Introduction
Microconchids were small encrusting tubeworms that originated during the Late Ordovician and went extinct at the end of the Middle Jurassic (late Bathonian) (e.g., Taylor and Vinn, Reference Taylor and Vinn2006; Zatoń and Vinn, Reference Zatoń and Vinn2011). Until Weedon (Reference Weedon1990, Reference Weedon1991) affiliated the microconchids with tentaculitoids, these spirorbiform or serpuliform tubeworms were generally treated as sedentary polychaetes or vermetid gastropods (e.g., Peryt, Reference Peryt1974; Burchette and Ridding, Reference Burchette and Riding1977; Beus, Reference Beus1980; Bełka and Skompski, Reference Bełka and Skompski1982). During their long evolutionary history, microconchids were very successful in colonizing various paleoenvironments, ranging from normal marine, through brackish to more freshwater settings (e.g., Dreesen and Jux, Reference Dreesen and Jux1995; Caruso and Tomescu, Reference Caruso and Tomescu2012; Zatoń et al., Reference Zatoń, Vinn and Tomescu2012a; Zatoń and Peck, Reference Zatoń and Peck2013). Their wide paleoenvironmental tolerance seemed to be a key factor in surviving mass extinctions and biotic crises, in the aftermaths of which they became the dominant opportunistic epibionts (Fraiser, Reference Fraiser2011; Zatoń and Krawczyński, Reference Zatoń and Krawczyński2011a; He et al., Reference He, Wang, Woods, Li, Yang and Liao2012; Yang et al., Reference Yang, Chen, Wang, Ou, Liao and Mei2015; Zatoń et al., Reference Zatoń, Borszcz and Rakociński2017).
Although the majority of microconchids were characterized by Spirorbis-like, planispirally coiled tubes, several genera produced unique morphologies. For example, some Carboniferous (Mississippian) species formed long, helically uncoiled tubes (e.g., Burchette and Riding, Reference Burchette and Riding1977), whereas the Lower Triassic Spathioconchus Zatoń et al., Reference Zatoń, Niedźwiedzki, Blom and Kear2016b formed straight, trumpet-like tubes (Zatoń et al., Reference Zatoń, Niedźwiedzki, Blom and Kear2016b) and the Permian Helicoconchus Wilson, Vinn, and Yancey, Reference Wilson, Vinn and Yancey2011 had long, helically uncoiled tubes that showed budding (Wilson et al., Reference Wilson, Vinn and Yancey2011).
Irrespective of tube morphology, all microconchids recognized so far possessed diminutive, millimeter-sized attachment portions and small tube diameters. Some species possessed uncoiled portions of significant size, e.g., the Permian bioherm-building Helicoconchus (Wilson et al., Reference Wilson, Vinn and Yancey2011), and the Carboniferous biostrome-forming ‘Serpula’ cf. S. advena Salter, Reference Salter1863 (Burchette and Ridding, Reference Burchette and Riding1977). However, the majority of microconchid species were rather tiny, inconspicuous encrusters.
Here, we describe a new microconchid species from the Mississippian Cracoean reefs of the United Kingdom that possessed a robust tube. Its large, planispirally coiled attachment portion, as well as its large tube diameter, make it a giant among Paleozoic and Mesozoic microconchids recognized so far.
Geological setting
Geology and stratigraphy
Mississippian Cracoean reefs of the UK formed marginal facies to rimmed shelves developed on stable basement blocks (Mundy, Reference Mundy, Beauchamp, Embry and Glass1994, Reference Mundy, Vennin, Aretz, Boulvain and Munnecke2007; Aitkenhead et al., Reference Aitkenhead, Barclay, Brandon, Chadwick, Chisholm, Cooper and Johnson2002). In North Yorkshire, the Cracoean reef tract (‘Craven Reef Belt’ of Hudson, Reference Hudson1930) defined the southern limit of the Asbian shelf limestones of the Askrigg Block and bridged the transition into the Craven Basin (Fig. 1). Exposures occur in three separate outcrops along a 23 km west-to-east tract (Fig. 1), with the intervening areas covered by Serpukhovian siliciclastics of the Bowland Shale and Pendleton formations. This once-continuous reef belt was broken into fault slices during movements on the Craven Faults (Arthurton et al., Reference Arthurton, Johnson and Mundy1988) and was substantially eroded prior to burial by Bowland Shale mudrocks (Hudson, Reference Hudson1930, Reference Hudson1932, Reference Hudson1944). Remnants of a shelf-contiguous ‘apron reef,’ together with isolated reef mounds, are represented.
