or nine (Wallace Reference Wallace1995) targets. However, these authors differed slightly in their criteria for “success”. Most agreed that success should include target suppression (Hulme Reference Hulme1988; Wallace Reference Wallace1995), but establishment was also important for introduction and inoculative augmentation, unlike inundative augmentation releases. Here, we assessed the programmes listed in Table 1 and defined success as either: (1) the agent became established in the target’s range and caused a measurable contribution to the control of the target (e.g., reduced the pest below an economic threshold) or (2) the agent provided at least local and measurable control of the target by the released agent but failed to achieve long-term control (as in the case of inundative augmentation releases).
Using these two categories of success, we found that: 15 agents successfully controlled nine targets, with some agents controlling multiple targets (Table 3; Section A); and an additional seven agents had an important impact on six more targets, albeit with no long-term control (Table 3; Section B). Among the influential agents, Compsilura concinnata (Meigen) (Diptera: Tachinidae) was the only one to suppress two targets; the non-native browntail moth, Euproctis chrysorrhoea (Linnaeus) (Lepidoptera: Lymantriidae), and the satin moth, although it also influenced the control of the non-native European gypsy moth, Lymantria dispar dispar (Linnaeus) (Lepidoptera: Erebidae).
Table 3 Biological control agent species (A) successfully controlling their target species or (B) providing local and measurable short-term control of their target in biological control programmes against forest insects in Canada. See Table 1 for origin of agents and targets.
Four eras of biological control in Canada
The history of biological control programmes in Canadian forests can be divided into four broad eras, each defined by different factors affecting the strategies for forest pest management. Due to the large number of projects over this time (Table 1), we can only highlight the most influential projects or those that have demonstrated novel aspects in biological control development and practice. More detail on individual projects can be found in a sequential series of review monographs (McGugan and Coppel Reference McGugan and Coppel1962; Reeks and Cameron Reference Reeks and Cameron1971; Kelleher and Hulme Reference Kelleher and Hulme1984; Mason and Huber Reference Mason and Huber2002; Mason and Gillespie Reference Mason and Gillespie2013), and a review by Armstrong and Ives (Reference Armstrong and Ives1995). The history of the people and institutions responsible for biological control programmes in Canada have been also documented by Glen (Reference Glen1956), Beirne (Reference Beirne1973, Reference Beirne1975), Beirne and Kelleher (Reference Beirne and Kelleher1973), Riegert (Reference Riegert1980), Richmond (Reference Richmond1983), and Quiring et al. (Reference Quiring, Quiring, Quiring and Edwards2015).
The early era: 1882–1945
One of the earliest introductions against a tree pest in Canada was a parasitoid of the non-native European fruit lecanium, Parthenolecanium corni (Bouché) (Hemiptera: Coccidae), introduced from California, United States of America in 1896 to control an outbreak of a species of lecanium scale, Parthenolecanium Šulc, on elm, Ulmus (Ulmaceae), near Ottawa, Ontario, Canada (Beirne and Kelleher Reference Beirne and Kelleher1973). As in 1882, with the first introduction against currantworm, neither was considered successful, likely because both agents were already present in Canada and the introductions provided no additional mortality (Beirne and Kelleher Reference Beirne and Kelleher1973). Interestingly, the releases against currantworm and lecanium scale represent the first time insects were shipped between two countries for the purposes of biological control in North America, and the first to be tried in Canada (Simmonds et al. Reference Simmonds, Franz and Sailer1976). Surprisingly, it would take until 1910 before the permanent establishment of an introduced biological control agent in Canada could be shown.
Serious work on Canadian biological control of forest insects began in 1909 with the appointment of C. Gordon Hewitt as Dominion Entomologist. Since the 1880s, occasional outbreaks of the larch sawfly had occurred throughout eastern Canada, and Hewitt arrived in the middle of one. Immediately, he initiated a biological control programme against the sawfly aimed at importing Mesoleius tenthredinis Morley (Hymenoptera: Ichneumonidae), its natural enemy in the United Kingdom (Hewitt Reference Hewitt1912). Between 1910 and 1913, sawfly cocoons parasitised in the United Kingdom were shipped to Canada and set out in infested stands across Ontario, Québec, and Manitoba. Unfortunately, failure to screen the released material resulted in several species of parasitoids being introduced (McGugan and Coppel Reference McGugan and Coppel1962), along with a new European strain of the larch sawfly itself (Wong Reference Wong1974; Ives and Muldrew Reference Ives and Muldrew1984); this would have important consequences for later biological control efforts (see The intensive era: 1945–1970). By 1919, the programme was considered successful in reducing sawfly populations; for the first time, this established biological control as a viable tactic to manage forest pests in Canada (Turnbull and Chant Reference Turnbull and Chant1961).
Hewitt influenced later work by J.D. Tothill, who introduced at least three parasitoids and a predator into the Maritime provinces against the browntail moth around 1914 (Table 1) (Beirne Reference Beirne1973). Tothill made a series of introductions against both the browntail moth and the European gypsy moth by relocating parasitised larvae from New England (United States of America) into eastern Canada, including a number into Ontario and Québec (neither of which apparently became established) (McGugan and Coppel Reference McGugan and Coppel1962). Tothill’s intent was to get agents established on native species of Lepidoptera before the actual targets arrived. However, the effect of this anticipatory biological control effort was never examined. His introductions into the Maritime provinces coincided with a decline in the browntail moth but were considered a failure because no direct link could be made between the introductions and the decline of the target (Turnbull and Chant Reference Turnbull and Chant1961; Beirne and Kelleher Reference Beirne and Kelleher1973). Tothill later expanded his work by introducing Calosoma sycophanta (Linnaeus) (Coleoptera: Carabidae), a non-native predator of European gypsy moth, into British Columbia, Canada as an agent against the native hemlock looper. This was the first attempt in Canada to create a “new association” between a non-native agent and a native insect. Here again, the actual introduction was considered successful but not effective, as C. synchophanta failed to control its target (Munroe Reference Munroe1971). Subsequent work has shown that C. concinnata, likely introduced to Canada during these early Tothill releases, is one of the main causes for collapse of browntail moth populations across eastern North America (Elkinton and Boettner Reference Elkinton and Boettner2012).
