INTRODUCTION
Annually, an estimated 4·8 million episodes of gastroenteritis occur in the Dutch population of 16 million [Reference De Wit1]. With an estimated 80 000 cases per year, Campylobacter is the most common cause of bacterial gastroenteritis in The Netherlands [Reference De Wit1, Reference Mangen2]. At least one out of five cases with campylobacteriosis consults a general practitioner [Reference Mangen2–Reference De Wit4]. About 5650 are laboratory-confirmed each year, but this number may vary up to 10% between years [Reference Van Pelt5].
Apart from acute gastroenteritis, Campylobacter jejuni infection occasionally leads to serious sequelae such as Guillain–Barré syndrome and reactive arthritis [Reference Tam6, Reference Hannu7]. In addition, several studies have related Campylobacter infections to the development of irritable bowel syndrome and possibly inflammatory bowel disease [Reference Marshall8–Reference Karlinger12]. Due to these complications and the high incidence, Campylobacter infections cause considerable morbidity and economic costs [Reference Mangen2].
Numerous case-control studies in the past 20 years have focused on the identification of risk factors for sporadic Campylobacter infections, of which consumption of poultry is most frequently reported [Reference Stafford13–Reference Studahl and Andersson20]. Other frequently reported risk factors are consumption of unpasteurized milk [Reference Friedman14, Reference Neimann15, Reference Studahl and Andersson20], eating in a restaurant [Reference Friedman14, Reference Danis17, Reference Eberhart-Phillips19, Reference Gallay21], contact with pets, especially puppies [Reference Stafford13, Reference Friedman14, Reference Neal and Slack16, Reference Eberhart-Phillips19, Reference Carrique-Mas22, Reference Tenkate and Stafford23], contact with farm animals [Reference Stafford13, Reference Friedman14, Reference Danis17, Reference Eberhart-Phillips19, Reference Studahl and Andersson20, Reference Tenkate and Stafford23, Reference Potter, Kaneene and Hall24] and foreign travel [Reference Stafford13–Reference Neal and Slack16, Reference Eberhart-Phillips19, Reference Gallay21].
While these studies have contributed to the understanding of the epidemiology of campylobacteriosis, additional information may be obtained when Campylobacter spp. are differentiated. A case-case comparison in the UK revealed important differences in risk factors for C. jejuni and C. coli [Reference Gillespie25]. Aggregation of different species, which is done in most case-control studies, may mask important species-specific risks. In addition, risk factors may be age-related, seasonal or regional. To be able to study risk factors in these subgroups, large study sizes are needed.
Sources of Campylobacter and dietary habits may vary from country to country, resulting in slight differences in risk factors observed across countries. In The Netherlands, C. jejuni is the predominant species in broilers and dairy cattle, whereas C. coli is predominant in finishing pigs. In veal calves, mainly C. coli, but also C. jejuni can frequently be found [Reference Bouwknegt26]. Hardly any data on risk factors and transmission routes for human campylobacteriosis are available in The Netherlands.
Therefore a large case-control study, the CaSa study, was conducted to investigate risk factors for indigenous campylobacteriosis in The Netherlands, with a distinction between C. jejuni and C. coli infections. In addition, specific risk factors for C. jejuni according to age, season and degree of urbanization were studied. The study also aimed to quantify the contribution of different risk factors in order to predict the impact of control and intervention measures.
METHODS
A case-control study on risk factors for campylobacteriosis and salmonellosis, the CaSa study, was conducted from April 2002 to April 2003. This article is restricted to the Campylobacter part of the study. A detailed description of the methodology and the results of the Salmonella part are available elsewhere [Reference Doorduyn27]. In brief, cases were laboratory-confirmed patients with a Campylobacter infection, identified by the Regional Public Health Laboratories (RPHL) in The Netherlands, which covers about 50% of the Dutch population for Campylobacter. Campylobacter isolates were sent to the Central Veterinary Institute for molecular confirmation of the species [Reference Fermer and Engvall28, Reference Marshall29].
Based on historic surveillance information on the numbers of cases with Campylobacter and Salmonella infections in the RPHL, the expected numbers of cases by age, sex, degree of urbanization and season were obtained. Controls were selected from the population registries of 25 municipalities within the service area of the RPHL by frequency matching according to the expected numbers of cases by age, sex, degree of urbanization and season. Each first working day of the month questionnaires were sent to the controls. Cases and controls received a postal questionnaire with questions regarding food consumption, kitchen hygiene and food processing, contact with animals, occupational exposure, travel, water recreation, use of medication (during the previous 4 weeks) and contact with persons with gastroenteritis symptoms. Questions covered the 7 days prior to symptom onset (cases) or completion of the questionnaire (controls).