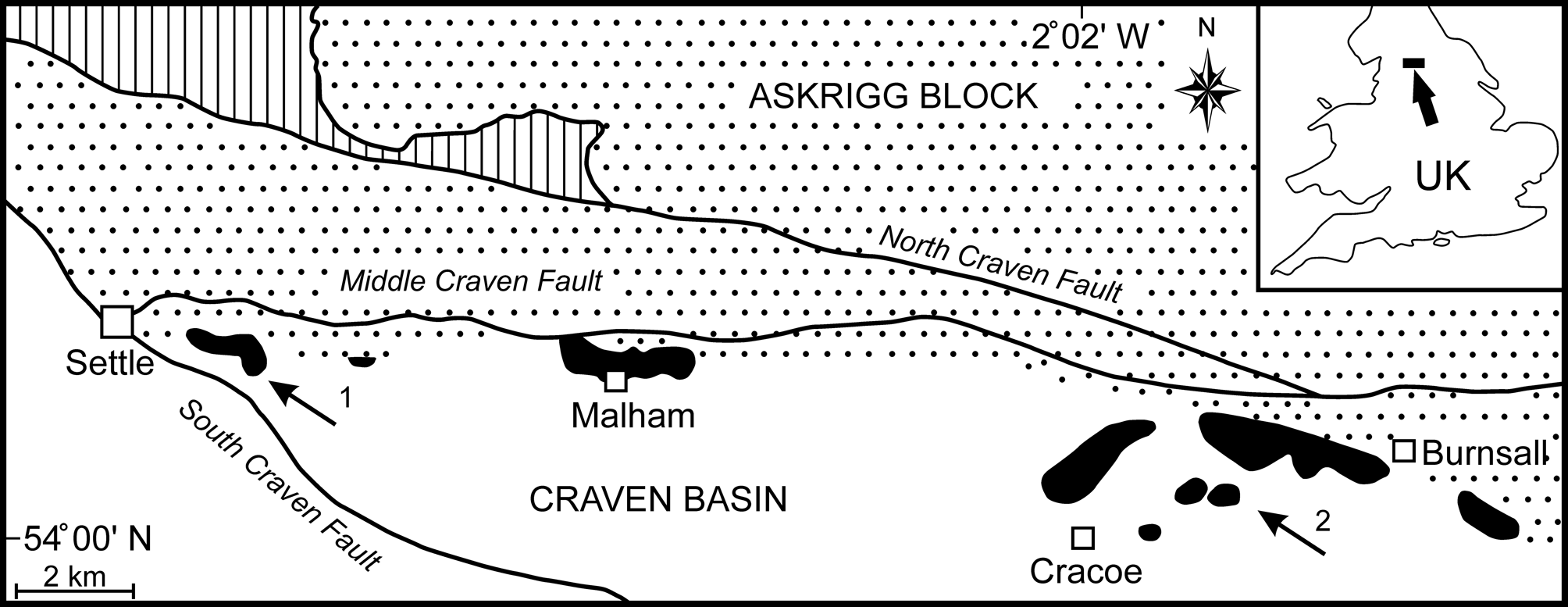
Figure 1. Simplified geological map of the Late Viséan Craven Reef Belt of North Yorkshire, UK (location marked by arrow in the inset), showing the main reef outcrops (black), the southern limit of shelf facies on the Askrigg Block (stippled), the basin facies (white), the Lower Paleozoic inlier (vertically ruled), and the Craven Faults. The outcrop of Serpukhovian (early Namurian) siliciclastics has been omitted for clarity (after Brunton and Mundy, Reference Brunton and Mundy1988). Localities yielding Microconchus cravenensis n. sp. are marked by arrows: (1) Scaleber; (2) Stebden Hill.
Lithostratigraphically, these reefs were traditionally assigned as a facies of the contiguous shelf succession, thus the Malham Formation on the Askrigg Block (Arthurton et al., Reference Arthurton, Johnson and Mundy1988). However, the name Cracoe Limestone Formation has been introduced for the Cracoean reefs and adjacent limestones (not all reefal) in the Cracoe-Burnsall area (Dean et al., Reference Dean, Browne, Waters and Powell2011, p.106; Waters et al., Reference Waters, Haslam, Cózar, Somerville, Millward and Woods2017). Chronostratigraphically, reefal deposition occurred mostly during the Asbian stage but extended into early Brigantian with representatives of the ammonoid biozones ?B1 to P1b (Bisat, Reference Bisat1924, Reference Bisat1934; Mundy, Reference Mundy1980, Reference Mundy2000; Riley, Reference Riley1990; Waters et al., Reference Waters, Haslam, Cózar, Somerville, Millward and Woods2017). Localities yielding the new microconchid range in age from Asbian B2a to early P1a.
Facies development
The term ‘Cracoean’ (Hudson and Philcox, Reference Hudson and Philcox1965) was introduced as a facies designation to apply to certain late Viséan ‘shallow-water’ reefs in a way similar to the usage of ‘Waulsortian.’ Cracoean reefs are hybrid buildups, an amalgam of ‘mudmound,’ substantial frameworks (both microbialite and lithostrotionid) and shelly bioaccumulations, which reflected a long period of growth, punctuated by frequent emergent episodes. Facies subdivision of the reefs (Fig. 2) was proposed by Mundy (Reference Mundy, Beauchamp, Embry and Glass1994, Reference Mundy, Vennin, Aretz, Boulvain and Munnecke2007). Shallow ramp bioclastic packstones with colonies of lithostrotionid corals, locally interbedded with crinoidal floatstones, formed the foundation of the reefs. These pass upward into prograding lenticular pack-wackestones containing a typical ‘reefal’ fauna and then into massive bedded-bank facies. The latter are bioclastic wackestones and floatstones (often with a clotted micritic matrix) that produced lenticular and tabular geometries and contain a shallow-water biota with a conspicuous component of in situ Gigantoproductus Prentice, Reference Prentice1950. Passage from ramp packstones to the bank facies was postulated to be microbially mediated (Mundy, Reference Mundy, Beauchamp, Embry and Glass1994, Reference Mundy, Vennin, Aretz, Boulvain and Munnecke2007).