The initiatives of Hewitt and Tothill greatly accelerated biological control in Canada, launching programmes against at least 12 targets between 1925 and 1940 (McGugan and Coppel Reference McGugan and Coppel1962) (Fig. 1). Beirne (Reference Beirne1973) suggested that this activity was precipitated by the successful control of cottony cushion scale in the United States of America, the larch sawfly and browntail moth in Canada, and similar successes in the suppression of agricultural insect pests (see McLeod et al. Reference McLeod, McGugan and Coppel1962). Some of the projects launched during this time continued for decades, one of the best examples being the non-native European pine sawfly, Neodiprion sertifer (Geoffroy) (Hymenoptera: Diprionidae), which had agents released against it in Canada for over 40 years (Griffiths et al. Reference Griffiths, Cunningham and Otvos1984). The success of biological control in Canadian forestry (and similar successes in Canadian agriculture) demonstrated its value and highlighted the need for a dedicated biological control organisation in Canada. As a result, in 1915 the first biological control laboratory was established at Fredericton, New Brunswick, moving eventually to Belleville, Ontario in 1928 where it became known as the Dominion Parasite Laboratory, and later the Entomology Research Institute (Glen Reference Glen1956; Beirne Reference Beirne1973). The work done at the Belleville laboratories would support most of the biological control programmes in Canada until their closure in 1972.
The non-native European spruce sawfly, Gilpinia hercyniae (Hartig) (Hymenoptera: Diprionidae), dominated forest insect problems in Canada during the 1930s (Glen Reference Glen1956), and programmes for its control exemplified biological control before World War II. This non-native sawfly was first reported in the Gaspé region of Québec in 1933, likely introduced from Europe, and provided an ideal opportunity for research. Unfortunately, early work showed that the sawfly was rare in Europe and that there were two additional congeneric species with similar morphology and ecology; Gilpinia polytoma (Hartig) and G. frutetorum (Fabricius). This led to the search for agents across a wide range of target species, including related genera within the Diprionidae (i.e., Gilpinia Benson, Diprion Schrank, and Neodiprion Rohwer) and other sawflies on spruce (Picea Miller; Pinaceae). In the end, ~220 potential agent species were identified, imported to the laboratories in Belleville, reared, and many released into Canadian forests (Table 1; Glen Reference Glen1956; McGugan and Coppel Reference McGugan and Coppel1962; Reeks and Cameron Reference Reeks and Cameron1971). By the end of the project, over 843 million individuals were released, with another 247 million released in the United States of America (Dowden Reference Dowden1962; McGugan and Coppel Reference McGugan and Coppel1962). Despite this massive effort, classical biological control agents were not responsible for the eventual decline of the European spruce sawfly, but instead it was attributed to a nucleopolyhedralvirus, GhNPV (formerly Borrelinavirus hercyniae), brought in with agents during the 1930s (Bird and Elgee Reference Bird and Elgee1957). This virus spread quickly to wild European spruce sawfly populations causing significant mortality (Bird and Elgee Reference Bird and Elgee1957), and resulted in a shift in biological control work to mass-production of GhNPV (reviewed by van Frankenhuyzen et al. Reference van Frankenhuyzen, Lucarotti and Lavallée2015). The virus became so successful that by 1943, classical biological control was scaled back to only production and release of a few agents. Complete suppression of the sawfly was achieved by the late 1940s and Canadian forests have not experienced a significant outbreak of this species since (Magasi and Syme Reference Magasi and Syme1984).
Classical biological control efforts against the European spruce sawfly clearly contributed to its long-term suppression even though most work pointed to GhNPV. Subsequent research showed that at low sawfly densities the virus was actually ineffective (Neilson and Morris Reference Neilson and Morris1964), and suppression was due to native predators and two non-native agents, Drino bohemica Mesnii (Diptera: Tachinidae) and Exenterus vellicatus (Cushman) (Hymenoptera: Ichneumonidae) (Neilson et al. Reference Neilson, Martineau and Rose1971). The complexity of this density-dependent relationship was revealed during the 1970s when the application of insecticides for control of the spruce budworm in New Brunswick led to sawfly outbreaks. Research showed that these insecticides caused local extirpation of agents attacking the sawfly (including GhNPV), and that this enabled sawfly populations to rebound unchecked until the agents could re-establish and dampen the rise (Magasi and Syme Reference Magasi and Syme1984). Oddly, in the United States of America, Dowden (Reference Dowden1962) attributed the control of high European spruce sawfly populations to two different parasitoids, D. fuscipennis and E. amictorius, a phenomenon that was not seen in Canada. To date, this difference has not been investigated.
The intensive era: 1945–1970
After 1945, biological control in Canadian forests shifted away from targets in coniferous forests, and forest pest management programmes became more reliant on synthesised insecticides (Glen Reference Glen1956; Beirne Reference Beirne1973). At the same time, there was a tendency for biological control targets to have more cryptic life histories (i.e., inside plant tissues such as phloem) since this attribute made them more difficult to control. There was also greater recognition during this era that host range testing could be used to improve the selection of potential release agents, and thus have more impact against the target. Finally, concepts from the expanding field of animal ecology during this period (e.g., Holling Reference Holling1959) started to filter into biological control, and greater emphasis was placed on the role of forest insect population dynamics in choosing targets and refining the selection process for agents.
The infrastructure and production of agents that supported the European spruce sawfly project during the 1930s influenced the direction of work against other targets during this era well into the late 1940s. This was due in part to World War II, which made new agents difficult to obtain from Europe (i.e., the native range of most targets during this period), and reduced the number of personnel available to work on biological control. These factors also led to a general pause in forest biological control until 1947, after which, Canada saw eight programmes launched within only three years. Despite this apparent returning interest, many of these programmes were only viable because agents produced by the Belleville laboratory for control of the European spruce sawfly became redundant following the success of the GhNPV (Table 4). As a consequence, the new introduction programmes tended to target species of minor importance, with some attempting classical biological control (targeted at the original non-native agent in Canada), while others sought to create “new associations”, usually native targets (Tables 1 and 4). In general, these were small efforts and were not successful (Munroe Reference Munroe1971), although a number of native species were colonised that were not the target of any programme (Table 4). Somewhat surprisingly, there was little concern expressed at the time for the potential impact of these agents on non-target species, perhaps because some were considered pests as well.