The incidence of laboratory-confirmed C. jejuni and C. coli enteritis was calculated using the total number of cases identified from the RPHL divided by the population covered by these laboratories. Adjustments in the denominator were made for the time each laboratory participated and for underreporting by the laboratories. The latter was based on a comparison between the reported number of Salmonella cases in this study and the regular laboratory-based surveillance of Salmonella, because in The Netherlands no regular surveillance data with regional information for Campylobacter were available at that time.
Missing values were handled using multiple imputation [Reference Rubin30]. Five imputed datasets were created. With these datasets, five different logistic regressions (or other analyses) were performed and the five results were pooled using SAS proc mianalyse in order to obtain a single final result.
Analyses were performed using cross-tabulations, χ2 tests and univariable logistic regression models (which also included the matching variables and level of education) for significance testing. For further analyses, only cases and controls who had not travelled abroad were included. Variables which reached a significance level of P⩽0·10 in the univariable analyses were selected for inclusion in a multivariable logistic regression model. Multivariable models were developed for C. jejuni and C. coli separately. A manual backwards selection procedure was used in which variables that the likelihood ratio test gave a P value ⩽0·05 were kept in the multivariable model. For C. jejuni, multivariable submodels were developed for food consumption, occupational exposure, animal contact, water recreation activities, kitchen hygiene and food processing, and contact with persons with diarrhoea or vomiting. Finally, the submodels were combined in one final model. The population attributable risk (PAR) of each risk factor in the final multivariable logistic regression models was calculated based on multivariable odds ratios (ORs) and the frequency of exposure in cases. In the same way, confidence limits of the PARs were derived from the confidence limits of the multivariable ORs.
To detect specific risk factors for C. jejuni according to age, season and degree of urbanization, univariable logistic regression analyses were performed for each age group (0–4, 5–17, 18–29, 30–44, 45–59, ⩾60 years), season (April–June 2002, July–September 2002, October–December 2002, January–March 2003) and degree of urbanization (categorized as ‘urban’: >2500 addresses per km2; ‘urbanized’: 500–2500 addresses per km2; ‘rural’: <500 addresses per km2). To test formally if risk factors were different between strata, we tested the interaction between age, season and degree of urbanization with the risk factor of interest for significance in a univariable logistic regression model including all C. jejuni cases. In these univariable analyses, only for some risk factors were differences observed in season and degree of urbanization. We therefore expanded the final multivariable model for C. jejuni with the interaction between these variables. For age, differences in risk factors were observed in young children (0–4 years) and the elderly (⩾60 years) compared to other age groups. We therefore developed separate multivariable models for C. jejuni infection in young children and the elderly.
Finally, a case-case analysis was performed in which C. coli patients were designated as a ‘case’ and C. jejuni patients were designated as controls. Ten cases were excluded from this analysis, because the species determination was ambiguous.
RESULTS
The RPHL identified 3178 cases with campylobacteriosis, of which nine cases did not live in The Netherlands. Of the remaining 3169 cases, 2858 (90%) were C. jejuni and 257 (8%) were C. coli cases. The overall incidences of C. jejuni and C. coli enteritis were 36 and 3/100 000 person-years, respectively, including travel-related cases (Table 1).
Table 1. Incidence of Campylobacter jejuni and C. coli campylobacteriosis per 100 000 person-years and questionnaire response by demographic variables, including travel-related cases and controls and cases where species determination was ambiguous, The Netherlands, April 2002 to April 2003
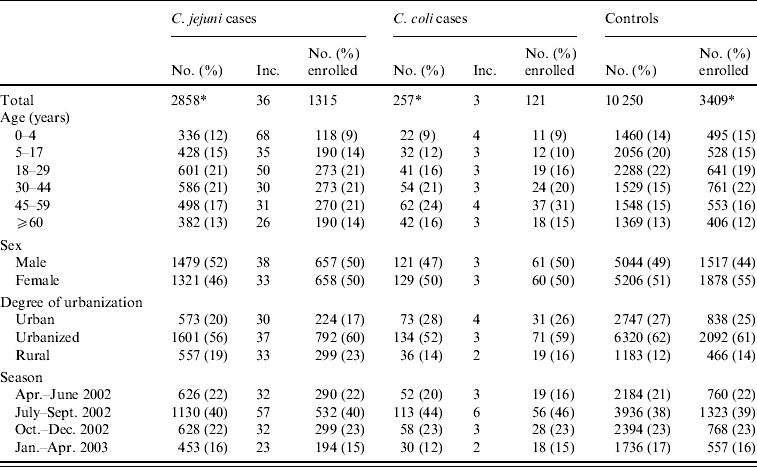
Inc., Incidence per 100 000 person-years.