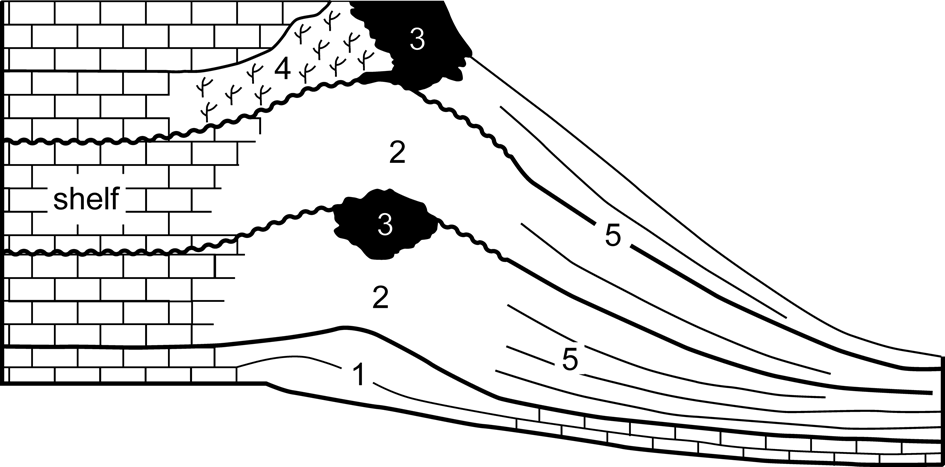
Figure 2. Component subfacies of the Cracoean reefs (after Mundy, Reference Mundy, Vennin, Aretz, Boulvain and Munnecke2007): (1) foundation; (2) bank; (3) microbialite framework; (4) Siphonodendron (coral) thicket; (5) flank.
At intervals during the growth of the buildups, microbialite frameworks developed, attaining thicknesses to 40 m, and often initiated during flooding recolonization following emergence. The frameworks were constructed by microbialite and an encrusting consortium of bryozoans, tabulate corals, and lithistid sponges (Mundy, Reference Mundy, Beauchamp, Embry and Glass1994; Rigby and Mundy, Reference Rigby and Mundy2000) that locally bound in situ groves of small solitary rugosans. A unique shelly fauna is present, consisting of attached productoids and cemented pseudomonotid bivalves (Mundy and Brunton, Reference Mundy and Brunton1985; Brunton and Mundy, Reference Brunton and Mundy1988). Thickets of Siphonodendron McCoy, Reference McCoy1849 developed on the leeside of some frameworks.
Basin-facing foreslopes of the reefs consist of bedded flank facies that attained depositional dips of 35° and could span a paleobathymetry of 100–170 m. Lithologies are bioclastic wackestones and floatstones that locally grade to cementstones where radiaxial fibrous calcite is significant. These yield a distinctive and diverse fauna for which the reefs are renowned. Brachiopods, quasi-infaunal productoids, and pediculate taxa (spriferoids and rhynchonelloids) dominate the fauna at most bathymetric levels, but there are pronounced changes in community from upper to lower (shallow to deeper water) flank. Characteristically, the upper (shallow) flank limestones contain large productoids (Gigantoproductus and Linoprotonia Ferguson, Reference Ferguson1971) and rostroconchs, yield proportionally more gastropods, and locally have abundant green algae. Lower (deeper) flank communities are notably crinoidal, contain large fenestellid bryozoan colonies, have abundant ammonoids and nautiloids, and are associated with the solitary rugosan Amplexus Sowerby, Reference Sowerby1814.
The microconchid specimens occurred mostly in shallow-flank facies, with a single specimen located in a microbialite framework and the holotype from bank- or possibly shallow-flank facies.
Materials and methods
Materials
The microconchid specimens were collected by one of us (DM) during fieldwork along the Craven Reef Belt between 1971 and 1981. These specimens were obtained from two localities in the Craven Reef Belt: Scaleber, east of Settle, and Stebden Hill, near Cracoe (Fig. 1). The material consists of one well-preserved, albeit still incomplete, specimen, and three fragmentary specimens, together with a specimen observed in thin section. Ornamentation patterns and microstructure of the tubes are well preserved in the specimens.
Methods
Microstructure was observed on uncoated specimens using a Philips XL 30 environmental scanning electron microscope (ESEM) in back-scattered mode; comparative observations were made from a thin section. The specimens were too large to be photographed using the ESEM, thus they were coated with ammonium chloride and photographed using a Canon digital camera.
Repositories and institutional abbreviations
NHM PG = Department of Earth Sciences, The Natural History Museum, London, UK; TS = thin section collection, D.J.C. Mundy, Calgary, Alberta, Canada.