Table 4 Parasitoid agents released in Canada for the control of European spruce sawfly, Gilpinia hercyniae (Hartig), 1933–1951, which were also released against other native and non-native targets.
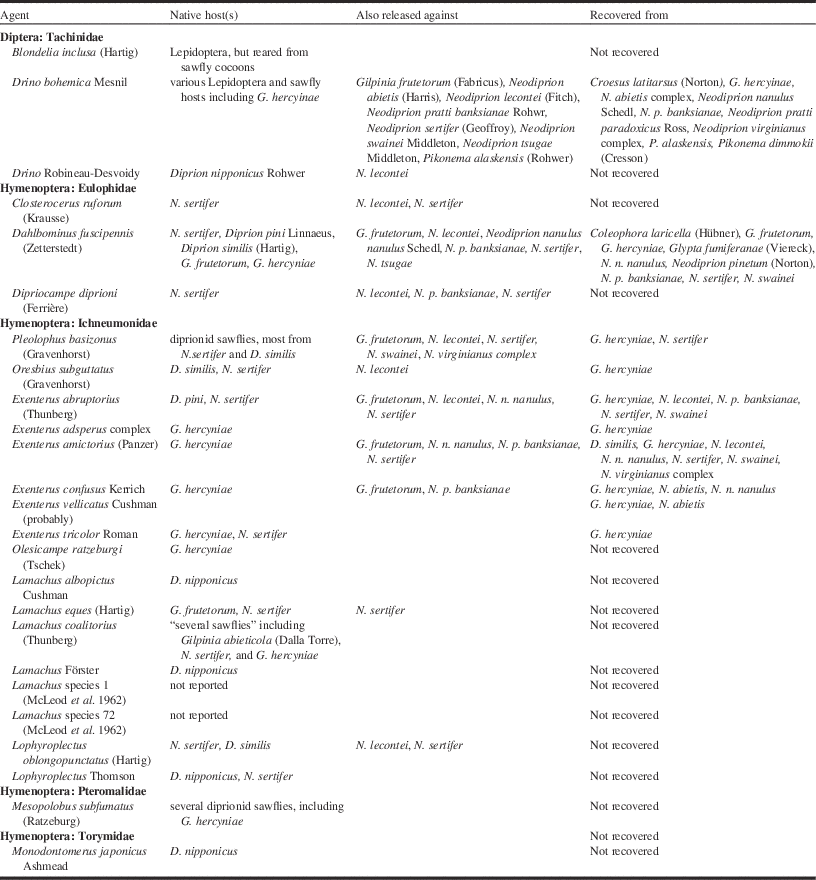
Where only the genus name is given indicates one or more unidentified species were released.
Major programmes during this post-war period continued those begun before 1939. For example, work against the balsam woolly adelgid that had started in 1933 eventually led to the introduction of 13 generalist predators (Table 1), eight of which established (McGugan and Coppel Reference McGugan and Coppel1962). These agents continued to be released in Newfoundland, British Columbia, and throughout the Maritimes during the 1960s, ending only in the early 1970s when it became apparent that they were ineffective against the adelgid (Clark et al. Reference Clark, Greenbank, Bryant and Harris1971; Schooley et al. Reference Schooley, Harris and Pendrel1984). Introductions against the European pine sawfly also continued during this time, again as an offshoot of the European spruce sawfly programme. Interestingly, agents targeted at the European pine sawfly had been first collected from this species in Europe as possible agents for the European spruce sawfly, and now were being redirected against their original host. Much like work targeting the balsam woolly adelgid, many agents were investigated and released against European pine sawfly for over 40 years with little or no significant impact. Control of the European pine sawfly was eventually achieved, but only through the development of a nucleopolyhedralvirus (Griffiths et al. Reference Griffiths, Cunningham and Otvos1984). This European pine sawfly programme is noteworthy in that it did see the first use of pre-release testing for biological control agents (Griffiths et al. Reference Griffiths, Rose and Bird1971), and it was the subject of intensive population ecology studies (Holling Reference Holling1959). Work on the dynamics of the European pine sawfly later influenced two successful projects that typified this post-war period: one against the non-native winter moth, Operophtera brumata (Linnaeus) (Lepidoptera: Geometridae), in Nova Scotia, and a renewed interest in the larch sawfly.
One of the major introduction programmes in Canada during this era was aimed at the winter moth, a defoliator of broadleaved, deciduous trees. This target was studied extensively in Europe before its discovery in North America during the early 1950s, and was known to be attacked by at least 63 parasitoids in its home range. The six most abundant species were selected for introduction into Canada (Embree Reference Embree1971), two of which eventually established; Cyzenis albicans (Fallén) (Diptera: Tachinidae) and Agrypon flaveolatum (Gravenhorst) (Hymenoptera: Ichneumonidae). Releases of the other four agents were unsuccessful and discontinued to avoid competition with the first two agents that had established. Life table studies on winter moth populations in Nova Scotia were later used to quantify the impact of these released agents, along with other ecological factors (Embree Reference Embree1966; Varley and Gradwell Reference Varley and Gradwell1968). This work showed that the agents were able to keep moth populations low only if local weather conditions accelerated overwintering emergence before bud burst and led to larval starvation (Embree Reference Embree1966). Roland (Reference Roland1988) and Roland and Embree (Reference Roland and Embree1995) later found that the released agents caused simple indirect mortality to the winter moth pupae by lengthening their time in the soil, thereby increasing their odds of predation by small mammals and ground-dwelling invertebrates.
The 1950s also saw a renewed effort against the larch sawfly, because M. tenthredinis, the most effective agent controlling it, had become ineffective by 1957. This programme was actually the fifth effort targeting the sawfly, and the fourth since Hewitt’s work in the 1910s. Throughout the 1920s, 1930s, and 1940s, parasitised sawfly larvae were collected near one of the first releases in Manitoba and redistributed to other parts of Canada (McGugan and Coppel Reference McGugan and Coppel1962). Wong (Reference Wong1974) eventually showed that the sawfly’s resistance to M. tenthredinis was because it could encapsulate the parasitoid’s eggs and prevent them from hatching. The source of this resistance was thought to have come from the accidental introduction of a non-native sawfly strain from Europe during Hewitt’s initial releases in the early 1900s (McGugan and Coppel Reference McGugan and Coppel1962). Although yet to be confirmed, this suggests that the spectacular success of Hewitt’s releases was in fact due to the target being the native strain of larch sawfly and therefore susceptible to M. tenthredinis, whereas after 1957, sawfly outbreaks were likely caused by the non-native sawfly strain that was resistant to the parasitoid.
