INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) continues to be an important human and veterinary pathogen and a significant burden for healthcare systems worldwide. MRSA was first reported by two groups in the UK less than 2 years after the introduction of the synthetic penicillin, methicillin [Reference Jevons1, Reference Barber2]. Although many MRSA are not multidrug-resistant, they often display clinically relevant resistance to key compounds frequently used in prophylaxis and therapy and thus reduce treatment success.
Resistance to methicillin is conferred by an altered penicillin-binding protein (PBP)2a which has a low affinity to the whole class of penicillins and makes MRSA inherently resistant to all β-lactam antibiotics [Reference de Lencastre3]. PBP2a is encoded by the mecA gene which is located on a mobile genetic element designated staphylococcal cassette chromosome (SCCmec). This large element has been introduced into the S. aureus genome as foreign genetic material on very few occasions and possibly originated from animal-adapted bacteria [Reference Couto4]. At least seven types plus subtypes of SCCmec have been identified and their identification can be used to epidemiologically characterize isolates and investigate their relatedness [Reference Deurenberg and Stobberingh5]. The expression of mecA is regulated by associated repressor and inducer genes (mecR, mecI) and by various other S. aureus genes (fem, aux) [Reference de Lencastre and Tomasz6]. Laboratory confirmation of MRSA typically requires either the demonstration of PBP2a by latex agglutination tests or of mecA after replication by polymerase chain reaction.
Two epidemiologically, clinically and genetically distinct entities of MRSA infection are recognized in people. First, MRSA is one of the most common causes for nosocomial infections. People who, for example, are immunocompromised, elderly, exposed to antimicrobial agents or undergo surgery are most at risk of acquiring MRSA infection. Such infections are difficult to treat as the bacteria are resistant to the most useful antimicrobial agents [Reference Harbarth7]. These risk factors are associated with hospitals and other healthcare facilities (HA-MRSA) and infections typically involve genetically distinct lineages. Second, the incidence of MRSA infection in the community seems to have increased dramatically over the past 10 years. Such community-associated MRSA infections (CA-MRSA) emerged in the late 1990s in young and healthy people without the typical hospital connections [Reference Chambers8]. Around the same time MRSA also became recognized as an important veterinary pathogen and since then animal hosts have been implicated as reservoirs and vectors for human infections outside hospitals [Reference Tomlin9–Reference Manian12]. It has become clear that important genetic and epidemiological differences exist among the infecting strains of MRSA encountered in the different animal host species. While MRSA strains isolated from pets tend to be of human hospital origin, those from horses are of a more varied genetic background and their origin remains largely unknown; lineages most often associated with food-producing animals seem to have evolved only recently and independently from common human S. aureus clones.
This review will focus in particular on dogs, cats and horses and their role in the epidemiology of MRSA outside human hospitals. These species are typically kept for companionship, are often handled closely by humans and thus, provide ideal opportunity for exchange of zoonotic pathogens such as MRSA. Furthermore, the identification of MRSA in companion animals has raised concern over the use of antimicrobial agents in veterinary practice as this may lead to selection for resistant organisms and thus have important implications for human health. In contrast, MRSA in livestock, laboratory and working animals may differ.
