Introduction
A concussion is caused by a traumatic force to the head or body through mechanisms such as contact injury or rapid acceleration or deceleration movements (Esterov & Greenwald, Reference Esterov and Greenwald2017). Although the incidence of concussion is high in sports-related trauma, the Centers for Disease Control and Prevention (2019) Surveillance Report of Traumatic Brain Injury (TBI)-related Hospitalizations and Deaths states that unintentional falls was the most common cause of TBI-related hospitalizations, followed by motor vehicle accidents (Peterson, Zhou, Thomas & Daugherty, Reference Peterson, Zhou, Thomas and Daugherty2021). Individuals aged 15–34 accounted for the highest rate of motor vehicle accident related TBI hospitalizations in 2017 (Peterson et al., Reference Peterson, Zhou, Thomas and Daugherty2021). It is estimated that 70–90% of TBIs can be classified as “mild”, also known as “concussion” (Cassidy et al., Reference Cassidy, Carroll, Peloso, Borg, von Holst, Holm and Kraus2004; Numminen, Reference Numminen2011) and 10–15% demonstrate persistent symptoms (McCrory et al., Reference McCrory, Meeuwisse, Aubry, Cantu, Dvorak, Echemendia and Tator2013). Given the incidence rate of concussion across many demographics, research is indicated to explore the examination and management of post-concussive symptoms in expanded populations, including those with protracted recovery.
Concussions result in a variety of impairments that can fall under multiple categories including cervical musculoskeletal, vestibulo-oculomotor, autonomic/exertional tolerance, motor function, psychological, sociological, emotional, and sleep-related disturbances (Esterov & Greenwald, Reference Esterov and Greenwald2017; Quatman-Yates et al., Reference Quatman-Yates, Hunter-Giordano, Shimamura, Landel, Alsalaheen, Hanke and McCulloch2020). Signs and symptoms can include headaches, dizziness, gait and balance difficulties, nausea, vomiting, light and sound sensitivities, fatigue, as well as alterations in mental processing, concentration, memory, and mood (Esterov & Greenwald, Reference Esterov and Greenwald2017; Pertab et al., Reference Pertab, Merkley, Cramond, Cramond, Paxton and Wu2018). Presentation varies greatly between individuals post-concussion, thus in 2014 Collins and colleagues presented a model illustrating 6 categories of common examination findings. Also known as “clinical profiles”, they include cognitive fatigue, vestibular, ocular, migraine, anxiety/mood, and cervical (Collins, Kontos, Reynolds, Murawski & Fu, Reference Collins, Kontos, Reynolds, Murawski and Fu2014; Kontos, Sufrinko, Sandel, Emami & Collins, Reference Kontos, Sufrinko, Sandel, Emami and Collins2019). Awareness is growing for symptoms associated with autonomic nervous system (ANS) dysfunction post-concussion, which has fueled recommendations to add assessment and treatment of autonomic dysfunction in standardized concussion management (Quatman-Yates et al., Reference Quatman-Yates, Hunter-Giordano, Shimamura, Landel, Alsalaheen, Hanke and McCulloch2020).
Current research, in conjunction with statements from the American Academy of Neurology, supports the conclusion that concussion likely causes anomalies within the ANS (Callaway & Kosofsky, Reference Callaway and Kosofsky2019; Pertab et al., Reference Pertab, Merkley, Cramond, Cramond, Paxton and Wu2018). The ANS is primarily responsible for the regulation of vital functions including stabilization of the cardiovascular system, particularly during changing environmental demands (Esterov & Greenwald, Reference Esterov and Greenwald2017; Pertab et al., Reference Pertab, Merkley, Cramond, Cramond, Paxton and Wu2018; Thompson & Hagedorn, Reference Thompson and Hagedorn2012). The National Institute of Neurological Disorders and Stroke defines dysautonomia as a “disorder of ANS function that generally involves failure of the sympathetic or parasympathetic components of the ANS, but dysautonomia involving overactive ANS actions also can occur.” (The National Institute of Neurological Disorders and Stroke, 2019). It is suspected that dysautonomia plays a role in symptom exacerbation after concussion due to cardiovascular vessel dilation and constriction throughout the body, including cerebral and brainstem perfusion (Worley et al., Reference Worley, OʼLeary, Sackett, Schlader, Willer, Leddy and Johnson2021). This can lead to protracted recovery and/or onset of dysautonomia symptoms such as balance impairments, noise or light sensitivity, shortness of breath, dizziness, abnormal fatigue, gastrointestinal/genitourinary disturbances, heart palpitations, brain “fog”, mood swings, and weakness, all of which can overlap with traditional concussion symptoms (Cleveland Clinic Medical Professionals, 2020; Miranda, Boris, Kouvel & Stiles, Reference Miranda, Boris, Kouvel and Stiles2018).