A number of options were explored to suppress the renewed outbreaks of larch sawfly. For the first time, host testing was done to determine the best parasitoids to release and to estimate the potential for non-target effects (Ives and Muldrew Reference Ives and Muldrew1984). However, some of these tests were done after the agents were released so there would have been little chance to prevent any unintended consequences. A rather unique programme was implemented against the sawfly in Newfoundland and Labrador when the masked shrew was released as an agent. Life table work in New Brunswick had shown that overwintering larch sawfly pupae suffered significant predation from small vertebrates, but Newfoundland lacked small predatory mammals. Unfortunately, once released, the masked shrew had no effect on larch sawfly populations, although it did contribute to European spruce sawfly control (Magasi and Syme Reference Magasi and Syme1984). Of greater concern was that the masked shrew populations expanded to become a nuisance over much of the island (Beirne and Kelleher Reference Beirne and Kelleher1973), illustrating the importance of non-target screening before release. In the rest of Canada, two new agents were released, M. tenthredinis from a European population that was not encapsulated by the sawfly, and Olesicampe benefactor (Thomson) (Hymenoptera: Ichneumonidae), another host-specific parasitoid showing promise (Turnock and Muldrew Reference Turnock and Muldrew1971). Based on initial success, O. benefactor was released across Canada up until 1981. It was later determined that a hyperparasitoid of O. benefactor, originally thought to be native only in Europe, also occurred in Canada and attacked O. benefactor, thereby limiting its effectiveness against the larch sawfly (Ives and Muldrew Reference Ives and Muldrew1984).
As with winter moth, interactions between the larch sawfly, O. benefactor, and M. tenthredinis were examined in detail following their release, including studies on dispersal and life tables (Ives and Muldrew Reference Ives and Muldrew1984). Unfortunately, larch sawfly populations collapsed during the late 1970s and this biological control programme ended without being fully assessed. No other larch sawfly outbreaks have been detected since, and the fate of O. benefactor and M. tenthredinis remains unknown. In a final wrinkle, Wong (Reference Wong1974) showed that the larch sawfly was a Holarctic species and likely had been present in North America since the Permian (250 mya). Early dendrochronological studies from across North America (reviewed by Ives and Muldrew Reference Ives and Muldrew1984), and recent work from northern Canada by Nishimura and Laroque (Reference Nishimura and Laroque2010), both suggest that the larch sawfly is native to North America, although its actual distribution remains uncertain. If Wong’s hypothesis is correct, then the non-native agents introduced by Hewitt during the early 1900s actually formed a “new association” that initially suppressed the native larch sawfly (Hewitt Reference Hewitt1912; Glen Reference Glen1956), but this was then disrupted by the inadvertent contamination and release of a resistant strain of European sawfly and its hyperparasitoids.
The declining era: 1970–2000
After 1970, biological control programmes in Canada became significantly smaller in both size and scope. The Belleville laboratory closed in 1972 as part of broader reductions across the federal government, and this severely constrained the resources available for forestry programmes. At the same time, aerial insecticide programmes expanded exponentially with focus on both chemical and biological applications (reviewed by Holmes and MacQuarrie Reference Holmes and MacQuarrie2016 and van Frankenhuyzen et al. Reference van Frankenhuyzen, Lucarotti and Lavallée2015). Biological control projects that began after 1970 were of much smaller scope than earlier. However, this reduction also correlated with a higher degree of success than seen in the past.
Starting in 1970, the relocation of established non-native agents became a regular practice in Canada. Relocations had always been used as a technique to obtain parasitoids dating back to the first releases against the imported currantworm. However, because established agents were now readily available, this technique became popular, especially given limited resources. Numerous relocation examples could be cited here, including those for the spruce budworm and winter moth, but for brevity’s sake, the following focusses on only a select few. Two egg parasitoids of European gypsy moth were released in Ontario from New Jersey during 1976 and 1980 (Griffiths and Quednau Reference Griffiths and Quednau1984). Successful introductions of Olesicampe geniculatae Quednau and Lim (Hymenoptera: Ichneumonidae) were made from Europe into Québec for control of the non-native mountain ash sawfly, Pristophora geniculata (Hartig) (Hymenoptera: Tenthredinidae) (Quednau Reference Quednau1990), and this agent was subsequently relocated to Newfoundland during the mid-1970s (West et al. Reference West, Dixon, Quednau, Lim and Hiscock1994, Reference West, Dixon, Quednau and Lim2002a). Four species of parasitoids from Europe and Japan were introduced into British Columbia between 1969 and 1980 against the non-native larch casebearer, Coleophora laricella (Hübner) (Lepidoptera: Coleophoridae), and two of these were later relocated from Montana to British Columbia (Otvos and Quednau Reference Otvos and Quednau1984). There were also a number of relocations from Ontario to the island of Newfoundland of Lophyroplectus luteator Thunberg (Hymenoptera: Ichneumonidae) and P. basizonus, two agents originally released against the European spruce sawfly (Table 1), but now targeting the European pine sawfly. Finally, successful relocations of L. thomsoni from Alberta to the Northwest Territories, British Columbia, and Alaska against the non-native ambermarked birch leafminer were also made at the beginning of the 2000s (MacQuarrie et al. Reference MacQuarrie, Langor, Digweed and Spence2013a, Reference MacQuarrie, Williams and Langor2013b; Soper et al. Reference Soper, MacQuarrie and van Driesche2015).