* Totals do not always add up because of missing values.
The incidences of C. jejuni and C. coli were clearly higher for children aged 0–4 years and during the summer. For C. jejuni, higher incidences were also found in young adults (18–29 years) and in urbanized places compared to urban and rural regions. For C. coli, higher incidences were found in the 45–59 years age group and in urban regions (Table 1).
Questionnaire response and clinical observations of cases
Of the C. jejuni and C. coli cases 1315 (46%) and 121 (47%), respectively, completed a questionnaire. The questionnaire response was higher in the 45–59 years age group (54% and 60%, respectively) and in urbanized (49% and 53%, respectively) and rural (54% and 53%, respectively) areas (Table 1). Of C. jejuni cases, the response was lower for children aged 0–4 years (35%) and for C. coli cases the response was lower for children aged 5–17 years (38%). From April to June 2002, a lower response was observed in C. coli cases (37%), whereas in January to April 2003, a higher response was observed (60%).
The majority of cases reported diarrhoea (96%), abdominal cramps (85%), stomach ache (75%), fever (59%), mucus in the stool (55%) and nausea (53%), whereas 40% had blood in the stool and 29% reported vomiting. C. jejuni cases more frequently reported diarrhoea than C. coli cases (97% and 92%, respectively; χ2 test, P=0·005), as well as fever (60% and 50%, respectively; χ2 test, P=0·05) and blood in the stool (41% and 33%, respectively; χ2 test, P=0·01).
At the time the questionnaire was completed, 70% of the Campylobacter cases had recovered. The median duration of symptoms for recovered cases was 10 days [25th–75th percentile (P25–75): 7–14 days]. Of the cases that had not yet recovered, the median time between symptom onset and completion of the questionnaire was 21 days for C. jejuni infections (P25–75: 13–31 days) and 25·5 days for C. coli infections (P25–75: 18–51 days). Of the C. jejuni and C. coli cases, 124 (9%) and 16 (13%), respectively, were admitted to hospital (χ2 test, P=0·14).
Travel history and demography of cases and controls
In total 10 250 controls were approached and 3409 (33%) completed the questionnaire. Twenty-five controls did not provide demographic data. Of the remaining 3384 controls, 244 (7%) had travelled. Travelling abroad within 7 days prior to symptom onset was reported by 287 C. jejuni (22%) and 39 C. coli cases (32%). For nine C. jejuni cases, three C. coli cases and 21 controls, the travel history was unknown. In a univariable analysis, foreign travel was strongly associated with campylobacteriosis (C. jejuni: OR 3·8, 95% CI 3·2–4·7; PAR 16%, 95% CI 16–17; C. coli: OR 7·4, 95% CI 4·8–11·3; PAR 29%, 95% CI 26–30).
Compared to controls who had not travelled, patients with indigenous C. jejuni infections were more often male (50% vs. 44% of controls) and from rural areas (24% vs. 14%). They were less often from urban areas (17% vs. 24%). Indigenous C. coli patients were more often in the 45–59 years age group (30% vs. 16%) and returned the questionnaire more often in the summer (52% vs. 37%). All risk analyses were adjusted for differences in demography between cases and controls.
Risk factors for C. jejuni infection
Several food and non-food factors were associated with indigenous C. jejuni infections (Table 2). With a PAR of 28%, consumption of chicken was the most important risk factor, followed by consumption of meat prepared at a barbecue, grill or microwave oven (12%), eating in a restaurant (10%) and consumption of undercooked meat (9%). Less important risk factors were consumption of steak tartare (3%) and undercooked seafood (4%).
