All principles of intensive care management apply to the patient on ECMO. Some aspects need special consideration. These are discussed in this chapter.
Sedation and paralysis
Most patients are heavily sedated and often paralysed when ECMO support is started.
Muscle relaxants should be discontinued at the earliest opportunity. The risk of awareness is increased in patients with a sudden change in the volume of distribution of drugs when ECMO is commenced.
Continued sedation is not required to support a patient with ECMO and should be discontinued at the earliest opportunity. Most patients will, however, require sedation for several days, often in the context of distressing multiorgan failure and an intense inflammatory response.
The pharmacokinetics and bioavailability of most drugs seem to be modified, but little is known about the specifics (see Pharmacology and ECMO, this chapter).
Analgesia must be continued and titrated to provide comfort and allow pain-free interventions and nursing care.
The presence of ECMO renders daily sedation breaks easier, as the respiratory drive can be controlled by adjusting CO2 removal. Interrupting the sedation allows an assessment of neurological function. This is a key step in ensuring that ECMO is not futile, for example in patients with neurological injuries such as an intracranial bleed.
Ventilation and haemodynamic support during ECMO
These are discussed extensively in most other chapters of this book.
Renal function and ECMO
Acute kidney injury (AKI) is common in patients supported with ECMO, with approximately 50% requiring renal replacement therapy (RRT).
The need for RRT may reflect inadequate renal perfusion or may result from a direct injury to the kidneys. These can be caused by the underlying insult, such as sepsis, respiratory failure or cardiac failure with high vasopressor requirements. If the insult is short lived, the kidneys can recover fully. Renal replacement therapy can be used to manage fluid balance in these very ill patients.
The criteria for starting RRT used for other critically ill patients are applicable to the patient supported with ECMO. These include significant acidaemia (pH <7.25), hyperkalaemia resistant to other therapy, pulmonary oedema due to fluid overload, and significant uraemia. There is no consensus regarding the optimal timing of RRT in patients with AKI, and this extends to the patient supported with ECMO.
The management of RRT is similar to that used in all critically ill patients.
Impact of ECMO on renal function
The rapid haemodynamic changes altering renal blood flow may cause ischaemia or trigger a reperfusion injury in the kidney. This can lead to AKI.
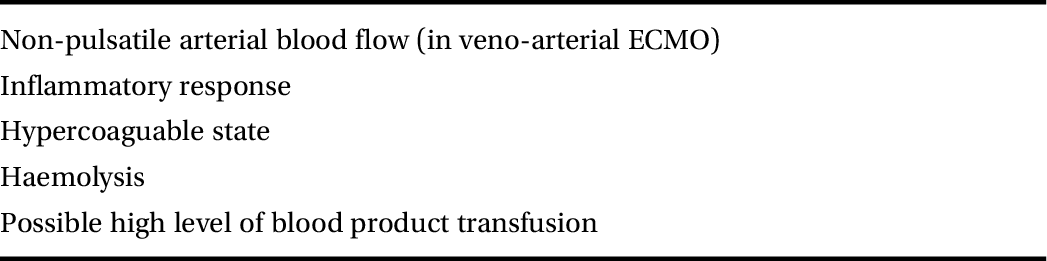
Table 12.1 lists the possible causes of AKI during ECMO.
Table 12.1 Possible causes of AKI during ECMO
| Non-pulsatile arterial blood flow (in veno-arterial ECMO) |
| Inflammatory response |
| Hypercoaguable state |
| Haemolysis |
| Possible high level of blood product transfusion |
A high arterial blood pressure not responding to treatment is often observed in ECMO patients. The mechanism is unknown but is possibly multifactorial. Contributing factors include fluid retention, a reduction in nitric oxide plasma level (due to an increase in the level of plasma-free haemoglobin mediating nitric oxide scavenging), variation in blood levels of drugs and a possible alteration of the renin–angiotensin system.
Indications for RRT in patients treated with ECMO
The combination of ECMO and RRT has several potential benefits: it allows optimization of the fluid balance, which permits the administration of adequate nutrition, intravenous drugs and blood products by preventing fluid overload; and it may decrease the inflammatory response.