Systematic paleontology
Class Tentaculita Bouček, Reference Bouček1964
Order Microconchida Weedon, Reference Weedon1991
Family Microconchidae Zatoń and Olempska, Reference Zatoń and Olempska2017
Genus Microconchus Murchison, Reference Murchison1839
Type species
Microconchus carbonarius Murchison, Reference Murchison1839
Microconchus cravenensis new species
Figures 3–5, 6.3

Figure 3. Microconchus cravenensis n. sp. from the Mississippian Cracoean reefs: (1–3) holotype, NHM PG 10009, Scaleber, east of Settle, North Yorkshire, UK, three views (arrows indicate repaired injuries); (4) paratype, NHM PG 10007, Stebden Hill, near Cracoe, North Yorkshire, UK. Both specimens show the planispirally coiled tube followed by the uncoiled stage. Scale bars = 2 mm.
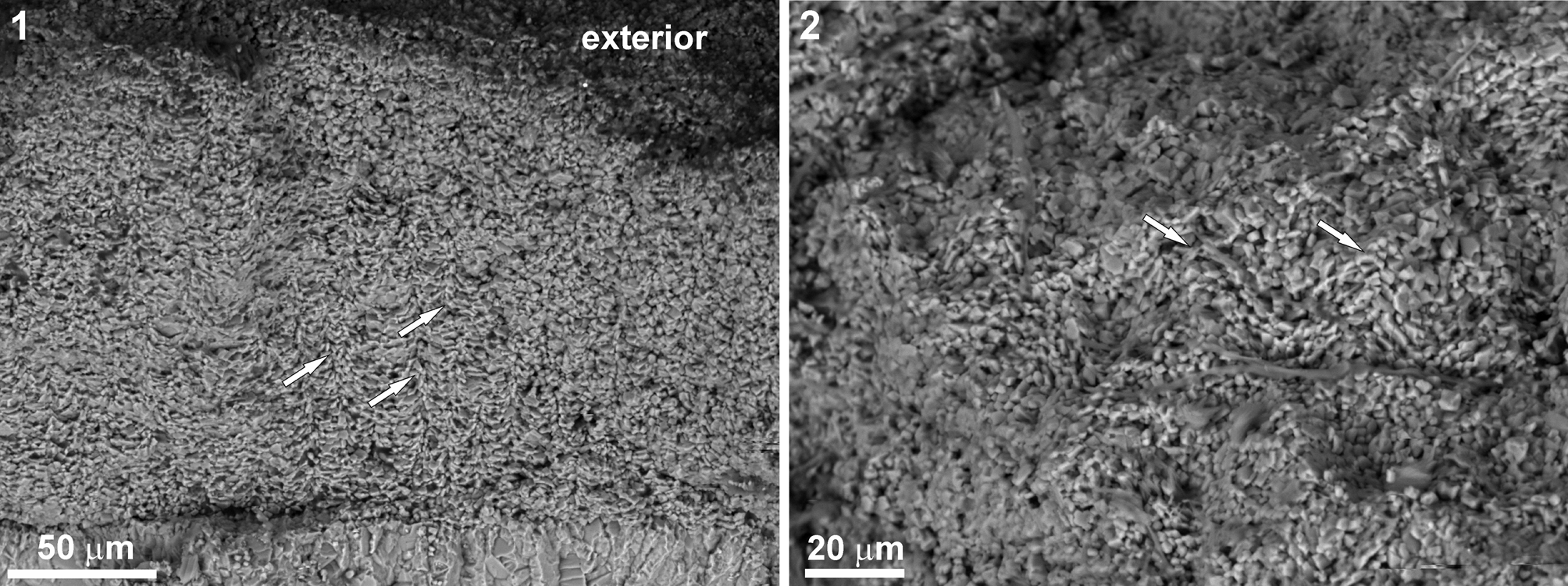
Figure 4. Tube microstructure of Microconchus cravenensis n. sp. from the Mississippian Cracoean reef of Stebden Hill near Cracoe, North Yorkshire, UK, ESEM photomicrographs showing microlamellar fabric interrupted by cone-like punctae (arrows): (1) NHM PG 10007, with exterior indicated; (2) NHM PG 10008, tube interior.
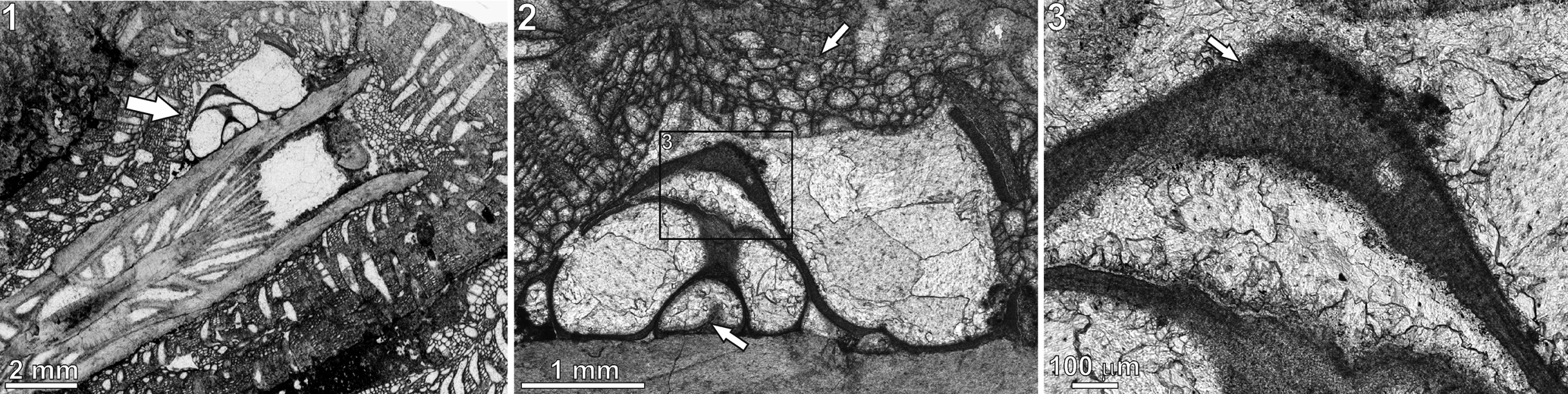
Figure 5. Microconchus cravenensis n. sp., TS 25, Stebden Hill, near Cracoe, North Yorkshire, UK, in thin section: (1) specimen (arrow) encrusting the coral Cyathaxonia cornu; (2) same specimen as (1) under higher magnification, showing the bryozoan Fistulipora incrustans that encrusted the tube after death of the microconchid (upper arrow) and putative septum inside the tube (lower arrow); (3) enlarged portion of the tube, indicated by rectangle in (2), showing visible punctation (arrow).