Table 2. Univariable and multivariable logistic regression analyses and population attributable risk of risk factors associated with indigenous Campylobacter jejuni campylobacteriosis. A case-control study in The Netherlands, April 2002 to April 2003
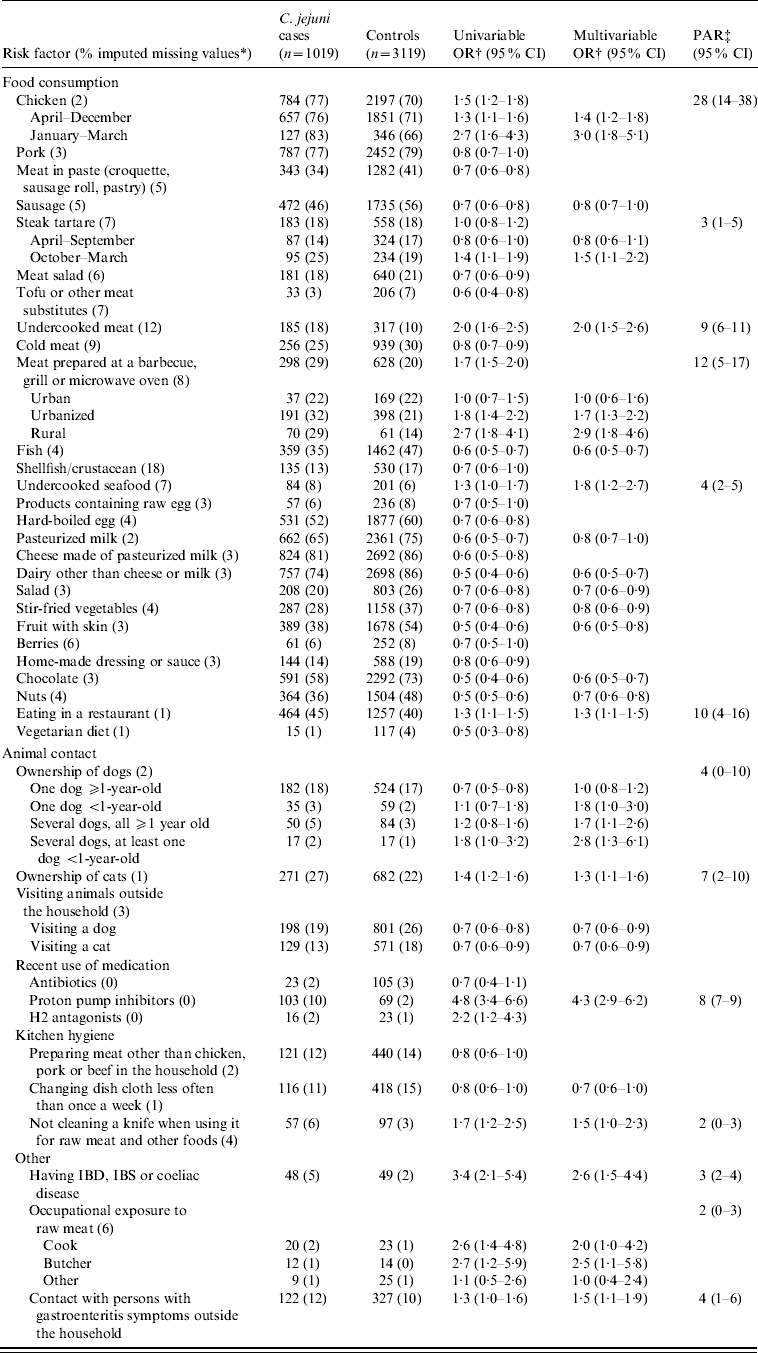
CI, Confidence interval; OR, odds ratio; PAR, population attributable risk; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
* Fraction of imputed missing values in cases and controls together.
† Adjusted for age, sex, degree of urbanization and level of education.
‡ Based on the multivariable odds ratio.
Of the non-food factors, strong associations were found for use of proton pump inhibitors, occupational exposure to raw meat and having one of the following chronic intestinal illnesses: inflammatory bowel disease (IBD), irritable bowel syndrome (IBS) or coeliac disease. However, because only limited numbers of cases were exposed to these risk factors, the corresponding PARs were relatively low. Ownership of dogs, especially several young dogs, and ownership of cats were identified as risk factors with relatively low PARs.
For some risk factors significant differences were observed between seasons and degrees of urbanization: consumption of chicken in the spring was more strongly associated with C. jejuni infections than in the rest of the year; consumption of steak tartare was only a risk factor in autumn and winter and meat prepared at a barbecue, grill or microwave oven was a stronger risk factor in rural areas compared to urban areas.
Many exposures were negatively associated with C. jejuni campylobacteriosis, such as consumption of sausage, fish, pasteurized milk, fruit, salad, stir-fried vegetables, chocolate and nuts and visiting dogs or cats outside the household (Table 2).
Risk factors for C. jejuni infection in young children and the elderly
In Table 3 the results of the separate risk analyses for young children (0–4 years) and the elderly (⩾60 years) are displayed. Consumption of undercooked meat and meat prepared at a barbecue, grill or microwave oven remained risk factors in these age-specific models. Visiting farm animals, contact with persons with gastroenteritis symptoms and ownership of farm animals were predominant risk factors for C. jejuni enteritis in young children: an estimated 19%, 12% and 9% of the cases in this age group were attributable to these factors, respectively. Consumption of products containing raw egg was a unique risk factor for young children and was not associated with illness in any other age group. Predominant risk factors for C. jejuni enteritis in the elderly were eating in a restaurant (PAR 19%), use of proton pump inhibitors (PAR 14%) and having a chronic intestinal illness (PAR 14%). Consumption of ready-to-eat sandwiches was a unique risk factor for the elderly.