First reports of MRSA in companion animals
The earliest report of a methicillin-resistant S. aureus in companion animals describes isolation from 2/109 healthy dogs screened for staphylococcal carriage in Nigeria in 1972 and phage-typing of the two isolates suggested a human origin for both [Reference Ojo13]. This report preceded the description of S. intermedius as the main coagulase-positive Staphylococcus isolated from dogs and speciation of the two isolates must be interpreted with care [Reference Hajek14]. However, both strains were resistant to methicillin and other β-lactam antibiotics including the cephalosporins. This strongly supports the view that they were indeed methicillin-resistant S. aureus as resistance to cephalosporins has been exceedingly rare in S. intermedius and S. pseudintermedius until recently. During the 1980s and early 1990s, sporadic case reports of MRSA isolated from animals were published, mainly in human medical journals. These referred to MRSA-contaminated or carrier pets implicated as vectors for human infection where infection control during human outbreaks proved difficult [Reference Scott10, Reference Cefai, Ashurst and Owens11]. In the veterinary field, MRSA received attention in the late 1990s, when infections due to methicillin-resistant staphylococci were recognized in dogs and horses in the UK, USA and in Asia [Reference Tomlin9, Reference Gortel15–Reference Pak, Han and Shimizu19]. Since then, MRSA has been isolated from many other companion animal species including cats, rabbits, a guinea pig, a turtle and a chinchilla and also from birds, and has included healthy and infected individuals [Reference Rankin20–Reference Rich and Roberts23]. In addition, infection and carriage of MRSA in companion animals are now recognized worldwide, particularly in areas where MRSA is widespread in human hospitals, including South America, Australia, New Zealand, Canada and Germany and also in The Netherlands where HA-MRSA has remained rare [Reference Strommenger22, Reference Lilenbaum, Nunes and Azeredo24–Reference Malik28].
Even though the incidence of infection remains largely unknown, MRSA can still be considered an uncommon pathogen in companion animals based on the frequency of MRSA isolation from clinical submissions to veterinary diagnostic laboratories. During 2003, MRSA was isolated from 95 companion animals by one UK veterinary laboratory; all but two were from dogs and cats, and accounted for 1·5% of 6519 coagulase-positive staphylococci isolated from microbiology submissions [Reference Rich and Roberts23, Reference Rich and Roberts29, Reference Chiers30]. Based on hospital admissions, nosocomial MRSA infections in horses were recognized in 1·8/1000 and in 4·8/1000 on admission to a Canadian and an Austrian hospital, respectively [Reference Weese31, Reference Cuny32].
More information is available on MRSA carriage in companion animals but prevalence rates vary between countries, regions and groups of animals sampled. MRSA carriage appears rare in healthy populations in the community without known contact with MRSA but may be more frequent in hospitalized animals especially those sampled during MRSA outbreaks, similar to observations in people. No MRSA was isolated from healthy companion animals including 200 dogs and 300 horses in Slovenia [Reference Vengust33], 100 dogs and 100 horses in Denmark [Reference Bagcigil34], 200 horses in The Netherlands [Reference Busscher35], 22 dogs, 24 cats and 40 horses in the UK [Reference Baptiste36], 50 dogs in the USA [Reference Griffeth37], 581 horses in Ontario and New York state on farms without a history of previous MRSA [Reference Weese38] and 497 horses in Atlantic Canada [Reference Burton39]; all studies were published between 2005 and 2008. Others authors have reported infrequent MRSA isolation from companion animal populations, including 3/148 cats in Brazil in 1998 [Reference Lilenbaum, Nunes and Azeredo24], 6/815 dogs in Hong Kong [Reference Boost, O'Donoghue and James40] and 1/193 dogs in Canada [Reference Hanselman, Kruth and Weese41], both in 2008. In contrast, in animals admitted to referral hospitals, carriage rates have ranged from 9% in dogs where no MRSA cases were hospitalized concurrently [Reference Loeffler42] to 20% in a small animal [Reference Weese43] and 16% in an equine hospital [Reference Baptiste36] during outbreak conditions in 2005 and 2007. Most recently in 2009, a new lineage, MRSA ST398, has been recognized in horses in various countries [Reference Van den Eede44] and also from a dog in Germany [Reference Niehoff45]. This lineage has been able to spread rapidly between individual animals in pig herds and as this may also be the case in other animal species, the position in dogs and horses will need to be kept under review.
