Introduction
Freshwater and terrestrial molluscs are highly threatened and have suffered the largest number of documented extinctions of any taxonomic group. However, only a small percentage of species (2% in 2004) have had their conservation status assessed (Lydeard et al., Reference Lydeard, Cowie, Ponder, Bogan, Bouchet and Clark2004). In many biodiversity studies molluscs are often ignored in favour of more charismatic fauna (Cameron, Reference Cameron1998; Lydeard et al., Reference Lydeard, Cowie, Ponder, Bogan, Bouchet and Clark2004), even though protected area designation for other taxa rarely adequately represents land snail diversity (Tattersfield, Reference Tattersfield1998; Fontaine et al., Reference Fontaine, Gargominy and Neubert2007). In common with many other taxa, land snail diversity is affected by habitat change and land use (Raheem et al., Reference Raheem, Naggs, Preece, Mapatuna, Kariyawasam and Eggleton2008) and unregulated harvesting is a further threat to some terrestrial molluscs. Land snails are an important part of the local diet in parts of West Africa (Memel et al., Reference Memel, Otchoumou, Kouassi and Dosso2008), where some people are full-time snail gatherers (Agbelusi & Ejidike, Reference Agbelusi and Ejidike1992). Organized trade in land snails has been recognized as a major reason for their decline in Nigeria, and availability for human consumption has been falling because of over-collection and habitat destruction (Osemeobo, Reference Osemeobo1992). Island mollusc faunas are particularly at risk and have suffered mass extinctions because of factors such as the introduction of invasive non-native species (Cowie & Cook, Reference Cowie and Cook2001; Coote & Loève, Reference Coote and Loève2003).
The islands of São Tomé and Príncipe, in the Gulf of Guinea, are one of the 25 global biodiversity hot spots (Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000) and the archipelago is recognized as a Terrestrial Ecoregion by WWF (Gascoigne, Reference Gascoigne, Burgess, D’Amico Hales, Underwood and Dinerstein2004). Most research on the islands has focused on their birds (Jones & Tye, Reference Jones and Tye2006; Dallimer et al., Reference Dallimer, King and Atkinson2009) and vascular flora (Figueiredo, Reference Figueiredo1994). Nevertheless, there is also a high level of mollusc endemism. Príncipe alone has 19 single-island endemics (plus a further six species shared with just São Tomé) of 32 land snail species (Gascoigne, Reference Gascoigne1994a). Although the urgent need for distribution surveys and monitoring of the mollusc community was first highlighted in the mid 1990s (Gascoigne, Reference Gascoigne1994a), almost nothing is known about the conservation requirements of the various species. Of the 27 species of mollusc listed by the IUCN on São Tomé and Príncipe, only two have been given an IUCN status (IUCN, 2008). One of these is the giant land snail Archachatina bicarinata (endemic to São Tomé and Príncipe), which is categorized as Vulnerable solely because of its restricted range and the presumed threats from harvesting (Gascoigne, Reference Gascoigne1995). The species is an important part of the local diet on both islands, where it is preferred to Archachatina marginata, which was introduced from the African mainland, because of its larger size, superior taste and texture.
Here, we report the first systematic survey of the distribution of A. bicarinata. In addition, we collate anecdotal accounts of A. bicarinata abundance and distribution to document changes in population size and range.
Study area
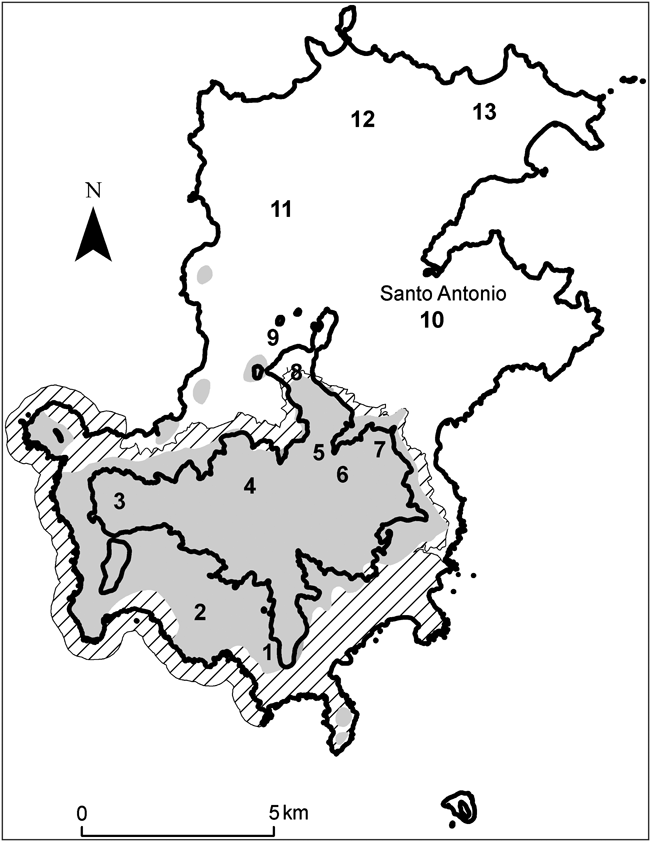
Príncipe (area 139 km2) is the second largest oceanic island in the Gulf of Guinea. It lies 220 km from the African coast and 146 km north of São Tomé (Fig. 1). The north of the island is relatively flat and contains most of the island’s human population and agriculture. To the south and centre the island is rugged and includes the highest mountain, Pico de Príncipe (948 m). Significant tracts of primary rainforest (33% of the island’s total land area) remain in the south and west, which form the bulk of the Parque Natural Obô do Príncipe. However, the protected area has received little active conservation management and the forest is still subject to many of the pressures associated with unprotected areas, such as hunting and harvesting of forest products. Although it is believed that A. bicarinata is restricted to relatively undisturbed forest, no formal surveys of its distribution have previously been carried out. We therefore visited 13 sites, covering representative areas of the island, including primary and secondary forest and plantations (Fig. 2, Table 1).