Table 3. Univariable and multivariable logistic regression analyses of risk factors associated with indigenous Campylobacter jejuni campylobacteriosis in the very young (0–4 years) and the elderly (⩾60 years). A case-control study in The Netherlands, April 2002 to April 2003
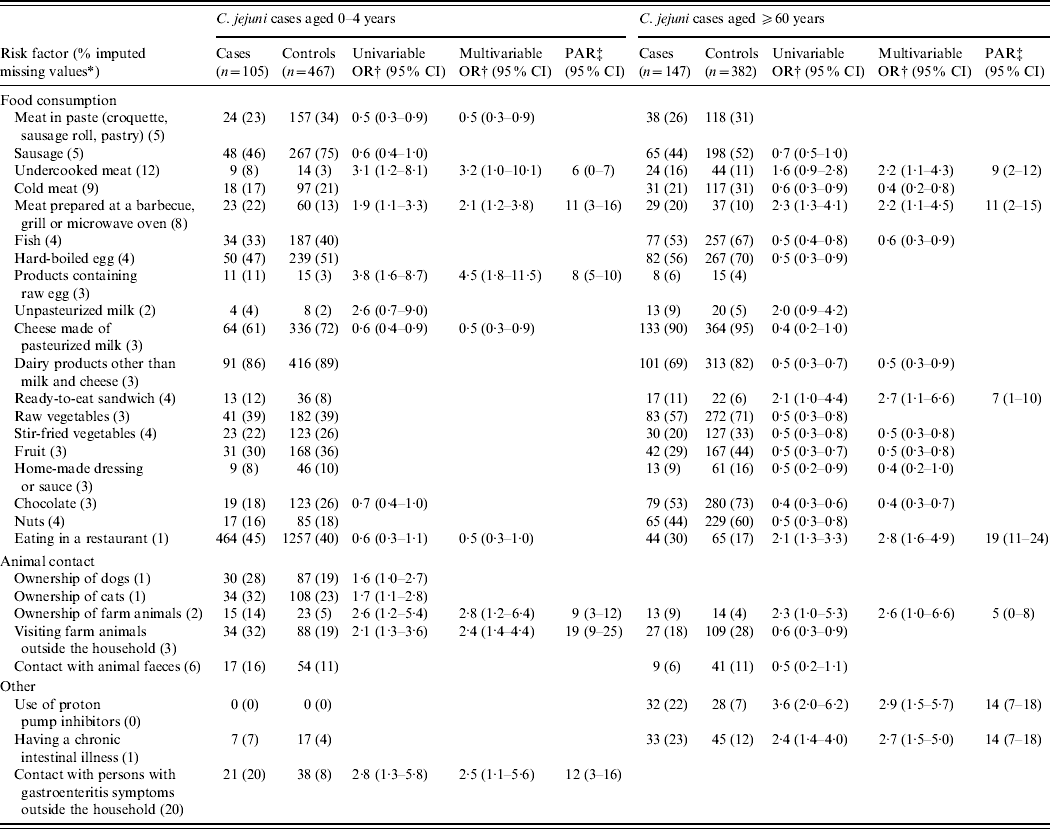
CI, Confidence interval; OR, odds ratio; PAR, population attributable risk.
* Fraction of imputed missing values in C. jejuni cases and controls of all ages together.
† Adjusted for sex, degree of urbanization and level of education.
‡ Based on the multivariable odds ratio.
Risk factors for C. coli infection
Consumption of undercooked meat, meat prepared at a barbecue, grill or microwave oven, ownership of cats and use of proton pump inhibitors were not only identified as risk factors for indigenous C. jejuni campylobacteriosis, but also for indigenous C. coli campylobacteriosis (Table 4). For C. coli infections, consumption of game, tripe and foods bought from a stall, e.g. a mobile caterer or market stall, and swimming were also identified as risk factors. The PAR was highest for consumption of undercooked meat (25%), followed by consumption of meat prepared at a barbecue, grill or microwave (19%), use of proton pump inhibitors (18%), ownership of cats (15%) and swimming (14%).