Epidemiology of MRSA in companion animals
S. aureus is a highly clonal organism so that epidemiological typing studies which investigate genetic relatedness between strains allow insight into the origins and spread of MRSA. Epidemiological typing of MRSA isolates and strain comparison have revealed important differences between MRSA isolated from the individual animal species. Typing technologies originally included the use of bacteriophages. This was superseded by pulsed-field gel electrophoresis (PFGE). Now other techniques such as multilocus-sequence typing (MLST), SCCmec typing, spa-typing or rapid PCR-based analysis of lineage-specific DNA segments have become available and these have been reviewed previously in a veterinary context [Reference Leonard and Markey46].
While MRSA infection and carriage isolates from dogs and cats have mostly been identical to the HA-MRSA lineages prevalent in each country or region, the genetic background of equine isolates is more varied (Table 1). It has been shown for many countries that typically two S. aureus clones are responsible for the majority of human HA-MRSA infections [Reference Cockfield47]. In the UK, successful clones are currently represented by the epidemic strains EMRSA-15 and EMRSA-16 [Reference Johnson48], and the majority of canine and feline isolates have been of these lineages [Reference Rich and Roberts29, Reference Weese43]. In other countries too, most canine and feline isolates have been indistinguishable genetically by PFGE from the local HA lineages and display the same multidrug resistance as human HA-MRSA [Reference Baptiste36, Reference Niehoff45, Reference O'Mahony49]. Only occasionally, unusual or ancestral S. aureus lineages have been recognized from dogs and cats and this mirrors the findings in people [Reference Niehoff45, Reference Boost, O'Donoghue and Siu50–Reference Enoch52].
Table 1. Origin, lineage and characteristics of MRSA typically isolated from companion animal species
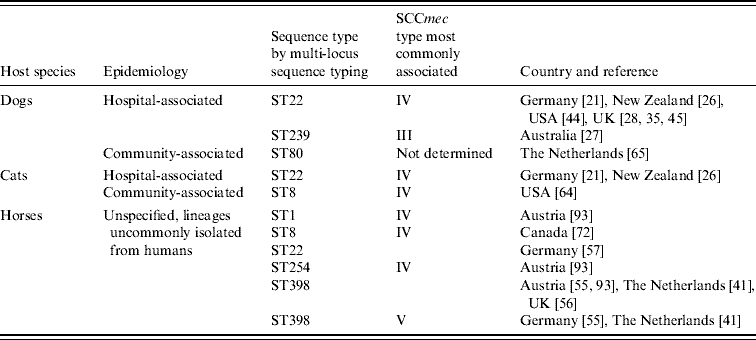
In horses, however, older or less prevalent MRSA lineages which are different from the local hospital lineages have predominated in both infection and carriage isolates from most countries. This pattern was first reported from Japan in 1997 where 15 equine infection isolates with identical PFGE patterns were different from the commonly identified human lineages based on comparison of coagulase isotypes [Reference Shimizu53]. Similarly, O'Mahony and colleagues found that antibiogram-resistogram types of eight equine MRSA were distinct from the most frequently isolated human strains in Ireland [Reference O'Mahony49]. More recently, additional genetic analyses have identified one predominant clonal complex (CC8) in horses represented by several different sequence types (ST), including ST1, ST254, ST247 and ST8, mostly lineages that had been associated with human HA-MRSA in the past but which have since been superseded [Reference Weese31, Reference Cuny32, Reference Moodley54–Reference Christianson56]. More recently, the livestock-associated MRSA lineage, ST398, has also been isolated from healthy and diseased horses in The Netherlands, Austria, Germany and the UK [Reference Van den Eede44, Reference Witte57, Reference Loeffler58]; endemic HA-MRSA lineages are only rarely reported in horses as demonstrated in a recent German analysis where only 1/19 infection isolates was of hospital origin (ST22) [Reference Walther59].