Most ECMO patients will receive RRT either because of worsening renal failure (as indicated by the biochemistry results) or to control their fluid balance. Timely administration of fluids is life-saving, but fluid in excess will have a negative effect on outcome.
Patients will often not tolerate significant fluid removal immediately after initiation of ECMO, probably due to the intense capillary leak resulting from the inflammatory response. Eventually it will be possible to filter out large volumes, and this will be beneficial in relation to cardiac and/or lung recovery.
ECMO blood flow may be compromised by RRT if the filtration rate is excessive. An intravascular volume depletion can be observed in patients on RRT, and this may precipitate pre-renal azotemia, subsequent AKI and lengthening of the duration of ECMO support. We usually advocate removing a maximum of 2 L of fluid over 24 h in an average 70 kg person and aim to return to the pre-disease weight.
The patient’s condition will ultimately affect how fluid management is conducted and how the patient may respond to fluid shifts.
Methods of RRT during ECMO support
Renal replacement therapy can be conducted with continuous peritoneal dialysis. This provides less effective clearance of electrolyte and waste products. Intra-abdominal haemorrhage can be an issue.
Intravascular access for RRT can be provided via a separate dedicated central venous catheter. This allows RRT to be continued once ECMO support is removed.
Introduction of a haemofiltration filter into the ECMO circuit is possible. The filter inlet is connected after the pump, and the blood can be returned at various points in the circuit. This system provides slow continuous ultrafiltration, and continuous convective clearance with replacement fluids is possible. The technique is simple, cheap and uses a smaller blood volume than a conventional machine. It carries a significant risk of monitoring error with uncontrolled volume shifts or sudden failure of the haemofilter. Access by connecting to the ECMO circuit is possible (Figure 12.1). This is necessary where vascular access is difficult. It is the preferred method in some centres. One option is to connect the RRT device to the inlet and outlet ports of the oxygenator. The inflow of RRT is connected to the arterial tubing of the ECMO circuit just after the oxygenator and the outflow to the tubing of the ECMO circuit just before the oxygenator. The system returns the blood to the oxygenator.
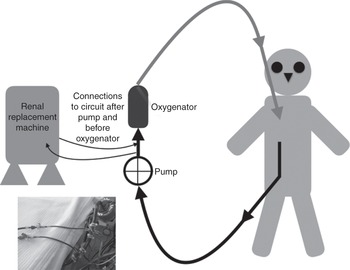
Figure 12.1 Connection of the RRT machine to the ECMO circuit.
The RRT circuit may need to be reconfigured slightly to take account of access and return vascular pressures, as the machine safety mechanisms may not allow the use of high-pressure systems. Connection and disconnection from the ECMO circuit increases the risk of air entrainment, leakage and infection. It is important to remember that air can be entrained in the ECMO circuit from any indwelling vascular line open to the air. Connection of the RRT device to the ECMO venous circuit (Figure 12.1) allows the use of all the potential modes including continuous veno-venous haemofiltration, continuous veno-venous haemodialysis and continuous veno-venous haemodiafiltration. Connecting the return blood from the continuous RRT device to the tubing before the oxygenator allows air and thrombi to be trapped in the oxygenator, and avoids venous admixture into the oxygenated tubing of the ECMO circuit. Connecting a full RRT system allows accurate monitoring of any mode of filtration, increases the accuracy of fluid balance and keeps a constant blood flow through the filter. Finally, filters can easily be changed without disruption of the ECMO flow.
Renal replacement therapy can be performed by connecting the RRT device to the venous line before the centrifugal pump, but this low negative pressure increases the risk of haemolysis and microembolization. Air embolism is more likely to happen at the time of connection/disconnection.
Independent vascular access can be used. The advantages of this approach include less interference with the ECMO circuit. The insertion of a large catheter in an anticoagulated patient increases the risk of bleeding. Veins may already be in use with other lines. It is important that impaired cerebral venous return is considered in patients with large catheters inserted in all neck vessels. The RRT circuit and vascular catheters can be a source of major air embolism, even when not directly connected to the ECMO circuit.
Anticoagulation with RRT and ECMO
The anticoagulation used in patients with ECMO is sufficient to prevent thrombi forming in the RRT circuit. Additional anticoagulation is not routinely used.