Table 4. Univariable and multivariable logistic regression analyses and population attributable risk of risk factors associated with indigenous Campylobacter coli campylobacteriosis. A case-control study in The Netherlands, April 2002 to April 2003
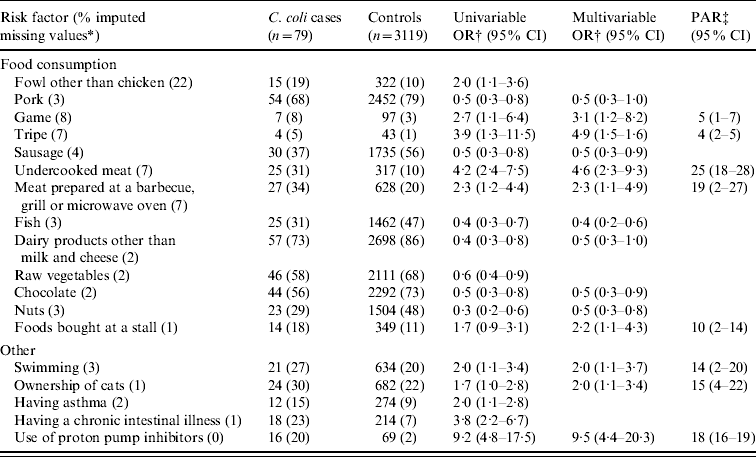
CI, Confidence interval; OR, odds ratio; PAR, population attributable risk.
* Fraction of imputed missing values in C. coli cases and controls together.
† Adjusted for age, sex, degree of urbanization and level of education.
‡ Based on the multivariable odds ratio.
Case-case comparison
The case-case analysis highlighted the differences in risks between C. coli and C. jejuni infections (Table 5). Compared to C. jejuni infections, consumption of poultry other than chicken, tripe and undercooked meat were strongly associated with C. coli infections, as well as eating foods bought from a stall, contact with animals outside the household and swimming.
Table 5. Case-case comparison of Campylobacter coli and C. jejuni campylobacteriosis: Univariable and multivariable logistic regression analyses of risk factors for C. coli campylobacteriosis. A case-control study in The Netherlands, April 2002 to April 2003

CI, Confidence interval; OR, odds ratio.
* Fraction of imputed missing values in C. coli and C. jejuni cases together.
† Adjusted for age, sex, degree of urbanization and level of education.
‡ Cases where species determination was ambiguous are excluded from the analyses.
DISCUSSION
This is the first case-control study of risk factors for sporadic C. jejuni and C. coli campylobacteriosis in The Netherlands. Extrapolation of the incidences of these species found in this study, according to the Dutch population at 1 January 2003, yields an estimate of 5829 and 527 laboratory-confirmed cases, respectively, and a total of around 81 300 and 7350 community cases, respectively, per year [Reference De Wit1, Reference Mangen2].
We included a large number of cases and controls in this study, enabling us to study risk factors for different Campylobacter spp. and age-, season- and urbanization-specific risk factors for C. jejuni enteritis. The PAR provided information about the impact of each risk factor on the incidence, whereas the OR provided information about the individual risk of infection after exposure. In general, in order to reduce Campylobacter incidence, the highest impact may be expected from public health interventions targeted at those risk factors displaying the highest PARs.
Based on the PAR, the dominant risk factor for C. jejuni enteritis was consumption of chicken. The higher risk from consumption of chicken in spring was unexpected. We hypothesized that the risk of chicken consumption would be higher in the summer, because the prevalence of Campylobacter in broilers peaks in the summer months, corresponding with a peak in Campylobacter-contaminated poultry products in this period [Reference Van de Giessen31]. It is conceivable that in spring chickens encounter uncommon Campylobacter strains from the environment for which humans do not yet have protective immunity.
Consumption of steak tartare, a raw beef product, was associated with illness in autumn and winter only. In recent years, we experienced several outbreaks of Shiga toxin-producing Escherichia coli (STEC) and Salmonella due to consumption of steak tartare in The Netherlands, all occurring in autumn and winter [Reference Kivi, Hofhuis and Notermans32–Reference Greenland34]. The risk of contamination of steak tartare may not only be confined to Salmonella and STEC and may also include Campylobacter. However, because Campylobacter is not able to multiply in foods and has a longer and more variable incubation period, contamination would less often lead to outbreaks. In addition, it has been shown that pork, beef and veal products are rarely Campylobacter contaminated and where contamination exists, it is at a low dose [Reference Ghafir35].
Consumption of undercooked seafood was associated with an increased risk of C. jejuni infection, as was also found in the Foodnet case-control study [Reference Friedman14]. Although Campylobacter has been isolated from shellfish and crustaceans [Reference Wilson and Moore36], the predominant species identified was C. lari [Reference Endtz37]. The risk of undercooked seafood consumption may also mirror the effect of cross-contamination of undercooked food products in general.