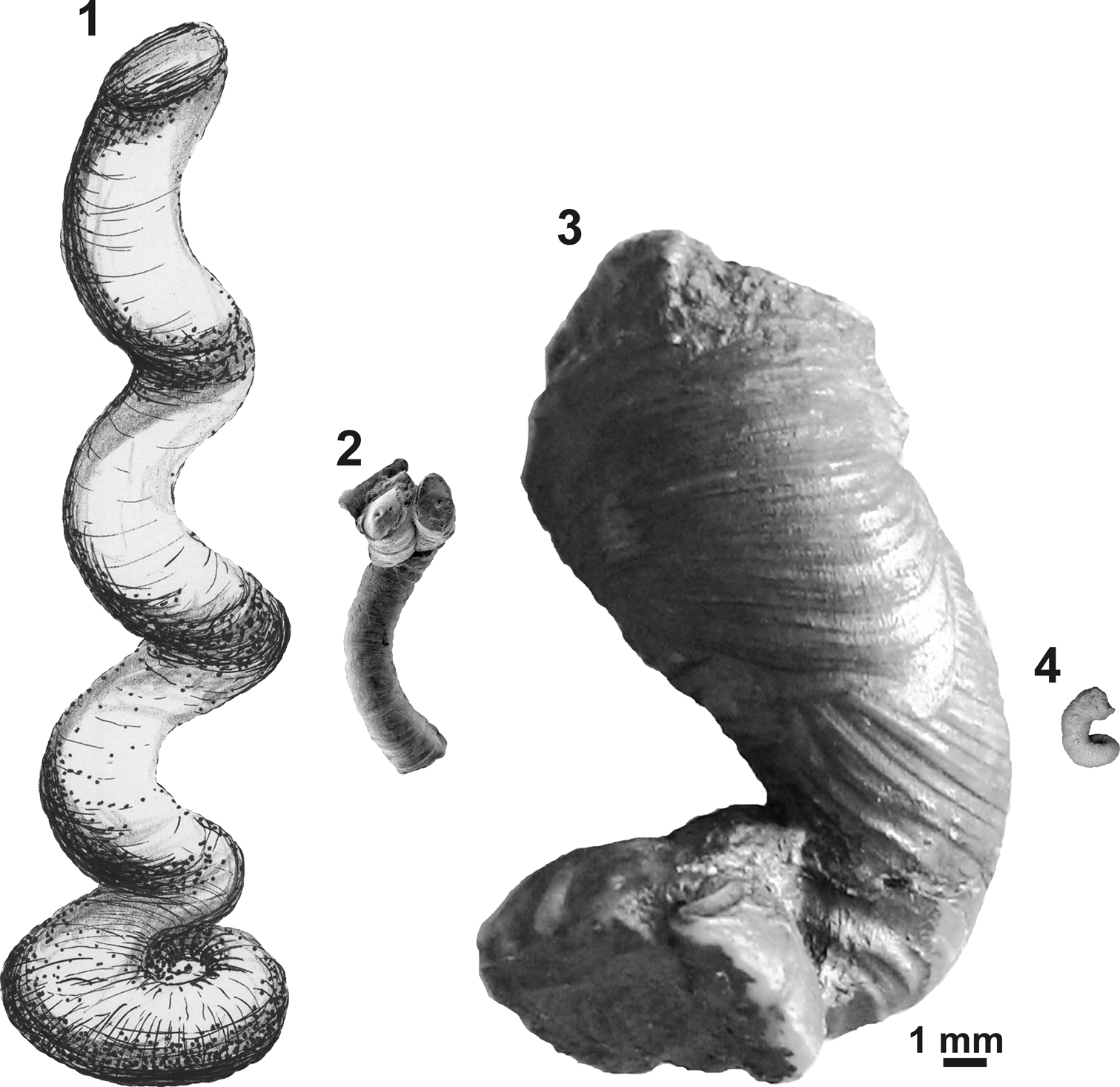
Figure 6. Comparative apertural size of selected microconchids showing the giant nature of the new species: (1) ‘Serpula’ cf. S. advena, Mississippian, UK (redrawn from Burchette and Riding, Reference Burchette and Riding1977); (2) Helicoconchus elongatus Wilson, Vinn, and Yancey, Reference Wilson, Vinn and Yancey2011, Lower Permian, USA (courtesy of Mark A. Wilson via Wikimedia Commons); (3) Microconchus cravenensis n. sp., Mississippian, UK (NHM PG 10009, holotype, this paper); (4) Microconchus hintonensis, Mississippian, USA (from Zatoń and Peck, Reference Zatoń and Peck2013). All specimens presented to scale with reference to apertural diameters.