If ECMO support is provided without systemic anticoagulation, the RRT circuit is at high risk of occlusion with thrombi (the blood flow through an RRT circuit is much lower than in the ECMO circuit). Techniques reliant on the anticoagulant running exclusively in the RRT circuits are possible (such as citrate anticoagulation).
Sepsis on ECMO
ECMO during refractory septic shock
Septic shock in the adult patient is usually associated with low systemic vascular resistance and refractory hypotension with preserved cardiac output. This distributive shock is related to a maldistribution of blood flow at a microvascular level, and veno-arterial ECMO is of little value in restoring vascular tone.
This is different from what is observed in children, and the international guidelines for the management of severe sepsis and septic shock in children recommends considering veno-arterial ECMO for circulatory collapse unresponsive to all conventional treatment. This remains controversial in adult patients suffering from refractory septic shock. Veno-arterial ECMO might prove useful if the cause of the shock is cardiogenic in addition to distributive.
Nosocomial infections in patients supported with ECMO
There is a long definition of nosocomial infection in patients supported with ECMO: an infection not present at the start of support but detected more than 24 h after ECMO commencement, or within the first 48 h after ECMO discontinuation, and with a pathogen different from those detected within 7 days before ECMO initiation.
The risk of infection is markedly increased in the patient supported with ECMO because of the presence of multiple indwelling devices. Activation of the inflammatory response by the ECMO circuit, coupled with the primary insult, often leads to a relatively immunosuppressed status that may decrease the ability to respond to secondary insults.
Nosocomial infection is the second most common complication of ECMO after haemorrhage, and affects up to two-thirds of patients supported by ECMO. Ventilator-associated pneumonia and bloodstream infections are the most common causes, followed by surgical wounds, urinary tract infection and cannulation-related infection.
The most commonly identified organisms include coagulase-negative Staphylococcus, Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Enterobacter, Klebsiella, Enterococcus and Escherichia coli species are also possible.
The risk of nosocomial infection is increased if the ECMO support is continued for a long time, in cases of mechanical complication, if the patient has an autoimmune disease and if veno-venous ECMO is used.
All the standard measures used in intensive care to decrease the risk of nosocomial infections are applicable. Elevation of the head of the bed, oral prophylaxis and medical treatment of reflux should be strictly followed. All unnecessary lines should be removed. Strict aseptic techniques should be used to access all indwelling catheters.
The diagnosis of newly acquired infection can be challenging because of the intense inflammatory response that mimics sepsis itself. The temperature of the blood is maintained by the circuit’s heat exchanger, and fever can easily be masked. Physical examination and radiographic changes may be difficult to interpret. Subtle changes in clinical condition and signs of poor perfusion manifested by metabolic acidosis, increasing lactate levels, decreasing urine output and a rise in hepatic transaminases are all indices of possible sepsis.
Blood, urine and tracheal cultures should be obtained from patients on ECMO at the earliest suspicion of a possible secondary infection.
Antibiotic therapy
The principles of good antibiotic stewardship apply to all critically ill patients, and include appropriate initial therapy, regular reviews, de-escalation where possible, appropriate prophylaxis, and use of local guidelines and specialist advice.
Treatment of documented infections should follow the same principles as for patients who are not on ECMO support. The increased volume of distribution and impaired drug clearance may affect the dosage of antibiotics. Drug level monitoring in the blood is appropriate where possible.
The underlying diagnosis in the majority of patients who receive ECMO for severe acute respiratory failure is bacterial or viral pneumonia, although a definitive microbiological diagnosis is not reached in approximately one-third of patients. Antibiotic therapy for severe pneumonia should initially be broad spectrum and then more focused when a definitive microbiological diagnosis is reached. National and local patterns of disease and antimicrobial resistance will guide initial and subsequent therapy, and local advice should be sought.
It is common practice to administer single-dose prophylactic antibiotics on ECMO cannulation, decannulation and when changing components of the ECMO circuit, although there is limited evidence to support this.
Pharmacology and ECMO
Effective treatment of the primary disease and subsequent complications is required in order to cure those patients supported with ECMO.
Drug pharmacokinetics may be altered in patients on ECMO because of an increased volume of distribution and reduced drug clearance, due at least in part to the binding of drugs to the ECMO circuit (Figure 12.2).