Healthy cats and dogs are carriers of different Campylobacter spp. and high prevalences of Campylobacter are found in young animals and animals with diarrhoea [Reference Acke38–Reference Hald and Madsen40]. This corresponds well with our observation that having dogs and especially several young dogs poses a risk for C. jejuni enteritis and ownership of cats is a risk for both C. jejuni and C. coli enteritis.
Use of proton pump inhibitors has previously been associated with Campylobacter infections [Reference Neal and Slack16] and was identified as a risk factor for Salmonella infections in The Netherlands [Reference Doorduyn27]. The neutralization of gastric acid by anti-secretory drugs may facilitate Campylobacter (and other bacteria) to survive this hostile environment. The current study showed that the use of these drugs is frequent in the elderly, resulting in a relatively high PAR in this age group.
Chronic intestinal illnesses such as IBD, IBS and coeliac disease appeared to increase the risk for C. jejuni infections, which suggests that patients with these chronic diseases are more susceptible to infection. This seems paradoxical to observations of other studies that indicated that gastrointestinal infections may be a cause of chronic intestinal illnesses [Reference Marshall8–Reference Karlinger12]. However, it is conceivable that patients with IBD or IBS have a disturbed intestinal function which may facilitate enteric pathogens to cause infection. Especially in elderly cases, chronic intestinal illnesses, also including other illnesses than those mentioned above, were prevalent and a relatively high proportion of elderly cases were attributable to this risk factor.
Occupational exposure to raw meat was strongly associated with C. jejuni infections. Due to the low frequency of exposure in cases, the corresponding PAR remained low. A large public health impact from regulations to reduce transmission of Campylobacter for persons working with raw meat is therefore unexpected, but the regulations may have impact at the individual level and may reduce illness and absence from work.
Person-to-person transmission of Campylobacter is considered uncommon. However, a Danish study showed that household outbreaks of Campylobacter are more common than expected [Reference Ethelberg41]. This raises the question whether all of these outbreaks are related to a common source exposure or whether person-to-person transmission to some extent may play a role. The risk of contact with persons with gastroenteritis symptoms outside the household found in the current study was particularly pronounced in young children, which may be in favour of the hypothesis that person-to-person transmission is more common than believed.
In studies focusing on risk factors for children with Campylobacter infections, contact with farm animals has been associated with illness [Reference Friedman14, Reference Tenkate and Stafford23]. In our study, based on the PAR estimates, we concluded that contact with farm animals is the dominant source of infection in children.
Cross-contamination and poor kitchen hygiene within the household is considered to play a major role in the transmission of Campylobacter. However, this is difficult to measure in a case-control study, since questionnaires may not be adequate to measure these risks and study participants may not be willing to disclose unhygienic behaviour. In the current study, many questions about kitchen hygiene were asked. Of these, only using a knife for raw meat and other foods without cleaning was found as a risk factor for C. jejuni infections. This indicates that poor kitchen hygiene does play a role in the transmission and it is likely that the true association is underestimated in this study.
The unique association between consumption of ready-to-eat sandwiches and C. jejuni infections in the elderly may also mirror cross-contamination, since the preparation of ready-to-eat sandwiches involves considerable handling of food.
Consumption of products containing raw egg was a risk factor for young children only. In a previous study, consumption of mayonnaise, possibly made of raw egg, was associated with Campylobacter infections in infants [Reference Tenkate and Stafford23]. Since contamination of eggs with Campylobacter is very unlikely [Reference Sahin, Kobalka and Zhang42], this finding might be the result of cross-contamination.
Drinking unpasteurized milk has been identified as a risk factor for sporadic Campylobacter infections [Reference Friedman14, Reference Neimann15, Reference Eberhart-Phillips19, Reference Studahl and Andersson20] and outbreaks [Reference Harrington43, Reference Lehner44]. Although Dutch outbreaks of C. jejuni due to unpasteurized milk are also described [Reference Heuvelink45, Reference Teunis46], it was not identified as a risk factor in the current study.
Some distinct risk factors were found for C. coli infections, compared to C. jejuni infections. The risk of consumption of the internal organs of animals, e.g. tripe, for C. coli enteritis has been confirmed in a previous study [Reference Gillespie25]. Swimming was an important risk factor for C. coli infections. In a Dutch investigation, Campylobacter spp. was found in 58–92% of the samples of recreational water, with C. jejuni, C. coli and C. lari found in equal amounts [Reference Ruiter47]. In our study, a higher proportion of C. coli cases swam in open water or the sea compared to controls, who more often swam in swimming pools (data not shown).