Type specimens
Holotype, NHM PG 10009, Scaleber, east of Settle, North Yorkshire, UK, west bank of Stockdale Beck, Mundy Locality 136 (National Grid Reference SD 8416 6319), Mississippian (Asbian Stage, B2a ammonoid biozone), Cracoean Facies, Malham Formation. Paratypes from Stebden Hill, Cracoe, North Yorkshire, UK, NHM PG 10006, Mundy Locality St 25A (NGR SE 0030 6076); NHM PG 10007 and 10008, Mundy Locality St 116 (NGR SE 0017 6083), both localities Mississippian (Asbian Stage, B2b ammonoid biozone), Cracoean Facies, Cracoe Limestone Formation.
Diagnosis
Large microconchid with helically uncoiled tube, ornamented by thin, transverse riblets.
Occurrence
Mississippian (upper Viséan) of Scaleber near Settle, and Stebden Hill near Cracoe, North Yorkshire, UK.
Description
The attachment portion of the tube is planispiral, dextrally (clockwise) coiled, 5.4–7.7 mm in diameter. Later, the tube helically uncoils to the preserved height of 16 mm in the holotype. The aperture is round and the tube diameter increases rapidly. In the attachment portion, the aperture can be ~4 mm (PG 10007) to 5 mm in diameter (holotype), whereas in the terminal, helically uncoiled part, it increases to 8.3 mm in diameter (holotype, Fig. 3.1–3.3). However, this is a minimum size because the tube is incomplete. The umbilicus is open, ~2 mm in diameter, with a gently dipping, rounded umbilical slope. The exterior of the tube is ornamented with fine, closely spaced transverse riblets of varying width that can be thickened at the flank of the planispirally coiled tube, and form well-spaced rib-like structures (Fig. 3). The riblets run sinuously across the tube from the umbilical slope to the attachment base and around the helically uncoiled portion of the tube. In the uncoiled part of the holotype, signs of tube regeneration occur, manifested by a distinct interruption of ornament pattern (Fig. 3.2). No longitudinal striae have been observed.
Tube microstructure is lamellar and punctate (Figs. 4–5.3), with the punctae clearly deflecting the laminae throughout the tube thickness (Fig. 4). The presence of septa is not excluded (Fig. 5.2).
Etymology
For the Craven Reef Belt (North Yorkshire, UK) where the species was found.
Materials
Type specimens, plus thin section TS 25, Stebden Hill, Mundy Locality St 55 (NGR SE 0026 6083), age and formation as above.
Remarks
The lamellar tube microstructure and the presence of tiny punctae suggest that the microconchids can be classified in the family Microconchidae. Although the punctae on the cross section of the tubes observed under ESEM look like deflections of the laminae (Fig. 4), these seem better discernible in the thin section (Fig. 5.3). The manifestation of punctae on the tube exterior of other representatives of the family Microconchidae is simply due to exfoliation of the tube exterior (e.g., Zatoń and Peck, Reference Zatoń and Peck2013; Zatoń et al., Reference Zatoń, Hagdorn and Borszcz2014b; Zatoń and Olempska, Reference Zatoń and Olempska2017), which was not observed on the specimens studied here. Thus, classification of the new species in the family Microconchidae seems justified. The punctae present in much younger (Jurassic) representatives of the family Punctaconchidae occur in the form of large pores (Vinn and Taylor, Reference Vinn and Taylor2007; Zatoń and Olempska, Reference Zatoń and Olempska2017) and thus differ markedly from those present in Microconchidae.