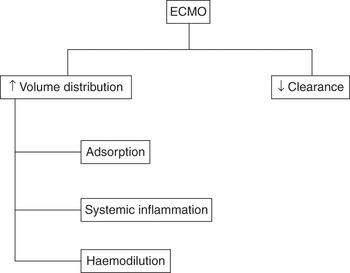
Figure 12.2 Pharmacokinetic changes of drugs during ECMO.
It is not possible to predict the effect of ECMO on pharmacokinetics, and it is impossible to integrate this with the effects of critical illness, drug interactions and RRT.
Therapeutic drug monitoring helps prevent toxicity and monitor efficacy.
Intravenous drugs should be administered directly to the patient and not via the ECMO circuit. This reduces the risks of air entrainment during manipulation of the connectors, or inadvertent rapid drug delivery due to negative pressure in the drainage limb of the circuit. Clotting factors and lipid-rich solutions, such as propofol and parenteral nutrition, should not be given directly in the ECMO circuit, as the high concentration of lipids may block the oxygenator.
Drug availability changes during ECMO
The ECMO circuit will increase the volume of distribution because its material can bind circulating proteins and drugs. This will be affected by the type of components used in the circuit. Reduced adsorption is observed in hollow-fibre oxygenator membranes. Less adsorption is observed in circuits with shorter tubing and those using centrifugal pumps. Drug molecular size, degree of ionization, lipophilicity and plasma protein binding may also influence the adsorption to circuit components.
Adsorption of lipophilic drugs to ECMO membranes and tubing is common and likely to rapidly reduce plasma concentrations. Highly lipophilic drugs such as fentanyl or midazolam will disappear almost completely in an ECMO circuit. However, not all drugs are affected, and the extent of sequestration is not consistent.
The volume of the ECMO circuit increases the total blood volume, and this is compounded by the haemodilution due to repeated blood transfusions, loss in the circulating blood volume during changes in the equipment and the administration of fluids to maintain ECMO flow. This will mainly affect hydrophilic drugs.
The inflammatory response induced by the exposure of blood to foreign material and sepsis causes a redistribution of albumin that is disproportionate, resulting in a low plasma albumin concentration. The proportion of unbound drugs is then increased, with a higher extravascular distribution.
Prolonged elimination is multifactorial, but the reduction of renal function is the primary determinant. Prolonged half-lives of gentamicin and vancomycin are seen in ECMO patients, and meropenem will often remain at a higher level. Adding haemofiltration or other modes of continuous RRT to the ECMO device may increase drug clearance, but this is disputed.
Regional blood flow changes in the liver during pulseless veno-arterial ECMO can also affect clearance of those drugs with a high extraction ratio, such as propranolol.
A decreased drug elimination rate predisposes patients to toxicity, especially for the drugs with a narrow therapeutic window.
Available pharmacokinetic studies have many limitations because they have been performed ex vivo and in neonates with immature enzymatic and elimination systems.
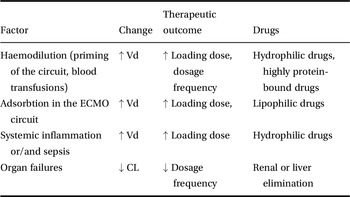
A summary of the changes in pharmacokinetic caused by the ECMO circuit is shown in Table 12.2.
Table 12.2 Pharmacokinetic changes during ECMO support
| Factor | Change | Therapeutic outcome | Drugs |
|---|---|---|---|
| Haemodilution (priming of the circuit, blood transfusions) | ↑ Vd | ↑ Loading dose, dosage frequency | Hydrophilic drugs, highly protein-bound drugs |
| Adsorbtion in the ECMO circuit | ↑ Vd | ↑ Loading dose, | Lipophilic drugs |
| Systemic inflammation or/and sepsis | ↑ Vd | ↑ Loading dose | Hydrophilic drugs |
| Organ failures | ↓ CL | ↓ Dosage frequency | Renal or liver elimination |
Vd, volume of distribution; Cl, clearance.
A few specific drugs and ECMO
Many of the statements in this section are assumptions based on in vitro studies or observations in the paediatric population. They are important enough to appear in a book about ECMO in the adult patient. They also illustrate the great variability and unknowns when using them in a patient on ECMO support.