Consumption of pork was associated with a reduced risk for C. coli campylobacteriosis, although C. coli is highly prevalent in finishing pigs [Reference Bouwknegt26]. On the other hand it has been shown that Campylobacter contamination of red meat is rare and contamination involves low doses [Reference Ghafir35].
A variety of foods were negatively associated with C. jejuni and C. coli infections. Such ‘protective’ effects have been observed in many previous case-control studies [Reference Stafford13, Reference Neimann15, Reference Kapperud, Espeland and Wahl18]. Frequently mentioned explanations are differences in food preferences or immune status between cases and controls, statistical coincidences or bias. For fruits and vegetables it has been proposed that consumption may have a truly protective effect, as these foods contain high levels of antioxidants and carotenoids which inhibit bacterial growth and enhance general immunity to infection. In addition, these foods may alter the intestinal microflora in a way that would prevent infection [Reference Stafford13, Reference Neimann15, Reference Kapperud, Espeland and Wahl18]. However, for most of the negative associations in the current study, we were unable to find a biologically plausible mechanism that could explain the effect.
It has been postulated that repeated exposure to different Campylobacter strains may lead to sufficient immunity to provide at least partial protection against clinical illness [Reference Havelaar48, Reference Belongia49]. In case-control studies, this protective immunity would lead to misclassification, since part of the control group may consist of persons in whom exposure to Campylobacter does not lead to clinical illness because of protective immunity. This would result in biased OR and PAR estimates towards the null and thus in underreporting and underestimation of risk factors. This may also explain the fact that in most case-control studies the majority of the cases remain unexplained. In addition, mathematical models have shown that in epidemiological studies negative associations may be found for risk factors where exposure is consistent over years and at low dose, given the assumption that lifelong immunity occurs [Reference Swift and Hunter50]. Therefore, case-control studies may better identify risk factors where exposure is only occasional and in high doses or involves uncommon Campylobacter strains like C. coli [Reference Havelaar48, Reference Swift and Hunter50].
Other concerns in case-control studies are recall and selection bias. In our study, the recall period for cases was longer than for controls: cases answered questions about the 7 days prior to symptom onset, which was a median 20 days before completion of the questionnaire, whereas controls answered questions about the 7 days before completion of the questionnaire. We used multiple imputation to handle missing values. Before using this statistical method, in several questions cases more frequently answered ‘I don't know’ than controls. However, after using multiple imputation, results of the risk analyses were similar. This suggests that missing values were randomly distributed over the response categories and independent from exposure status. From the approached controls, we obtained a 33% response for the postal questionnaire. Interested controls may have a healthier lifestyle including a preference for eating fruits, vegetables, nuts, fish and less takeaway foods or eating out. This bias may provide an alternative explanation why we found a reduced risk for these food products and an increased risk for foods bought at a stall, ready-to-eat sandwiches and eating in a restaurant.
An advantage of conducting a case-case analysis is that selection bias and recall bias is less likely to occur than in a case-control design, because C. jejuni and C. coli cases are selected in exactly the same way and have a similar recall period. Results from the case-case analysis corresponded with the risk factors found for C. coli in comparison with controls, supporting our belief that recall bias and selection bias had limited impact on our results.
In conclusion, this large case-control study on campylobacteriosis identified several and distinct risk factors for indigenous C. jejuni and C. coli infections. This study also confirms that risk factors differ dependent on age, season and degree of urbanization. PAR estimates provided insight in the relative importance of different risk factors on public health.
ACKNOWLEDGEMENTS
We are grateful to Dr M. A. S. de Wit for her contribution in the design of the study, and to the laboratory staff of the Central Veterinary Institute, especially Mr E. Pothoven. We also thank the participating Regional Public Health Laboratories (RPHL) for their contribution to the data collection, especially Dr F. Vlaspolder, Dr J. H. Sloos, Dr J. Spaargaren, Dr J. Peereboom, Dr M. A. Schouten, Dr R. W. Brimicombe, Dr F. W. Sebens, Dr Ph. H. Rothbarth, Dr L. J. M. Sabbe, Dr H. Mulder, Dr Veenendaal, Dr E. Ijzerman, Dr J. H. T. Wagenvoort, Dr J. H. van Zeijl, Dr B. M. de Jongh, Dr M. Tersmette, Dr P. Voorn, Dr A. M. Horrevorts, Dr J. Buitenwerf, Dr B. G. A. Hendrickx, Dr M. Peeters and Dr A. R. Jansz. We are also grateful to Dr H. C. Boshuizen for help and advice in the data analysis and the multiple imputation method.
DECLARATION OF INTEREST
None.