The 2020 American Physical Therapy Association (APTA) Clinical Practice Guidelines reported Level II evidence that physical therapists should utilize graded exertional assessments to evaluate exercise intolerance in individuals diagnosed with concussion (Quatman-Yates et al., Reference Quatman-Yates, Hunter-Giordano, Shimamura, Landel, Alsalaheen, Hanke and McCulloch2020). Currently, the Buffalo Concussion Treadmill Test (BCTT) is the established gold standard to assess exercise intolerance associated with concussion, which the authors suggest as evidence supporting dysautonomia. A review of the literature revealed that the majority of studies completed on the BCTT evaluate sports-related concussions (SRCs) with predominantly young male participants (Chizuk, Willer, Horn, Haider & Leddy, Reference Chizuk, Willer, Horn, Haider and Leddy2021; Haider, Johnson & Mannix, Reference Haider, Johnson and Mannix2019; Haider et al., Reference Haider, Leddy, Wilber, Viera, Bezherano, Wilkins and Willer2019). The APTA guidelines discuss the need for additional research to determine specific guidelines for modes, protocols and interpretation of exertional assessments, as well as studies to assess these protocols in non-athletes (Quatman-Yates et al., Reference Quatman-Yates, Hunter-Giordano, Shimamura, Landel, Alsalaheen, Hanke and McCulloch2020). The objective of this study was to identify trends of provoked signs and symptoms consistent with dysautonomia during the BCTT.
Methods
Participants
This is a retrospective cohort study of 101 patient charts post-concussion between August 1, 2019 and May 7, 2020, after approval from the Intermountain Healthcare Institutional Review Board. The inclusion criteria were patient charts: (1) with a diagnosis of concussion, (2) were administered a BCTT based on clinic protocol utilizing a Borg Rating of Perceived Exertion Scale (RPE; Borg, Reference Borg1998), (3) documented signs and/or symptoms of suspected dysautonomia, as outlined by Pertab et al, Reference Pertab, Merkley, Cramond, Cramond, Paxton and Wu2018. Exclusion criteria included: (1) BCTT not completed due lost to follow up or deemed inappropriate based on clinic protocol, (2) patients who completed the BCTT per established guidelines, asymptomatically reaching the target heart rate (HR) zone for their estimated HR max, (3) charts with incomplete data, (4) utilization of a modified RPE.
Measures
Leddy and Willer, Reference Leddy and Willer2013 states the BCTT may be administered “safely and reliably” in the post-concussive population to determine a safe level of exercise during concussion treatment, aid in differential diagnoses of concussion-like symptoms, recognize physiological signs and symptoms related to recovery time, and to appraise exercise capacity. The BCTT protocol states that the test is terminated upon the presence of one or more of the following criteria: (1) an increase of three or more points on the Visual Analog Scale (VAS) scale from resting VAS score, (2) a RPE of >17 without significant symptom exacerbation, (3) a clinical judgment that continuing the test constitutes a significant health risk for the patient, (4) the patient has reached 90% or more of age-predicted maximum without any increase in symptoms and still reporting low RPE, or (5) the patient requests to stop for any reason (Haider et al., Reference Haider, Johnson and Mannix2019).
Procedure
In this clinic, patients with a diagnosis of concussion were administered a baseline BCTT if they demonstrated (1) protracted recovery which defined as longer than three weeks (Iverson, Brooks, Collins & Lovell, Reference Iverson, Brooks, Collins and Lovell2006) and (2) suspected difficulty with ANS regulation, demonstrating symptoms such as hot flashes or temperature intolerance, digestive changes, weight loss or gain, or abnormal fatigue with activities of daily living and exercise. Baseline concussion symptoms were reported using the Post-Concussion Symptom Scale. Recordings were completed every 60 seconds for HR and oxygen saturation using a medical digit pulse oximetry, RPE, exacerbation of baseline symptoms using a 0–10 VAS scale, subjective reports of concussion rebound symptoms during recovery, and observation of ANS responses. Peak HR was recorded at the workload of concussion symptom exacerbation, representing the individual’s HR threshold which is used to determine appropriate workloads during future treatments. The delta difference of 80% of maximum beats per minute (bpm) achieved compared to 80% of age-expected HR max for each individual’s test was also collected.