Although MRSA infections in animals tend to occur in the community or outside human healthcare facilities and, as such, have been described by some authors as community associated [Reference Weese38, Reference Maeda60] a differentiation from the genetically and clinically distinct CA-MRSA is warranted for better understanding [Reference Weese61, Reference Boyle-Vavra and Daum62]. In humans, CA-MRSA are clonally unrelated to HA-MRSA lineages and originate from many clonal complexes (CC), even those occurring within one country [Reference Chambers8]. They typically carry the smallest SCCmec types IV or V [Reference Diederen and Kluytmans63] and they are less drug-resistant; it is suggested that this is because resistance genes can present a burden for the bacterial metabolism in less competitive environments [Reference Ender64]. In addition, many carry Panton–Valentine leucocidin (PVL) toxin genes which may be responsible for severe skin and soft tissue infections in previously healthy people [Reference Vandenesch65]. Such CA-MRSA lineages (Table 1) and PVL toxin gene-positive MRSA have now been isolated from 12 infected animals including dogs, cats, a rabbit and a parrot in the USA and Europe [Reference Rankin20, Reference Vitale, Gross and Weese66] and from healthy pets in The Netherlands and Germany [Reference van Duijkeren67, Reference Sing, Tuschak and Hormansdorfer68].
Transmission of MRSA between humans and companion animals
Transmission of pathogenic staphylococci between humans and animals was first suspected for farm animals in the early 1960s combined with concerns about animals as a reservoir for such organisms [Reference Moeller69]. Early studies investigating inter-species transmission gave contradictory results though as both similar and distinct strains were isolated from animals and their in-contact people; however, conclusions may have been hampered by limited species definition for staphylococci [Reference Adekeye70, Reference Silberg, Blenden and Novick71]. Interest was renewed when MRSA proved difficult to control in the 1990s coinciding with the availability of improved phenotypic and genotypic analysis tools. Although there is no study published to date which has specifically investigated MRSA transmission between humans and companion animals, genetic analyses, several case reports and case series strongly indicate that such transmission can occur in both directions.
First, genetic analyses of canine and feline MRSA indicate, but do not prove, that MRSA isolated from these species has originated in human hospitals and that originally MRSA transmission must have occurred from humans to pets. Such a spillover from hospitals into the community and eventually to pets via patients or healthcare workers infected, carrying or contaminated with the organism seems plausible particularly in countries with a high burden of HA-MRSA. Healthcare links of pet-owners have not been investigated systematically to date but associations with animal infection have been reported, although inconsistently [Reference Oughton72, Reference Boag, Loeffler and Lloyd73].
Second, transmission of MRSA from animals to vulnerable people is also suspected based on indirect evidence [Reference Scott10–Reference Manian12, Reference van Duijkeren26, Reference Sing, Tuschak and Hormansdorfer68]. This direction is of particular concern where susceptible humans are exposed to contaminated or infected animals, where animals visit healthcare facilities for companionship to patients and where animals may be spreading PVL toxin gene-positive S. aureus strains. Healthy carrier animals have been implicated as sources and vectors for recurrent human infection [Reference Scott10, Reference Manian12, Reference Sing, Tuschak and Hormansdorfer68]. In addition, carrier animals may have been involved in promoting recurrent colonization in their owners [Reference Cefai, Ashurst and Owens11, Reference van Duijkeren26]. Although other causes such the effect of contaminated environments were not directly investigated in these settings, indirect evidence for the role of animal vectors was provided when human re-infections and re-colonization ceased after routine treatment and hygiene measures were combined with elimination or decontamination of the suspected animal vectors.
For horses, the origin of MRSA remains unclear; the infrequent isolation of HA-MRSA from this species despite close contact with humans indicates that factors other than exposure to human carriers are involved in the acquisition of MRSA by horses. However, equine-to-human transmission has been suggested by several groups. MRSA with indistinguishable PFGE patterns were isolated from 3/5 in-contact veterinary staff sampled for carriage in response to a series of suspected veterinary hospital-acquired infections [Reference Seguin17]. The zoonotic potential of MRSA from equine infections was further demonstrated by a case report on three human skin infections in people caring for an MRSA-infected and -colonized foal at a veterinary teaching hospital in Canada. All three human isolates were classified as CMRSA-5, a clone commonly isolated from infected and colonized horses in that region but relatively uncommon in humans, again supporting the concept of transfer [Reference Christianson56, Reference Weese74, Reference Weese75].