Ex vivo studies have demonstrated significant loss of fentanyl, diazepam, lorazepam and midazolam in an ECMO circuit. Morphine is less absorbed and therefore preferred.
Acetaminophen (paracetamol) is significantly less lipophilic and protein bound than fentanyl.
Propofol is a widely used, short-acting, hypnotic agent and is significantly sequestrated in the ECMO circuit.
Dexmedetomidine is a highly lipophilic α2-receptor agonist and up to 90% of the drug is lost in an ECMO circuit.
Vancomycin and gentamicin have an increased volume of distribution. Elimination half-lives for both drugs are prolonged during ECMO, and several studies have demonstrated a return to expected values after cessation of ECMO. Ex vivo studies did not find significant loss of vancomycin in the ECMO circuit.
No significant differences between ECMO and non-ECMO patients in serum concentrations, volume of distribution, total clearance and half-life has been found for meropenem and piperacillin.
Most drugs that are not usually highly protein bound or do not show a high degree of lipophilicity remain relatively stable in the ECMO circuit.
Caspofungin is freely water soluble and therefore sequestration to the ECMO circuit is not expected. Plasma caspofungin levels using loading and daily maintenance doses of 70 mg do not differ between ECMO and non-ECMO patients.
Voriconazole, a highly lipophilic drug, is significantly sequestered in the circuit, necessitating initial higher doses of the drug, which must later be reduced due to possible circuit saturation to avoid drug toxicity.
Suboptimal plasma concentrations of neuraminidase inhibitors may be associated with reduced antiviral effectiveness of the drug and the development of viral drug resistance. However, the pharmacokinetics of oseltamivir does not seem to be significantly influenced during ECMO support.
The estimated clearance for theophylline is significantly lower and the volume of distribution higher; these differences are probably a result of the expanded circulating volume during ECMO and altered renal and hepatic physiology. The increased volume of distribution and long half-life suggest that an initial loading dose is necessary, with the reduction of the maintenance dose to avoid toxic concentrations.
Furosemide is lost in the circuit components, but there seems to be no difference between intermittent and continuous administration.
Anti-epileptic drugs such as phenobarbital and phenytoin are highly sequestrated in the ECMO circuit. A higher phenobarbital loading and maintenance dose may be required during ECMO support. Levetiracetam is a first-line therapy for seizures in critically ill patients because of its clinical efficacy, minimal drug interactions and wide therapeutic window. It is hydrophilic and has minimal protein binding, and indeed does not seem to be affected by ECMO.
Amiodarone is highly lipophilic and is likely to be sequestered in the ECMO circuit. Higher doses may be needed. No changes are required in dosing hydralazine. Nicardipine requires higher doses due to the larger volume of distribution.
More than one-half of administered heparin is eliminated by the extracorporeal circuit itself or by blood components in the circuit.
Cyclosporine and insulin are likely to bind to the ECMO circuit.
Nutrition during ECMO
Malnutrition is associated with increased morbidity and mortality in critically ill patients. This is no different for the patient supported with ECMO.
Patients on ECMO demonstrate a marked catabolic stress response.
Patients on ECMO present several risk factors for peptic ulceration including multiple organ failure, coagulopathy, administration of corticosteroids and difficulties in establishing enteral feeding. Ulcer prophylaxis with a histamine type 2 receptor blocker or proton pump inhibitor is indicated.
Significant nasopharyngeal bleeding can be caused by the insertion of nasogastric tubes in patients supported with ECMO. The orogastric route may be a preferred option.
Metabolism and energy requirements for patients on ECMO
The metabolic response to illness is associated with a persistent increase of insulin concentration, catecholamine, glucagon and cortisol. Increased levels of cytokines released by activated macrophages promote catabolism and are associated with increased mortality.
Patients requiring support with ECMO usually present with an accentuated breakdown of skeletal muscle protein (the hallmark of the catabolic response to critical illness). This protein breakdown is required to provide gluconeogenesis, amino acids for the synthesis of acute-phase proteins and proteins for tissue repair. The progressive loss of skeletal muscle protein leads to respiratory compromise, cardiac dysfunction and increased susceptibility to infection.