There was very limited use of insect predators against forest pests in Canada post-1969. One bold biological control attempt was made against Swaine jack pine sawfly by introducing several predators into stands of jack pine, Pinus banksiana Lambert (Pinaceae), across Québec and Ontario (Finnegan and Smirnoff Reference Finnegan and Smirnoff1984). These predators included two species of red wood ant: (non-native Formica lugubris Zetterstedt (Hymenoptera: Formicidae) from Italy, and the native F. obscuripes Forel from Manitoba (Finnegan and Smirnoff Reference Finnegan and Smirnoff1984). The effectiveness of these ants on the target could not be assessed, but they were observed preying on other forest insect pests. Augmentative biological control with predators was also attempted against the holarctic balsam twig aphid, Mindarus abietinus Koch (Hemiptera: Mindaridae), using Chrysopa carnea Stephens (Neuroptera: Chrysopidae), Aphidoletes aphidimyaz (Rondani) (Diptera: Cecidomyidae), and Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). However, H. axyridis interfered with natural predation, and the establishment success of the other two species is unknown (Cloutier and Jean Reference Cloutier and Jean2002). Finally, attempts were also made to augment the native predator, Lonchaea corticis Taylor (Diptera: Lonchaeidae), against the native white pine weevil, Pissodes strobi (Peck) (Coleoptera: Curculionidae), in order to increase its density above a critical threshold, but the agent could not be reared in the laboratory and the project was abandoned (Hulme and Kenis Reference Hulme and Kenis2002). In the late 1990s, a similar effort was undertaken to assess the parasitoids associated with the terminal weevil, Pissodes nitidus Roelofs (Coleoptera: Curculionidae) in northeastern China with the intent of identifying agents that could be released against white pine weevil in Canada. Although the survey was completed (Hu et al. Reference Hu, Li, Wang, Langor, Yue, Liu and Han2000) and two species were identified as possible agents (D. Langor, personal communication), the programme was cancelled in 1999 due to declining interest and funding in Canada.
A classic and often-cited example of conservation biological control involved the management of the non-native European pine shoot moth, Rhyacionia buoliana (Denis and Schiffermüller) (Lepidoptera: Tortricidae). Several agents were released between 1969 and 1973 in Ontario for this target (Reeks and Cameron Reference Reeks and Cameron1971), but only one, Orgilus obscurator (Nees) (Hymenoptera: Braconidae), was found to be effective. Syme (Reference Syme1977) later showed that access to flowers, especially wild carrot, Daucus carota Linnaeus (Apiaceae), increased the parasitoid’s effectiveness against the target by extending its lifespan and allowing for maximum fecundity.
One major augmentation biological control programme using a native egg parasitoid targeted against the native spruce budworm also occurred during this era, with successful strategies developed over a 15-year period to commercially rear and release the parasitoid (Smith et al. Reference Smith, Wallace, Howse and Meating1990, Reference Smith, van Frankenhuyzen, Nealis and Bourchier2002). Efficacy studies and non-target risks showed this to be a promising option for protecting local, high value spruce forests. However, the programme was discontinued with the collapse of eastern spruce budworm across eastern North America in the mid-1990s.
Investigations continued into the biological control of European gypsy moth during the 1980s and 1990s, leading to the development of two innovative tactics. The first was aimed at reducing the high rate of hyperparasitism seen on the European gypsy moth parasitoid, Cotesia melanoscela (Ratzeburg) (Hymenoptera: Braconidae) in North America. Nealis and Bourchier (Reference Nealis and Bourchier1995) tested an Asian strain of C. melanoscela less vulnerable to hyperparasitism and recommended it for release (Nealis et al. Reference Nealis, Carter, Kenis, Quednau and van Frankenhuyzen2002). However, these releases were never carried out. The second tactic attempted to find new natural enemies for European gypsy moth in Europe, this time focusing on those attacking low density host populations, with the rationale that these agents might be more effective at suppressing the target than those from high host densities. Previous agents released against the European gypsy moth had come from outbreak populations in Europe, but research was starting to show that natural enemy communities differed depending on European gypsy moth density (Nealis et al. Reference Nealis, Carter, Kenis, Quednau and van Frankenhuyzen2002). In collaboration with CABI, a new agent, Aphantorhaphopsis samarensis (Villeneuve) (Diptera: Tachinidae), was discovered attacking low density populations in Europe, and interest shifted to introducing it against the European gypsy moth in Canada, as part of a new biological control strategy (Mills and Nealis Reference Mills and Nealis1992; Nealis and Quednau Reference Nealis and Quednau1996). Beginning in 1990, A. samarensis was released into Ontario and New Brunswick (Nealis et al. Reference Nealis, Carter, Kenis, Quednau and van Frankenhuyzen2002). However, few outbreaks of European gypsy moth have been seen in Canada since 1990, and the establishment of A. samarensis has not been evaluated.
In the 1970s and 1980s, Canadian biological control practitioners clearly recognised the importance of testing parasitoids under field conditions before their release. In at least three cases these assessments were done for potential agents that were subsequently rejected. For instance, extensive pre-release testing was done above for A. samarensis against the European gypsy moth (Fuester et al. Reference Fuester, Swan, Kenis and Hérard2004), and field cage releases of Japanese and Austrian strains of Cephaloglypta murinanae (Bauer) (Hymenoptera: Ichneumonidae) and a species of Japanese Lissonota Gravenhorst (Hymenoptera: Ichneumonidae), were used to test potential agents against spruce budworm, in New Brunswick (Varty Reference Varty1984). Two possible agents, Eubazus semirugosus (Ratzeburg) (Hymenoptera: Braconidae) and E. robustus (Nees), were tested against the white pine weevil but the latter was removed after laboratory and field cage studies found it was poorly synchronised with the target; the former was not pursued as funding stopped (Hulme and Kenis Reference Hulme and Kenis2002). Although not strictly pre-release testing, Myxexoristops hertingi Mesnil (Diptera: Tachinidae) was released into cages on plantation-grown trees infested by non-native pine false webworm, Acantholyda erythrocephala (Linnaeus) (Hymenoptera: Pamphiliidae), to prevent dispersal of the low numbers of available parasitoids (Lyons Reference Lyons2013a). No parasitoids were subsequently recovered.