MRSA within a companion animal reservoir?
How far companion animals provide a true reservoir for MRSA or whether they should only be considered as contaminated living vectors remains unclear. While the definition of a reservoir implies that the host animal can maintain the pathogen indefinitely [Reference Ashford76], this has not been investigated for any of the companion animal species to date. On the contrary, there are suggestions that MRSA carriage is not sustained for long periods by companion animal hosts in a clean environment. MRSA carriage resolved in 16 healthy rescue dogs identified during cross-sectional screening with daily cleaning and disinfection of the kennel environment alone [Reference Loeffler77]. Similarly, during an MRSA carriage eradication programme in two Canadian equine establishments, decolonization of human carriers and strict hygiene and isolation measures alone, without antimicrobial use on carrier animals, was associated with eradication of MRSA from all horses on one farm within 6 months [Reference Weese and Rousseau78]. In contrast, MRSA carriage was maintained over several weeks in a healthy dog; however, the dog lived with owners who had previously been infected with MRSA and continued to suffer from open wounds so contamination of the environment was highly likely [Reference Manian12]. No information on the persistence of MRSA in cats has been reported even though S. aureus may colonize and infect cats more frequently than dogs and horses [Reference Morris79, Reference Cox80]. In contrast, a true reservoir role seems likely in pigs for MRSA ST398 as it has been reported to spread rapidly between animals and occurs more frequently in pigs than in people [Reference de Neeling81, Reference van Duijkeren82].
Risk factors for MRSA acquisition by companion animals
With MRSA traditionally considered a human pathogen, identification of risk factors for animals could be highly significant. They are assumed to mirror those reported for HA-MRSA in people such as own carriage, contact with carriers, hospital admission and invasive procedures, and all of these risk factors have been identified in companion animals. For dogs and cats, a UK case-control study involving 182 animals with S. aureus infection showed that contact with human MRSA carriers was the most important risk factor for MRSA infection followed by repeated courses of antimicrobial therapy, surgery and several days of hospitalization at veterinary clinics predispose to MRSA infection when compared with methicillin-susceptible Staphylococcus aureus infection (R. Soares-Magalhaes et al., unpublished observations). In horses, MRSA carriage on admission to an equine hospital increased the risk for nosocomial MRSA infection in a population of 120 animals in one study (OR 38·9, 95% CI 9·49–160, P<0·0001) [Reference Weese31]. While contact with carriers has been reported inconsistently for individual animal patients, the significance of surgery and orthopaedic implants for the development of equine MRSA infection can be deduced from the large number of post-surgical infections reported in animals [Reference Seguin17, Reference O'Mahony49, Reference Oughton72, Reference Orsini83]. Antimicrobial therapy as a predisposing factor has been implicated in equine MRSA infection where ceftiofur or aminoglycosides appeared to predispose horses to MRSA carriage during hospitalization in one study [Reference Weese31]. Another equine study investigated risk factors for MRSA carriage prior to admission to veterinary hospitals in 67 carrier horses and identified contact with carriers, antimicrobial therapy and previous hospital admission as significant factors [Reference Weese and Lefebvre84]. More recently, acquisition of MRSA directly from human healthcare environments was suspected in healthy pet therapy dogs in the USA and in the UK [Reference Enoch52, Reference Lefebvre and Weese85], although two other screenings of therapy dogs failed to identify MRSA carriage or contamination in the UK and Hong Kong [Reference Boost, O'Donoghue and James40, Reference Leslie86].