Once the patient is liberated from ECMO, the caloric requirement needs adjusting. This is best achieved by measuring the energy expenditure using an indirect calorimeter, although this is rarely done in practice.
Nutrition initiation time and mode of delivery for ECMO patients
Adequate nutritional support in ECMO patients is challenging because of an active ongoing metabolic stress response, the clinical requirements for fluid restriction and often an intolerance of enteral feeding.
Early establishment of enteral nutrition is desirable in patients supported with ECMO. Enteral nutrition improves nitrogen balance, prevents gut mucosal atrophy, decreases frequency of bacterial translocation, improves immune function and reduces overall cost. Gastric feeding via a nasogastric tube is the preferred route. Jejunal tube placement should be considered if feed is not absorbed.
Various observational studies confirm that enteral nutrition at approximately 25 kcal/kg/day can be achieved within a week of initiation of either veno-venous and veno-arterial ECMO. This can be done in prone patients.
Controversies exist regarding the route of administration and time of initiation in haemodynamically unstable patients.
The two main barriers for delivery of enteral nutrition are interruption for a procedure and a high gastric residual volume. This can be solved by implementing strict protocols including the use of prokinetics allowing higher gastric residual volume, using post-pyloric feeding tubes and nursing the patient with their head elevated at 45°. Interruption of feed for procedures should be considered carefully. These interruptions can be decreased substantially with adequate planning and reassurance that some procedures will not need fasting as the stomach contents can be aspirated.
If tolerance of enteral feed decreases below 50% for at least 24 h after 1 week of ECMO, parenteral nutrition should be initiated but stopped as soon as absorption of enteral feed is greater than 75%.
Parenteral nutrition may be required in patients who cannot tolerate enteral nutrition. There is a theoretical risk of damage to the oxygenator from lipid-rich parenteral nutrition products, but this is very rarely seen in practice.
The volume of feed adds substantially to the overall amount of fluid administered to patients. This should be carefully considered and a compromise between volume and calories must sometimes be found.
Awake patients should be allowed to eat, but nutritional supplements might not be required.
Protein, carbohydrate and lipid requirements for ECMO patients
Critically ill patients supported with ECMO have a high protein turnover used to synthesize the proteins needed for the inflammatory response and tissue repair. Protein catabolism leads to a progressive loss of diaphragmatic, intercostal and cardiac muscle. Amino acid nutritional supplementation may reduce this overall negative protein balance. Patients receiving RRT have an even higher protein requirement due to amino acids lost through the filter.
Excessive protein administration should be avoided, especially in patients with marginal renal or liver function. Of note, enteral or parenteral glutamine supplementation in order to reduce the septic complications in critically ill patients is no longer recommended.
The catabolism of skeletal muscle generates glucose, as it is the preferred substrate for the brain, red blood cells and renal medulla, and provides the energy required by injured tissue. Septic patients have a threefold increase in glucose turnover, glucose oxidation and elevation of gluconeogenesis. Provision of dietary glucose is relatively ineffective in reducing gluconeogenesis. The excess glucose is converted to fat, resulting in the generation of CO2. The normal ketone metabolism is impaired in the stressed metabolic state, thus making glucose the main fuel for the brain.
Lipid metabolism is accelerated in critically ill patients. The process involves the recycling of free fatty acid and glycerol into triglycerides. Approximately one-third of released fatty acids are oxidized to release energy, and this is the prime source of energy in stressed patients. The glycerol portion of the triglycerides may be converted to pyruvate and then metabolized to glucose. Provision of dietary glucose does not diminish lipid recycling.
Electrolyte plasma levels (potassium, sodium, calcium, chloride and bicarbonates) must be measured frequently. Fluid shifts, the catabolic response to illness and multiple drugs will cause changes in electrolyte levels.
Potassium shifts may cause arrhythmias. Hypophosphataemia may cause thrombocytopenia and respiratory muscle dysfunction. Hypomagnesaemia may be the cause of cardiac arrhythmias. Hypochloraemia can result in metabolic alkalosis that inhibits the respiratory drive, leading to a potassium intracellular shift and a decrease in circulating ionized calcium. Chloride can be administered through a parenteral nutrition formula.