To maintain the safety of this patient population and compliance with testing criteria #3, the test administrators terminated the BCTT if they met termination criteria per the established protocol or if they demonstrated any of the following: poor HR stabilization defined as a drop or plateau in HR during exercise, exacerbation of concussion symptoms, rebound symptoms during recovery phases, decrease in oxygen saturation, or ANS responses such as abnormal shortness of breath, mood changes, changes in hand color, abnormal perspiration, dizziness, nausea, headache, etc.
Statistical analysis
Data was analyzed using descriptive statistics such as frequency, mean, standard deviations, and confidence intervals using SPSS version 27.
Results
Within the time frame of this study, 101 patient charts with a diagnosis of concussion were screened for inclusion criteria. Of the charts assessed, 4 (3%) were excluded secondary to completing the BCTT by asymptomatically reaching the target HR zone for their estimated HR max, 3 (2.9%) were excluded due to utilization of a modified RPE, 5 (4.9%) were excluded due to incomplete chart data and 45 (44%) patient charts did not have a BCTT due to lack of follow and/or deemed inappropriate based on clinic protocol. The remaining 44 patient charts (26 female, 18 male) met all inclusion criteria and were included in data analysis. The patients had an average age of 27.8 (± 14.98) years and were a mean of 268.84 (± 761.19) days post injury. The majority, n = 31 (70.5%), of patients incurred concussions from nontraditional sports (e.g. skiing or mountain biking), motor vehicle accidents and being struck by an object. Four patients (8%) had traditional SRCs. Approximately half, n = 20 (45.5%) of the patients had incurred a previous concussion and 25% (n = 11) did not have any contributing past medical history. All patient characteristics are outlined in Table 1.
Table 1. Patient Demographics

Outcomes of the BCTT are reported in Table 2. The average RPE reported during the BCTT was 16.42 (± 3.13). The average 80% of prescribed HR was 111.54 bpm (± 17.46). The average of 80% of expected HR was 153.50 (± 11.99). The average number of days from injury to BCTT was 268.84 (± 761.19). In reference to the maximum stage that patients were able to complete, the largest number of individuals were not able to complete the BCTT past stages 6, 8, 9, 10, n = 5 (11.5%) per stage.
Table 2. BCTT Outcomes

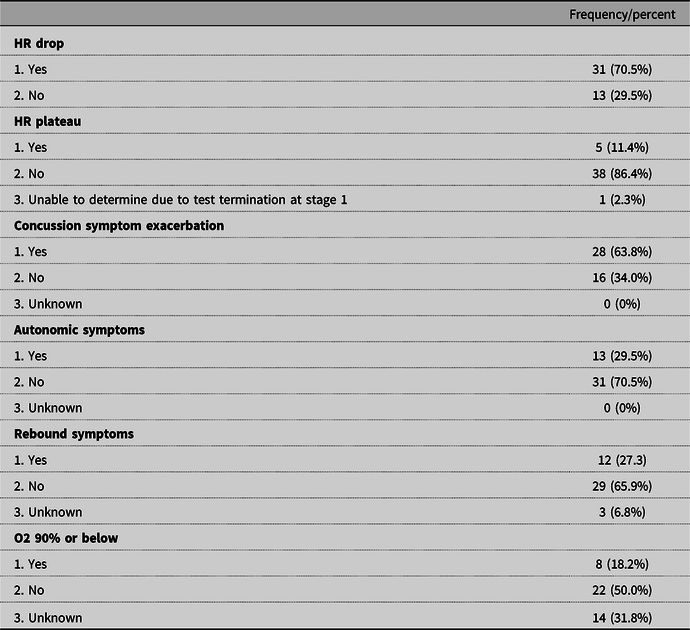
During the BCTT, the subjects demonstrated: 35 (79.5%) poor HR stabilization defined as a drop or plateau in HR during exercise, 28 (63.8%) exacerbated concussion symptoms, 13 (29.5%) ANS response such as hot flushed sensation, 12 (27.3%) rebound symptoms during recovery phases, and 8 (18.2%) desaturation of 90% or below. Reported concussion symptoms included headache, dizziness, feeling “foggy”, fatigue, pain, visual changes, pain, balance impairment, shortness of breath and nausea. The mean delta (80% expected HRmax – 80% achieved HR max) on the initial test was 80.66 (± 23.08) bpm. The distribution of dysautonomia signs or symptoms during the BCTT are outlined in Table 2.
There were 13 categories of reasons the BCTT was discontinued (see Table 3). The most common causes included exacerbation of concussion symptoms (n = 12) and reports of maximal effort indicated by a rating of 19/20 on the Borg (n = 9).