In view of the importance of contact with human MRSA carriers in the development of canine and feline MRSA infections, potential sources of contact need to be considered. In this context, the high MRSA carriage rates reported in veterinary staff are relevant. They exceed rates reported for healthy community members in many countries and affect veterinary staff with or without known contact with infected animal cases. While most countries have only very limited information on MRSA carriage rates in healthy people, estimates tend to be below 2% even where MRSA is endemic in hospitals such as in the UK, the USA, Italy or Portugal [Reference Abudu87–Reference Zanelli92]. Veterinary staff in two small animal referral hospitals in the UK showed carriage rates of 18% [Reference Loeffler42] and 27% [Reference Baptiste36] while cross-sectional sampling of small animal veterinary staff at veterinary conferences revealed 4·4% positive in the USA [Reference Hanselman93] and 3% positive in Denmark [Reference Moodley94]. Carriage was also high (9%), and mainly of HA-MRSA lineages, in 388 UK first opinion practice veterinary staff [Reference Loeffler95]. This is similar to an occupational risk identified in human healthcare workers but causes are less clear in veterinary staff [Reference Albrich and Harbarth96].
Similar high percentages have been identified in equine veterinary staff and handlers. The earliest indication of transmission between infected horses and their veterinary staff or handlers came from a case series of 11 post-surgical MRSA infections in the USA where 3/5 sampled staff volunteers were nasal carriers [Reference Seguin17]. Subsequently, 9·7% of 103 veterinary staff at an equine hospital in Canada and 4·6% of 43 veterinary staff at an Austrian university hospital were MRSA carriers at times when infected horses were hospitalized [Reference Weese38, Reference Cuny97]. In all three reports, human carriage isolates were of the same lineages as the equine infection isolates but distinct from the epidemic human clones in those areas. In contrast, no human carriers were identified in 12 veterinary staff sampled at a Liverpool equine referral hospital, UK, despite clinical MRSA infections being treated in three hospitalized horses during the study period [Reference Baptiste36]. However, sampling of veterinary staff unrelated to clinical infection cases at equine conferences in the USA and the UK identified MRSA in 10% of 257 [Reference Anderson, Lefebvre and Weese98] and in 8% of 276 [99] delegates.
MRSA contamination of veterinary clinic or hospital environments has also been recognized. MRSA was isolated from 10% of 30 sampling sites in a UK small animal referral hospital without known MRSA patients present at the time [Reference Loeffler42] and from 9·6% of 260 sites in a Canadian equine hospital while MRSA-infected horses were hospitalized [Reference Weese100]. A recent report from another UK university veterinary hospital demonstrated isolation of MRSA from 1·4% of 140 sites and from 3·1% of 64 staff [Reference Heller101].
Although the main direction of inter-species transmission and also the relevance and causes for this occupational risk in veterinary staff are still unclear, existing evidence indicates that the epidemiology of MRSA in companion animals and people caring for them are closely related.
Clinical aspects
The first reported cases of MRSA infections in companion animals involved post-operative wound infections including implant complications and chronic skin diseases in dogs [Reference Chambers8] and dermatitis and metritis in horses [Reference Anzai18] but ear, respiratory, urinary, arthritic and other infections have been recorded too [Reference Strommenger22, Reference Baptiste36, Reference O'Mahony49, Reference Oughton72, Reference Orsini83]. While these infection sites are typical for staphylococcal infections generally, some clinical features have been linked to MRSA in particular. In cats, two cases of abscess formation have been reported in association with MRSA infection both with HA-MRSA and CA-MRSA lineages [Reference Vitale, Gross and Weese66, Reference Bender102] and abscesses surrounded by eosinophilic inflammatory cells were also proposed as a feline species-specific reaction pattern associated with methicillin-resistant staphylococci [Reference Ozaki103]. In this retrospective analysis of 27 histopathological specimens from feline abscesses, 23 had Gram-positive cocci centrally and 15/17 such lesions showed immunoreactivity to PBP2a. Another MRSA-associated characteristic was proposed from a retrospective analysis of 749 staphylococcal isolates from small companion animals. The authors reported that the 39 MRSA were more frequently isolated from deep infections such as urinary tract or respiratory disease compared with methicillin-susceptible S. aureus (MSSA) (n=76) which tended to affect more the skin and ears [Reference Morris79]. They also reported that the rate of MRSA infection in cats was similar to that in dogs while other pathogenic staphylococci were less frequently identified in feline infection. However, disease frequencies derived from laboratory submissions need to be interpreted with care especially for multidrug resistant organisms due to submission bias and clinical disease characteristics. For example, non-MRSA canine bacterial skin infections will frequently respond to empirical antimicrobial therapy and samples may only be submitted after poor clinical response. Additional confirmation of these findings in a larger number of cases is warranted as this information could advance early recognition of MRSA infection in veterinary practice and thus minimize the spread of MRSA.