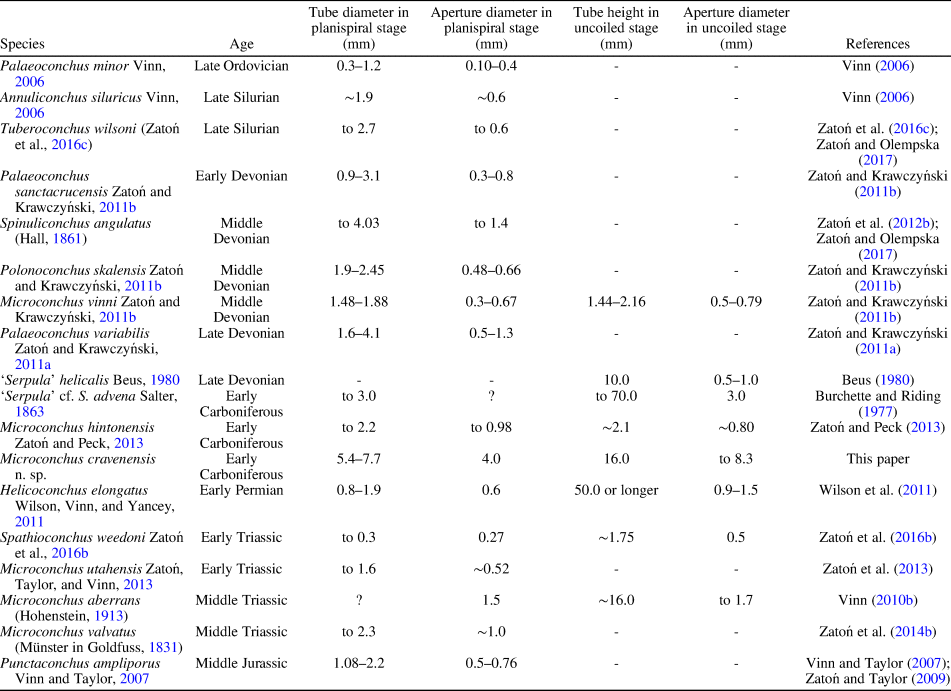
The ornament pattern and large size, especially of the planispiral portion of the tube and its robust uncoiled part with a wide aperture, make these specimens distinct from all other microconchid species described so far. Ornamentation of the Mississippian species still known by the informal name ‘Serpula’ cf. S. advena (see Burchette and Riding, Reference Burchette and Riding1977) appears similar, but no photographs of its external details have been presented. However, this species has a planispiral attached tube of smaller diameter and significantly smaller (nearly three times) aperture diameter, even in the helically uncoiled tube (Table 1). Moreover, aperture diameter of the new species increases more rapidly. Other Mississippian microconchids having uncoiled tubes, e.g., Microconchus hintonensis Zatoń and Peck, Reference Zatoń and Peck2013, from nonmarine deposits of the USA (Zatoń and Peck, Reference Zatoń and Peck2013) and an unnamed, marine microconchid (described as a vermiform gastropod) from Poland (Bełka and Skompski, Reference Bełka and Skompski1982), differ in their tiny sizes and ornamentation patterns, which includes additional longitudinal striae and widely spaced ridges, respectively. A helically uncoiled Mississippian ?tubeworm fragment illustrated by McCoy (Reference McCoy1844) and described under the name Serpula scalaris McCoy, Reference McCoy1844, shows distinct, widely spaced transverse ridges, unlike the closely spaced, thin riblets present in the new species. Moreover, the tube diameter of Serpula scalaris is ~4.2 mm (‘two lines’ of McCoy, Reference McCoy1844), two times smaller than in Microconchus cravenensis n. sp. Similarly, the Middle Devonian species Microconchus vinni Zatoń and Krawczyński, Reference Zatoń and Krawczyński2011b from the Holy Cross Mountains, Poland, is also several times smaller at each developmental stage (Table 1), and differs in having widely spaced, sharp transverse ridges (Zatoń and Krawczyński, Reference Zatoń and Krawczyński2011b). The differences between the new specimens and others, especially Carboniferous microconchids, justify the naming of this new species.
Table 1. Tube size of selected Paleozoic and Mesozoic microconchid species.
Discussion
The great majority of microconchids are small, inconspicuous tubeworms dwelling on various firm and hard substrata (e.g., Taylor and Vinn, Reference Taylor and Vinn2006; Zatoń et al., Reference Zatoń, Vinn and Tomescu2012a). In most cases, these are characterized by a dominant planispiral stage of tube development, with only a short uncoiled part to 2 mm in height (see e.g., Zatoń and Krawczyński, Reference Zatoń and Krawczyński2011b; Zatoń and Peck, Reference Zatoń and Peck2013). However, helical uncoiling, resulting in long, vertically oriented tubes, occurs in a few species, some of which are still undescribed. These species include (Table 1) the 1 cm long ‘Serpula’ helicalis Beus, Reference Beus1980, Microconchus aberrans (Hohenstein, Reference Hohenstein1913) with a 1.6 cm long tube, ‘Serpula’ cf. S. advena with tubes to 7 cm in height, and Helicoconchus elongatus Wilson, Vinn, and Yancey, Reference Wilson, Vinn and Yancey2011, which has an uncoiled tube 5 cm or more in length (Wilson et al., Reference Wilson, Vinn and Yancey2011). Although the preserved uncoiled tube of Microconchus cravenensis n. sp. is 1.6 cm in height, it could certainly have been larger when complete. However, there is a feature of the new species that surpasses all other microconchid species, even those having the largest tubes. This is the large aperture diameter, which gives this new species such a robust appearance. Its aperture in the uncoiled stage is not only five times larger than that in the similarly high Microconchus aberrans from the Middle Triassic (Vinn, Reference Vinn2010b), but nearly three times larger than the aperture in the highest tube of ‘Serpula’ cf. S. advena, and five and a half times larger than the aperture in the similarly long tube of Helicoconchus elongatus (Table 1; Fig. 6). If the aperture size reflects the size of the animal dwelling within the tube, then Microconchus cravenensis n. sp. is the largest among all known microconchids.