Fig. 1 The location of Príncipe in the Gulf of Guinea, West Africa.
Methods
At each site 4–10 150-m long, 2-m wide transects were sampled between 25 November and 20 December 2007 by scanning the forest floor and vegetation for both live and dead A. bicarinata. For many snail species the presence of empty shells is counted as evidence of their continued existence (Raheem et al., Reference Raheem, Naggs, Preece, Mapatuna, Kariyawasam and Eggleton2008) and hence we also recorded these. Transects were at least 150 m apart and their position was recorded with a global positioning system where possible. Together with extensive field notes this allowed all transects to be located on a digital map of the island (Gascoigne et al., Reference Gascoigne, Wojciechowski and Koo2004) and relevant environmental information to be gathered. Habitat type (primary forest, secondary forest, plantations with shade canopy cover, palm plantation and agriculturally diverse) and climatic zone (1, very humid zone, typical total annual rainfall ≥ 3,000 mm; 2, less humid zone, typical total annual rainfall c. 2,000 mm; 3, drier zone, typical total annual rainfall 1,000–1,500 mm) were classified following Diniz & de Matos (Reference Diniz and de Matos2002). Transect altitude and the relative productivity of the vegetation were assessed from satellite-derived remotely sensed data using a Digital Elevation Model and a measure of normalized difference vegetation index (NDVI), respectively. To quantify the accessibility of a site the distance to non-primary forest edge (which was negative for sites outside primary forest), distance to the nearest watercourse (as rivers often provide access routes into the forest) and distance to known settlements were also measured. Distance to the nearest road was not included in any analyses as it is a strong covariate of distance to settlements.
We used multiple regression, with both the abundance of live snails (snail abundance) and the occurrence of live or dead individuals (snail presence) as the response variables, and measures of habitat type, productivity and location as explanatory variables. For snail abundance a generalized linear model with Poisson errors was used, and for snail presence a binomial error structure. The full model set therefore included habitat and climate types, NDVI, altitude, and distances to primary forest edge, watercourse and settlement. A quadratic term was included for altitude to model the potentially humped relationship with this variable. The information theoretic approach (Burnham & Anderson, Reference Burnham and Anderson2002; Johnson & Ohmland, Reference Johnson and Ohmland2004) was used to model these data based on Akaike information criteria (AIC). All possible subsets of the variables were modelled. For the complete model set the difference in AIC for that model relative to the best fitting model with the minimum AIC (ΔAIC), the Akaike weight (wi) and the proportion of the variation explained by the model (D 2) were calculated. The best fitting model was defined as that with the lowest AIC, and models that differed by less than two AIC units were considered to have substantial support in terms of explaining the data (Burnham & Anderson, Reference Burnham and Anderson2002). The probability that each explanatory variable appeared in the best fitting model was also calculated (k). Parameter estimates and the relative contribution (partial D 2) of each explanatory variable to the overall predictive power of the model were calculated by model averaging across all models, weighting the estimates by wi. All analyses were performed in R v. 2.7.2 (R Development Core Team, 2008).