Table 3. BCTT Discontinuation Criteria
Discussion
The results of this study identified potential trends of dysautonomia signs and symptoms using the BCTT protocol in an expanded population of individuals with concussion that is not well represented in current literature. The 44 patients included in this study demonstrated an inability to complete the BCTT protocol due to limitations from signs and/or symptoms of suspected dysautonomia, most notably poor HR stabilization defined as a drop or plateau in HR during exercise, exacerbation of concussion symptoms, and ANS dysfunction responses. This study evaluated exercise intolerance, as a symptom of dysautonomia, in an expanded population of individuals with concussion. In contrast to the majority of current research, the participants in this study were 59.1% female, were an average of 27.8 years old, and 92% had a concussion unrelated to traditional sports.
Prior to implementation of this clinic protocol, patients were instructed to initiate gradient exercise without symptom exacerbation >2/10 for return to prior level of function post-concussion. A need was identified for individualized testing of exercise intolerance to assess severity of dysautonomia symptoms, and therefore better individualize exercise prescription. Due to the level of impairment identified on standardized testing and severity of exercise intolerance in this expanded population, this procedure was adapted as clinic protocol. The results of this study introduce the potential importance of objective measurement of autonomic dysfunction post-concussion.
It has been proposed that a reduction in cerebral perfusion identified in humans, persisting weeks to months after a concussion, may be the consequence of altered functioning of the ANS known as dysautonomia (Pertab et al., Reference Pertab, Merkley, Cramond, Cramond, Paxton and Wu2018). Nelson et al. (Reference Nelson, Temkin, Dikmen, Barber, Giacino, Yuh and Zafonte2019) found that although many athletes with SRC demonstrate full recovery in 1–2 weeks, more than half of individuals with mTBI report difficulties in day-to-day function for 1 year post injury and that recovery timelines differ across patient populations (Nelson et al., Reference Nelson, Temkin, Dikmen, Barber, Giacino, Yuh and Zafonte2019). All of the patients in this study (n = 44) were a mean of 268.84 (± 761.19) days post injury, exceeding the acute recovery timeline of less than 2 weeks. Clinicians may express concern regarding administration of the BCTT in its traditional format with the protracted recovery population. However, the BCTT has been deemed safe for individuals with symptoms persisting up to 6 months (Leddy et al., Reference Leddy, Kozlowski, Donnelly, Pendergast, Epstein and Willer2010), consistent with the chronicity of patients in this study who were able to achieve a mean of 6 stages despite a mean delta of 80.66 (± 23.08) bpm.
The purpose of the BCTT is to identify exercise intolerance accurately and therefore, it is not essential that all patients tolerate the test well nor achieve a maximal appropriate effort. Early termination of the BCTT allows for objective comparable measures over time to track progress of individuals recovering from concussion. The frequency of repeat testing and the significance of these changes will need to be validated with future studies of large sample sizes. However, it is the authors’ opinion that the BCTT in its original format is a feasible test for patients with suspected dysautonomia with the implementation of new termination criteria. The suggested modifications may assist clinicians in accurately identifying an autonomic component in patients’ concussion recovery process.
The strong influence of the ANS over the cardiovascular system (Gordan, Gwathmey & Xie, Reference Gordan, Gwathmey and Xie2015) may explain the cardiac symptoms associated with the majority of patients (66%) in this study who demonstrated a drop or plateau in HR. The ANS works to optimize local cerebral perfusion in response to changing demands and exertion by “increasing the efficiency of the brain by increasing blood supply to brain networks that are best suited to task demandsʼ” (Pertab et al., Reference Pertab, Merkley, Cramond, Cramond, Paxton and Wu2018, p. 399), and protect the brain from systemic hypertension (Franco, Reference Franco2007). Furthermore, the ANS influences the baroreflex, a mechanism responsible for maintaining blood flow to brain tissue. This feedback loop is responsible for cardiovascular changes responding to alterations in cerebral perfusion which restore stable pressure in the brain (Pertab et al., Reference Pertab, Merkley, Cramond, Cramond, Paxton and Wu2018).