Interestingly, all microconchids having helically uncoiled tubes were associated with organic buildups and some even formed their own bioconstructions—biostromes and bioherms (Leeder, Reference Leeder1973; Peryt, Reference Peryt1974; Burchette and Riding, Reference Burchette and Riding1977; Toomey and Cys, Reference Toomey and Cys1977; Beus, Reference Beus1980; Suttner and Lukeneder, Reference Suttner and Lukeneder2004; Wilson et al., Reference Wilson, Vinn and Yancey2011; Zatoń et al., Reference Zatoń, Niedźwiedzki, Rakociński, Blom and Kear2018). Such a niche could have been advantageous (see Vinn, Reference Vinn2010a), providing protection against overgrowth and sediment covering, and lessening competition for suspended food in a higher tier. The microconchids in this setting had the ability to keep pace with the growth of encrusting algae and microbialite with which they were typically associated (e.g., Peryt, Reference Peryt1974; Burchette and Riding, Reference Burchette and Riding1977; Dreesen and Jux, Reference Dreesen and Jux1995; Zatoń et al., Reference Zatoń, Niedźwiedzki, Blom and Kear2016b). Only such a growth mode allowed microconchids to develop primary frameworks (Vinn, Reference Vinn2010a). The microconchid described here did not form bioconstructions but was a minor component of the prolifically fossiliferous Cracoean reefs, which have yielded 568 known macrofaunal species (Mundy, Reference Mundy2000). This fauna is dominated by brachiopods and mollusks with a modest diversity of bryozoans, corals, arthropods, and echinoderms, together with rare sponges. The biota also includes microbialite, ‘skeletal’ microbes, and algae.
At present, Microconchus cravenensis n. sp. is only known from the Craven Reef Belt, where it is extremely rare. Just 10 specimens were recorded from six localities despite extensive collecting (from 378 exposures) along the reef tract (Mundy, Reference Mundy1980). One specimen (the holotype) came from an exposure at Scaleber, east of Settle, and nine specimens were recorded from five exposures on the Stebden Hill reef mound, Cracoe, of which four specimens are extant. In limestones of B2b zone age on Stebden Hill, a single specimen of Microconchus cravenensis n. sp. was located in a microbialite framework where it was attached to the epitheca of the small solitary rugosan Cyathaxonia cornu Michelin, Reference Michelin1847, and is sited just short of the calice (Fig. 5.1). The coral and the attached microconchid were postmortaly encrusted by the cystoporate bryozoan Fistulipora incrustans (Phillips, Reference Phillips1836) (Fig. 5.1, 5.2). Seven specimens were recovered from a coeval ‘shoal’ deposit in the upper flank facies that abuts the framework and its contiguous Siphonodendron thicket. There, the microconchids occur in a floatstone-grainstone that contains a typical upper flank (shallow-water) fauna, albeit with a high percentage of disarticulated shells. Conspicuous microbial (oncolitic) coatings by the microorganisms Aphralysia Garwood, Reference Garwood1914 and Girvanella Nicholson and Etheridge, Reference Nicholson and Etheridge1878 are present on many of the shell fragments. The attachment substratum for these microconchids is unknown but is inferred to be shells or shell fragments. A further specimen was located in younger lower flank limestones (P1a zone age) in a stressed community that was deposited immediately prior to an emergent episode. Here again, the substratum is unknown, but it is interesting that within this community and the overlying ‘lowstand’ brachiopod-dominant coquinas, attachment scars of small Microconchus spp. were evident, mostly attached to bivalve shells. Depositional setting at the Scaleber locality is unclear because the exposure occurs in a slab of the reef front displaced from the main reef trend and likely a late Mississippian slope failure. The occurrence of Gigantoproductus in this exposure suggests a bank- or shallow-flank facies setting.
The holotype of Microconchus cravenesis n. sp. bears distinct signs of repair and regeneration of the uncoiled tube. These occurred after a puncture or breakage and were characterized by deviation of the ornament pattern in the subsequently secreted tube material. Such regeneration occurred five times during the tube development. The first occurred at the beginning of the uncoiled part and was followed by the second one, which is the most severe tube breakage (Fig. 3.2). There, the epithelium, along with a large portion of the tube, must have been damaged. However, the individual survived and regenerated the tube. Subsequently, there was damage at three further locations, again with regeneration (Fig. 3.2). Such sublethal injuries are known in other Carboniferous microconchids and the percentage of damaged tubes varies widely from < 1–34% (Zatoń et al., Reference Zatoń, Grey and Vinn2014a; Zatoń et al., Reference Zatoń, Dębowiec and Peck2016a). The sublethal injuries were likely caused by external biological agents, namely failed attempts at predation (Vinn, Reference Vinn2009; Zatoń et al., Reference Zatoń, Grey and Vinn2014a, Reference Zatoń, Dębowiec and Peck2016a). In the Cracoean reefs, repaired predation injuries on brachiopods were documented by Mundy (Reference Mundy1982), who suggested that fish, crustaceans, and cephalopods were potential predators. These could also have been the perpetrators of damage in the robust Microconchus cravenensis n. sp., which could have provided a good food source for small durophagous animals. It cannot be entirely excluded that the paucity of Microconchus cravenensis n. sp. in the Craven Reef Belt could be the result of successful predation.
Acknowledgments
We gratefully acknowledge C. Sendino and Z. Hughes, curators at The Natural History Museum, London, for facilitating the loan of the specimens. P. Taylor (UK), an anonymous referee, and the journal Associate Editor J. Botting provided many constructive comments, useful remarks, and corrections, which is greatly appreciated.