By the 1980s, creating “new associations” among non-native agents and native insects had fallen out of favour in biological control programmes. Projects during the early 1930s and 1940s had used this technique, but these tended not to be successful (e.g., Table 4), and subsequent work suggested that there was little evidence to support their implementation (Nealis and Wallace Reference Nealis and Wallace1991; Wallace Reference Wallace1995). Only one attempt was made during this time to create a “new association” between a non-native agent and a native target with the introduction of Apanteles murinanae (Capek and Zwolfer) (Hymenoptera: Braconidae) against the spruce budworm (Mills Reference Mills1983). This species was shown to have a slower developmental rate and a longer pre-oviposition period than its native congener, suggesting that it might be better synchronised with its host. A small release was made in Québec (Nealis and Wallace Reference Nealis and Wallace1991), but it was not recovered and no further monitoring was done (Smith et al. Reference Smith, van Frankenhuyzen, Nealis and Bourchier2002). Spontaneous novel associations between native agents and non-native targets also started to be detected in the field during this era, and began to be exploited for introductions, most notably against the ambermarked birch leafminer (MacQuarrie et al. Reference MacQuarrie, Langor, Digweed and Spence2013a).
The century-old relationship between CABI in Europe and the Canadian Forest Service (CFS) was particularly important during the 1970s and 1980s. While the CFS had always funded CABI to collect and ship promising agents to Canada (Hulme Reference Hulme1982), during this era the relationship evolved into a stronger research collaboration, with CABI staff carrying out detailed biological studies on potential agents in Europe before shipping agents to Canada. Again, many projects could be cited here including most of the European gypsy moth work post-1980, but a few that have not been mentioned elsewhere include; the introduction of European parasitoids against the non-native birch casebearer, Coleophora serratella (Linnaeus) (Lepidoptera: Coleophoridae), into Newfoundland between 1968 and 1975 (Raske Reference Raske1984), and the introduction of M. hertingi against the pine false webworm (Kenis and Kloosterman Reference Kenis and Kloosterman2001). Work against the birch casebearer ended in the 1980s (Raske Reference Raske1984) and the parasitoid of pine false webworm has not been recovered, although the sawfly population did collapse after the agent was released (Lyons Reference Lyons2013a). CABI’s investigations on non-native agents for use against native seed cone maggots, Strobilomyia neanthracina Michelsen (Diptera: Anthomyiidae) and S. appalachenis Michelsen (Brockerhoff and Kenis Reference Brockerhoff and Kenis1997), suggested that introductions would not be warranted because the ecology of the parasitoids in Europe and North America were too similar (Sweeney et al. Reference Sweeney, Brockerhoff, Kenis and Turgeon2002). The same conclusions were reached for European agents targeted against the hemlock looper, spruce bud moth, and spruce seed moth, Cydia strobilella (Linnaeus) (Lepidoptera: Tortricidae). As a consequence, no biological control releases were attempted against these targets (West and Kenis Reference West and Kenis1997; Brockerhoff et al. Reference Brockerhoff, Kenis and Turgeon2002; West et al. Reference West, Kenis, Bouchier, Smith and Butt2002b).
One of the interesting aspects about biological control programmes during this era is the number of times decisions were made not to carry out releases, suggesting greater awareness of biological and ecological considerations, as well as potential risk to non-targets. While many of these projects were not successful at locating viable agents for control of native targets, the resources invested in these preliminary investigations likely resulted in significant savings compared to what would have been expended if full-scale, and likely unsuccessful, biological control programmes had been attempted. The lone successful project to occur during this period was the introduction of Lathrolestes nigricollis Thomson (Hymenoptera: Ichneumonidae) and Grypocentrus albipes Ruthe (Hymenoptera: Ichneumonidae) against the non-native birch leafminer, Fenusa pumila Leach (Hymenoptera: Tenthredinidae). These parasitoids were released during the 1970s in Québec and Newfoundland (Quednau Reference Quednau1984) and again into Alberta during the 1990s (Langor et al. Reference Langor, Digweed and Spence2002). One agent, L. nigricolls suppressed the leafminer in both eastern and western Canada, and the target is now much less dominant than it was before the release (MacQuarrie et al. Reference MacQuarrie, Langor, Digweed and Spence2013a).
The current era: 2000 onwards
The last 15 years have seen few biological control applications against forest insect pests in Canada. As evidence, only six of the 35 chapters in the review of Canadian biological control programmes between 2001 and 2012 were on insects attacking trees (Mason and Gillespie Reference Mason and Gillespie2013). This period includes efforts against relatively minor pests, such as the pine false webworm (Lyons Reference Lyons2013a) and the ambermarked birch leafminer (MacQuarrie et al. Reference MacQuarrie, Langor, Digweed and Spence2013a, Reference MacQuarrie, Williams and Langor2013b), but not against the most important pest species damaging Canadian forests, including the native mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae). During this period five non-native wood-boring insects were discovered in eastern Canada: the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae), the brown spruce longhorn beetle, Tetropium fuscum (Fabricius) (Coleoptera: Cerambycidae), the emerald ash borer, the European pine shoot beetle Tomicus piniperda (Linnaeus) (Coleoptera: Curculionidae), and Sirex noctilio Fabricius (Hymenoptera: Siricidae). Two of these species have arrived from Asia (Asian longhorned beetle and emerald ash borer) signalling a geographic shift in the origin of targets, from Europe to Asia. Of these five species, only the emerald ash borer has been targeted for biological control (Table 1).