Kozlowski, Graham, Leddy, Devinney-Boymel & Willer (Reference Kozlowski, Graham, Leddy, Devinney-Boymel and Willer2013) reported that patients with prolonged concussion recovery demonstrated a symptom-limited response to exercise. These findings are consistent with the results of this study where 63.8% reported exacerbation of concussion symptoms during the testing protocol. Worley et al. (Reference Worley, OʼLeary, Sackett, Schlader, Willer, Leddy and Johnson2021) proposed that exacerbation of concussion symptoms may be associated with elevated middle cerebral artery blood velocity found in symptomatic concussed individuals. In addition, the mean delta difference on the initial test in our study was 80.66 bpm. This indicates that the prescribed HR for these patients differed from a predicted normal result. Optimal testing regimens for exercise intolerance, including established objective measures of severity during the BCTT, have yet to be established for the post-concussion population with prolonged recovery times (Pertab et al., Reference Pertab, Merkley, Cramond, Cramond, Paxton and Wu2018; Quatman-Yates et al., Reference Quatman-Yates, Hunter-Giordano, Shimamura, Landel, Alsalaheen, Hanke and McCulloch2020). Outcome measures have yet to be established in current research. In some studies, findings during the BCTT are reported based on stages accomplished or minutes to symptom onset (Haider et al., Reference Haider, Johnson and Mannix2019). However, Leddy, Baker, Haider, Hinds and Willer (Reference Leddy, Baker, Haider, Hinds and Willer2017) defines physiological recovery as asymptomatic exercise for 20 minutes at or above 85% of the age-predicted maximum HR for 2 to 3 days in a row. Due to this discrepancy, the authors propose a newly identified objective measure, the mean delta difference, which may have more clinical significance as it provides a measurable comparison to age-predicted norms and may have potential to be predictive of recovery time.
A drop in oxygen saturation below 90% was the least common indicator for test termination (n = 8, 18.2%). The pulse oximetry method of data collection used in this study is an accepted and standardized method for determining oxygen saturation (DeMeulenaere, Reference DeMeulenaere2007). However, 14 patients (31.8%) had an unknown oxygen reading indicated in their chart, despite the use of 2–3 to attempt data confirmation during abnormal readings. Future studies are indicated to assess oxygen using medical-grade equipment in controlled settings to maximize accuracy of oxygen saturation readings and determine its prevalence during these protocols.
The authors acknowledge limitations to this study. The retrospective design and relatively small sample size likely affected data quality and analysis. A prospective study design with a repeat BCTT at a standard interval for patients meeting specific criteria would be beneficial to improve assessment of progression. There is limited generalizability of these findings to the broader concussion population, as this cohort is primarily represented by individuals with nontraditional sports as their mechanism of injury. The use of pulse oximetry to collect oxygen saturation and HR is applicable across settings and limits the financial barriers to completing the BCTT. This limitation provoked a clinical process change to HR assessment centrally with Polar H7 or newer chest strap and peripheral measurements with oximetry simultaneously. The accuracy of wearables has been demonstrated to be limited in the research but for patient education and management, are frequently used at home to track exercise. It is theorized that wearable devices and peripheral monitors provoked challenges in tracking HR due to altered peripheral blood flow visualized in oxygen saturation between 70–80% and altered coloration and temperature changes in patients hands. Because of these difficulties, education for the use of the RPE scale in daily cardiovascular exercise appears to be of the utmost importance particularly in participants with high HR fluctuations with dysautonomia which may increase the difficulty of tracking HR during daily exercise with wearable devices. Future research with newer, medical-grade data collection such as a Polar Heart Rate Monitor generation H7 and newer, a chest strap or electrocardiogram for collecting HR, would be beneficial for determining the necessity to include these observed changes and adding them to testing criteria for the BCTT.
Conclusion
This is the first study to introduce potential additional termination criteria for the BCTT including poor HR stabilization, exacerbation of concussion symptoms and onset of autonomic symptoms (e.g. atypical sweating patterns or mood changes), when dysautonomia is suspected after concussion. Although future research is necessary to validate these findings, the proposed criteria may provide guidance to clinicians to safely assess the BCTT in individuals with protracted concussion recovery and suspected associated dysautonomia.

Figure 1. Reasons for BCTT Discontinuation. Key: “Abn HR/sxs”: Abnormal Heart Rate and associated symptoms. “Conc sxs”: Concussion Symptom exacerbation using a 0-10 VAS scale. “Pt”: Patient. “Ans”: Autonomic Nervous System.
Acknowledgements
The authors thank Kate Minick, PT, DPT, Ph.D, OCS, Erik Davidov, MBA, Meaghan Dowdell, SPT and Elena Chermak, SPT for their assistance with this project.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Ethical standards
This study was approved by the Intermountain Rehabilitation Services Institutional Review Board. A waiver of consent was obtained, and the patient’s rights were protected.