Treatment of MRSA infections has relied on restoring the skin barrier function, removal of surgical implants and topical, systemic or occasionally intra-lesional antimicrobial treatment as indicated for the type of infection [Reference Tomlin9, Reference Owen, Moores and Coe104]. Although isolates from dogs and cats typically show multidrug resistance as expected for HA lineages, most canine and feline infections can be treated successfully. Antimicrobial drugs with efficacy in vitro against MRSA are available for use in companion animal species in many countries and include trimethoprim-potentiated sulphonamides, tetracyclines, possibly clindamycin for HA-MRSA [Reference Tomlin9, Reference Rich, Deighton and Roberts105] and fluoroquinolones for some CA-MRSA [Reference Vitale, Gross and Weese66, Reference van Duijkeren67]. Outcomes of MRSA infection in animals are only reported infrequently. However, the prognosis appears to be related to the severity of infection and to the prognosis of the underlying trigger [Reference Tomlin9, Reference Orsini83, Reference Morris106]. Fatal outcomes have been reported for individual debilitated patients and a causal link with MRSA has only been confirmed in one horse with severe osteomyelitis [Reference Weese55, Reference Boag, Loeffler and Lloyd73]. To date, there is no indication that HA-MRSA is more virulent in companion animals than other coagulase-positive staphylococci such as S. pseudintermedius or MSSA; this is supported by a study showing that the severity of clinical signs and the prognosis for MRSA infection in a group of 46 cats was no worse than for MSSA infection (n=33) [Reference Morris106]. As only 12 cases of CA-MRSA animal infection have been reported until now and their severity is unknown, it is not clear yet whether these strains may be associated with clinically distinct entities as in some humans infected with PVL-positive strains [Reference Rankin20, Reference Vitale, Gross and Weese66].
Future opportunities and conclusions
In summary, there is good but indirect evidence that companion animals can promote the recurrence of MRSA infection and carriage in humans in home environments. However, the extent of their role in MRSA transmission cannot be quantified from the information published to date. Further investigations, including longitudinal studies, into the dynamics of MRSA carriage or contamination in companion animals are now urgently required to advance our understanding of MRSA transmission between hosts and ultimately to develop better control and prevention strategies for this zoonotic pathogen.
At present, the predominance in dogs and cats of MRSA lineages that are successful in people and the worldwide pattern in horses of varied lineages belonging to CC8 suggest a degree of species-specificity of MRSA within companion animals. However, the recent emergence of CA-MRSA in pets emphasizes that the epidemiology of MRSA in pet animals is changing as it is in humans. Therefore, awareness of companion animals as possible vectors for highly virulent PVL toxin-positive MRSA strains may be critical for the success of infection control measures and monitoring of animals is warranted. In addition, the identification of antimicrobial agents as a predisposing factor for MRSA infection in companion animals, coupled with increasing use of β-lactam and fluoroquinolones in small animal practice, has renewed the discussion over the potentially dangerous implications that veterinary use of antimicrobial agents may have for human health [Reference Andrews107, Reference Guardabassi, Schwarz and Lloyd108].
However, the vital companionship that animals provide to people and the currently incomplete understanding of the role of animals in the spread of MRSA warrant a continued effort by human and veterinary clinicians and researchers to develop a better understanding for and new control strategies against MRSA.
DECLARATION OF INTEREST
None.