Exploration for potential biological control agents of emerald ash borer in China has resulted in the discovery, and eventual release into the United States of America of three species: Spathius agrili Yang (Hymenoptera: Braconidae), Tetrastichus planipennisi Yang (Hymenoptera: Eulophidae), and Oobius agrili Zhang and Huang (Hymenoptera: Encyrtidae) (Zhang et al. Reference Zhang, Huang, Zhao, Liu and Bauer2005; Yang et al. Reference Yang, Strazanac, Yao and Wang2006; Ulyshen et al. Reference Ulyshen, Duan and Bauer2010; Duan et al. Reference Duan, Bauer, Ulyshen, Gould and Van Driesche2011); T. planipennisi was obtained from the United States of America and released into Canada in 2013 and 2014 (D.B.L., personal observation). Spontaneous novel associations were also detected against the emerald ash borer and exploited for potential control (Lyons Reference Lyons2013b; Roscoe et al. Reference Roscoe, Lyons, Ryall and Smith2015). The parasitoids of Asian longhorned beetle have been investigated in China and some augmentative releases have taken place there, but as yet no releases have been made in North America because the beetle continues to be eradicated (Turgeon and Smith Reference Turgeon and Smith2013). No biological control agents have been released in Canada against the pine shoot beetle or S. noctilio. However, considerable information has been gained on their natural enemy assemblages, including “new associations” with native agents (Rudzik Reference Rudzik2009; Ryan et al. Reference Ryan, Smith and Turgeon2013). A parasitic nematode, Deladenus siricidicola Bedding (Tylenchida: Neotylenchidae), that attacks the eggs and sterilises females of S. noctilio has been shown to be already present in Ontario, as have two parasitoids from the target’s native range (Ryan et al. Reference Ryan, Smith and Turgeon2013). Moreover, native agents already appear to be regulating Canadian populations of both the pine shoot beetle (Rudzik Reference Rudzik2009) and S. noctilio (Ryan et al. Reference Ryan, Smith and Turgeon2013). No biological control programmes have been attempted against the brown spruce longhorn beetle.
Discussion
Biological control in Canadian forests has been subjected to a number of reviews over the past century. Two key papers published in the early 1960s were critical of its potential, expressing concerns about the relative ad hoc nature of past introduction programmes (Turnbull and Chant Reference Turnbull and Chant1961; McGugan and Coppel Reference McGugan and Coppel1962). These authors were uneasy with both the lack of information on what was released and the limited number of follow-up studies carried out. They contended that early work had failed to properly screen material before release, and that this had led to contamination by other organisms, including resistant or virulent pest strains, multiple agents, and hyperparasitoids, and that this had compromised the releases (e.g., larch sawfly; Table 1). They also noted that past studies had surprisingly made little attempt to assess an agent’s impact, in terms of its overall success. More importantly, these authors found there had been no real interest in examining the broader effects of the agents on possible non-targets. Their critiques also challenged the deliberate strategy of releasing multiple agents, as the potential for adverse competition among different agents was thought to be great. These two reviews appear to have been fairly influential, as later CABI reports attempted to address the issues raised by Turnbull and Chant (Reference Turnbull and Chant1961) and McGugan and Coppel (Reference McGugan and Coppel1962) (see Reeks and Cameron Reference Reeks and Cameron1971).
In his review, Munroe (Reference Munroe1971) agreed with many of the concerns expressed by the two early critiques, but he thought that they had somewhat overemphasised the importance of selectivity and need for caution, in part, based on a concern that criticism of biological control might lead to institutional paralysis and reduced capacity for future work. Beirne and Kelleher (Reference Beirne and Kelleher1973) avoided this discussion, and instead turned to the issues of non-target effects and success. While they accepted that some undesirable consequences had occurred as a result of releases in the past, they did not consider them harmful enough to warrant real concern (Beirne and Kelleher Reference Beirne and Kelleher1973). On the other hand, they were very critical of the “apparent record of success” for biological control in Canada, arguing that 90% of all projects (they did not distinguish between forestry and agriculture programmes) were not really successful because 70% of the agents did not even establish. They were also disapproving of the mass-rearing approach to the production of agents (e.g., European spruce sawfly), contending that the release of large numbers of agents was not always the best way to guarantee establishment, in particular when the agent had had no previous relationship with the target (e.g., Table 4).
It is difficult to evaluate the effect of these early assessments on biological control programmes in Canadian forests. Coincidently perhaps, around the same time as Turnbull and Chant’s (Reference Turnbull and Chant1961) review, biological control began to decline as an overall tactic in forestry (Figs. 1–2), and it is unclear whether these two events can be related. Recommendations made by Munroe (Reference Munroe1971) to improve biological control practice do not appear to have been adopted, and it is apparent that even though biological control studies continued, they were significantly smaller and releases much less frequent. During the 1970s, debate seemed to center around the relative merits of single versus multiple agent releases (Beirne and Kelleher Reference Beirne and Kelleher1973), but again, the discussion appears to have died out by the early 1980s without resolving this question. Unfortunately, disregarding these key biological control issues seems to have had little impact on the strategies used by subsequent programmes, as a decline in resources available to find, rear, and release large numbers of agents has only allowed a few agents to be considered at any one time since.
After the debates of the 1960s and 1970s, the reviews of biological control published during the 1980s and 1990s were more positive. Drawing from leading-edge ecological research, Hulme and Green (Reference Hulme and Green1984) emphasised the role of selecting the proper agents for release and taking into account the population dynamics of the target; they also supported a role for novel associations. In their later review, Nealis and Wallace (Reference Nealis and Wallace1991) described biological control against forest insects in Canada as “very successful”, arguing that agents tended to establish at higher rates in stable forest systems than in others, and that biological control was more likely to work in harmony with such natural systems, and be more accepted by the public. These authors also agreed that novel associations and inundative releases had a place in forest biological control. Wallace (Reference Wallace1995) later reiterated these views, and gave a generally positive assessment of biological control in Canadian forests, the last broad overview of its kind.
Our assessment of biological control in Canadian forests is cautiously optimistic. Clearly, a number of targets have been brought under control by these programmes, but many share undesirable characteristics when measured by today’s standards (Myers Reference Myers, Higgins and Kovacs1989). The goal of the earliest programmes was to establish, or re-establish, a natural balance between targets and their multiple agents, and as a result, projects during the 1930s and 1940s introduced as many species as possible in the hope that one would establish, and little attempt was made to find a strong ecological match; e.g., numerous agents were released against the European spruce sawfly with no known history of attack (Table 4). Oddly, the first successful biological control project against the larch sawfly was an exception to this multiple agent strategy in that studies from the target’s native range provided the rationale to introduce only one effective agent (Hewitt Reference Hewitt1912). The release of polyphagous agents with broad host ranges was another strategy used in these early programmes, notably against the European gypsy moth and browntail moth, based on the belief that this was a desirable trait and would facilitate survival when the target was not present (or had been driven to extinction). Perhaps one lesson learned during this period was that non-target effects were important, and that generalist predators, even when successful against their target (e.g., C. concinnata), were unlikely to be good agents for release because of this potential for broader, unanticipated impact (Buckner Reference Buckner1959; Schooley et al. Reference Schooley, Harris and Pendrel1984; Hulme and Kenis Reference Hulme and Kenis2002).