The need for vitamins and trace elements in the ECMO patient is similar to that in the healthy population. Excessive dosage is a risk.
Renal replacement therapy is often used during ECMO (see Renal function and ECMO, this chapter). This will result in further replacement requirements, as in other critically ill patients supported with RRT.
Enteral nutrition-related complications
Adverse events related to enteral nutrition include pulmonary aspiration, nosocomial pneumonia and abdominal complications.
Decreased blood flow to the abdominal organs is associated with ischaemic injury, bacterial translocation and multiple organ failure. This may be exacerbated by vasoactive drugs administered in the context of the primary insult.
Signs of intolerance (abdominal distention, increasing nasogastric tube aspirate or gastric residual volumes, decreased passage of stool and flatus, hypoactive bowel sounds, increasing metabolic acidosis and/or base deficit) should be closely monitored.
Nursing on ECMO
Nursing of the patient on ECMO is similar to nursing of the complex critically ill patient.
Aside from the various points raised throughout this book, special attention should be given to positioning and the increased risk of pressure sores from cannulas and tubing (Figure 12.3).
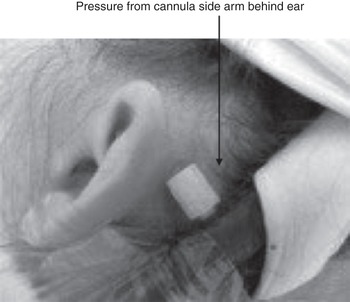
Figure 12.3 Risk of pressure sores is greatly increased in patients supported by ECMO.
Nurses will have a key role in the rehabilitation process (see Rehabilitation, this chapter).
Physiotherapy on ECMO
Patients on ECMO will require physiotherapy treatment as required. Physiotherapists will be part of the multidisciplinary team instituting rehabilitation (see Rehabilitation, this chapter), and ECMO patients should be assessed by a suitably qualified physiotherapist within a timely fashion.
Chest physiotherapy may be beneficial. It can reduce ventilator-associated pneumonia, improve static lung compliance, enhance sputum clearance, and address atelectasis and lobar collapse. The aim of chest physiotherapy is clearance of airway secretions in the early stages of admission and recruitment of lung volume in the later stages.
The only limitations to the treatment options that can be proposed are related to the ECMO circuit and anticoagulation.
Suction of the airways can cause life-threatening haemorrhage. Modification of intrathoracic pressure can affect ECMO blood flow. Cannula positioning may prevent adequate positioning.
Rehabilitation
There is increasing evidence showing that early rehabilitation of the critically ill patient leads to improved functional ability, decreased duration of mechanical ventilation, and decreased intensive care and duration of hospital stay. One could easily extrapolate that rehabilitation may decrease the duration of ECMO support. Early intervention decreases the incidence of delirium and mortality.
ECMO patients often have had prolonged bed rest, increased use of muscle relaxants and increased use of steroids (sometimes initiated before ECMO has been considered). These factors increase the risk of physical disability secondary to immobility and critical illness.
A multidisciplinary approach to provide rehabilitation to complex patients is required, including staff with experience of mobilizing patients on ECMO. Patients can be rehabilitated with internal jugular or femoral cannulation. Venous cannulation usually allows out-of-bed mobilization. Arterial cannulation of the femoral arteries, with the presence of a femoral line, usually precludes mobilization out of bed. This is another reason to move on from peripheral ECMO at an early stage.
The location of the ECMO cannula may impede the extent of the rehabilitation. Security of the cannula should be paramount and the position continuously checked. An ECMO cannula should be adequately supported by the use of a headband, tapes or other fixations.
Mobilization will always require several members of the team, including nurses, a physiotherapist and specialists who understand the ECMO circuit and are able to deal with emergencies.
The rehabilitation process should be clearly explained to the patient and/or family, and the risks and benefits of rehabilitation made clear.
Involvement of occupational therapists, speech and language therapists, and psychologists should be considered early.
Key points
ECMO support can be provided without sedation.
The principles of RRT are the same with or without ECMO.
The RRT circuit safely can be connected to the ECMO circuit.
Drug monitoring is required and would be ideal for all drugs given to the patient on ECMO.
Patients on ECMO demonstrate a marked catabolic stress response.