The motivation to suppress target populations, at least in the early days of biological control, was rooted in the desire to prevent damage to trees, rather than for economic or ecological reasons. This motivation is evident in the selection of targets from the 1930s through to the 1970s (Tables 1, 4; Fig. 1). Many targets did not kill trees or even cause economic damage, and certainly their ecological or social effects were rarely considered. Some programmes were only viable because “surplus” agents were available (e.g., those agents produced by the Belleville laboratory for the suppression of European spruce sawfly). What is most troubling at this time is that many of these “targets of opportunity” were native insects (Table 4), and while occasionally injurious to trees, were probably well adapted and co-evolved with many natural enemies. Over the years, economic considerations were often cited as the reason to start biological control programmes, but in the end, economic loss or benefit was never really evaluated. This apparent lack of desire (or need) to assess programmes strictly in terms of costs and benefits is surprising, although one might say not unique to biological control. In fact, the entire last century of forest pest management in Canada seems to have curiously missed requiring any real economic or environmental analysis to justify its use.
Classical biological control has been the dominant tactic used in Canadian forests over the past century, appropriately reflecting the emphasis on non-native targets introduced without their suite of natural enemies. This trend persists to the present day, even though work during the 1970s and 1980s attempted to include other strategies such as conservation and augmentation. Syme’s (Reference Syme1977) research on European pine shoot moth showed the value of working with vegetation and landscapes to conserve natural enemies, as did more recent studies of hemlock looper (Hebert and Brodeur Reference Hebert and Brodeur2002). Such examples of conservation biological control are the exceptions though, as no other efforts have been undertaken in Canadian forests. Augmentation strategies using inundative releases or the development of “new associations” in introduction biological control, have similarly been absent from biological control programmes since the 1980s. Inundative releases of a native egg parasitoid against the spruce budworm during the 1980s and 1990s showed that it could reduce populations and prevent defoliation (Smith et al. Reference Smith, Wallace, Howse and Meating1990); however, the project was discontinued when spruce budworm populations collapsed and before commercial production and application could be made viable. One new development in biological control for Canada has been the use of insect agents to suppress non-native vegetation in southern Ontario forests; i.e., dog-strangling vine, Cynanchum rossicum (Linneaus) (Apocynaceae) (S.M.S., personal observation). This project represents the first attempt at weed biological control in Canadian forestry, and if successful, could be significant for future restoration projects in disturbed natural habitats.
Success in classical biological control programmes is often measured by whether the agent becomes established and provides long-term, significant control of its target in the new range. Such a simple definition must be extended to include alternative strategies, such as conservation and augmentation biological control. In this review, we have defined success in two ways, one using the classic definition of established, long-term control, and a second that relaxes the standard for long-term suppression slightly to allow for better evaluation of augmentation strategies (Table 3). In the latter case, the agent may make a contribution to the control of the target, but other mortality factors may also be required in order to achieve complete suppression. Using these definitions provides for a more nuanced view of success in biological control programmes and a more comprehensive measure of effectiveness of agents. By refining the meaning of success, the significant historical role biological control has played in the management of Canadian forests becomes clearer.
Historically, Canada has been a world leader in the field of biological control for forest insects (Pschorn-Walcher Reference Pschorn-Walcher1977; Kelleher and Hulme Reference Kelleher and Hulme1984; Smith Reference Smith1993), and many of its biological control programmes have been considered successful (e.g., Munroe Reference Munroe1971; Hulme Reference Hulme1988; Wallace Reference Wallace1995). Much of this success is rooted in the extensive population studies and basic research typical of Canadian forest entomology (Nealis and Wallace Reference Nealis and Wallace1991; Quiring et al. Reference Quiring, Quiring, Quiring and Edwards2015). Yet despite this impressive track record, biological control programmes have been on the decline in Canada since the 1960s (Fig. 1). As of 2014, only a handful of active projects remain, all targeting the emerald ash borer in Ontario (Lyons Reference Lyons2013b). The last active programme was completed during the mid-2000s against ambermarked birch leafminer, and even this involved only the relocation of agents within Canada (MacQuarrie et al. Reference MacQuarrie, Williams and Langor2013b). All of this raises concern about Canada’s capacity to accomplish large-scale or concurrent biological control programmes into the future. Although critical infrastructure has been retained, along with new facilities capable of producing large numbers of biological agents (e.g., Natural Resources Canada’s CFS Insect Production Services Unit in Sault Ste. Marie, Ontario; Government of Canada 2013), few biological control practitioners remain in any research capacity, and there are no longer any formal university programs for training. Nealis and Wallace (Reference Nealis and Wallace1991) stated that the future of biological control introductions in forestry would depend on continuing strong, historic ties with CABI. While agriculture has retained these, the last major forestry project with CABI was in the early 2000s against the pine false webworm (Lyons Reference Lyons2013a).
All evidence suggests that when the right conditions are met, biological control is a viable option for managing Canada’s forest pests. Canadian practitioners also appear capable of significant innovation in the application of biological control methods. Despite this, biological control in Canadian forestry is at a present-day low, one not seen since the early 1900s when the first programmes began. Whether this is a permanent or temporary position is unknown. In either case, biological control seems unlikely to play a significant role in managing Canada’s non-native forest pests over the immediate future, with possibly the notable exception of the emerald ash borer. Many unanswered questions remain from the projects described here, and there is ample room for the research and development of novel methods and techniques against native and non-native pests alike (e.g., Smith et al. Reference Smith, Wallace, Howse and Meating1990; Mills and Nealis Reference Mills and Nealis1992; Hebert and Brodeur Reference Hebert and Brodeur2002). These are all potentially profitable areas for research and development that, if addressed, would help make better pest management decisions for our forests in the face of coming invasions.
Acknowledgements
The authors thank the volume editors for their invitation to take on this daunting task of reviewing the vast literature on biological control in Canadian forests, and two anonymous reviewers for their insightful comments. Most importantly, they extend a deep thanks to the hundreds of biological control researchers who have contributed to this important research over the past 130 years, and hope that this review, in some way, accurately represents their work.