While no formal surveys of terrestrial molluscs have previously been carried out on Príncipe, there are three indirect sources of data on recent population trends. Firstly, at remote sites A. bicarinata are processed by snail harvesters before returning home. The presence of piles of snail shells (middens) may therefore give an indication of the impact of collectors and some impression of the size of A. bicarinata populations. Secondly, some researchers, while not specifically working on A. bicarinata, have recorded relevant observations. Thirdly, local guides and snail harvesters were asked to report their observations of A. bicarinata numbers and distribution. We were thus able to compare our systematic survey results with these three indirect sources of evidence on A. bicarinata abundance.
Results
Field surveys
A total of 85 transects were surveyed across 13 sites (Fig. 2). Six sites were completely within primary rainforest. No A. bicarinata were observed outside primary forest and therefore habitat type was not included in the analyses. Snails were found in 22 transects, with 45 (mean 0.53, range 0–9 per transect) live snails encountered and an additional 20 empty snail shells found.
For snail abundance, total model-averaged D 2 was high (0.58; Table 2). The best model contained three variables, indicating that more live individuals were found on transects (1) further away from the nearest settlement (partial D 2 = 0.15), (2) with higher productivity (NDVI; partial D 2 = 0.23) and (3) at intermediate altitudes as both linear and quadratic relationships with altitude were retained in the best model (Table 2).
Table 2 Model-averaged parameter estimates for habitat variables used in modelling snail abundance and presence on the island of Príncipe, Gulf of Guinea (Figs 1–2). Figures in bold indicate variables that appeared in the best model and those in italics indicate the additional variables that appeared in models with a ΔAIC < 2.

* Normalized difference vegetation index
Half the variation in snail presence (D 2 = 0.50) was predicted by the model-averaged parameters, with three variables appearing in the best model. Snails were more likely to be present further from the nearest settlements (partial D 2 = 0.16) and at higher altitudes (partial D 2 = 0.11). Although predictive power was low (partial D 2 = 0.01), snail presence was more likely further away from watercourses.
Considering variables in the ΔAIC < 2 model set, both snail abundance and likelihood of occurrence increased with greater distance from the forest edge, although predictive power was low (partial D 2 = 0.09 and 0.08, respectively; Table 2). The only variable that did not appear in any model with a ΔAIC < 2 was the climatic zone within which transects were situated.
Anecdotal accounts of past occurrence and abundance
Anecdotal evidence from scientific expeditions, local snail harvesters and guides was limited to the few locations that have harboured large populations of snails in the past. Nevertheless, all suggest that there has been a marked decline in A. bicarinata abundance and range in the past 5–10 years. For A Mesa (Site 3), the only place where snails were still abundant (Table 1), the presence of several middens containing many hundreds of snail shells suggested that populations are suffering heavy harvesting pressure. One midden contained an estimated 515 recognizable snail shells along with many others in a more advanced state of disintegration. Within the area covered by the transects there were a further two of similar size and two additional, smaller, middens containing 100–150 shells. Therefore, within this small area, c. 3,000 A. bicarinata individuals had been collected in recent years. Middens could potentially represent the build-up of shells over a long period of time. However, in this instance, the condition of many of the shells was similar, indicating that a only a few harvesting trips may have been responsible for the majority of the shells.
Traditionally, both Pico do Príncipe (Site 4) and Boca do Inferno (Site 5) have been visited by local snail harvesters as these sites have supported large snail populations. At Boca do Inferno no live A. bicarinata were observed, and snail harvesters commented that they instead have to collect from deeper in the forest around Pico do Príncipe, where population sizes remain higher. One guide stated that in 2002 at Pico do Príncipe A. bicarinata was so common that they were ‘like stones’. This site has been visited twice by scientific expeditions in the past 10 years, both of which noted the abundance of A. bicarinata. In 2001, an expedition collected 20 A. bicarinata individuals on a single night for dinner (Californian Academy of Sciences, 2001). Baillie (Reference Baillie1999) was ‘struck by the abundance of giant snails. At one point we were finding snails at one metre intervals.’ In contrast, we observed only a single live snail (Table 1).
Discussion
A. bicarinata is both more likely to occur and be more abundant at sites that are less accessible and at higher altitude. A similar pattern has been observed for the globally threatened birds of Príncipe (Dallimer & King, Reference Dallimer and King2008). A. bicarinata does not occur in human-modified landscapes. The snail is therefore harvested both opportunistically when people are in the forest and during specific collecting trips, both for personal consumption and commercial sale. The small size of Príncipe means that all the forest is within only a few km of the nearest entry points. Even snail populations at the more remote sites are therefore vulnerable to collection pressure from harvesters, local guides and research scientists. Nevertheless, the less accessible forest has acted as a refuge, allowing populations of A. bicarinata to persist.
As no previous systematic surveys of A. bicarinata have taken place, our data provide a baseline for future monitoring. Although we are only able to present anecdotal evidence of population decline the records indicate a population collapse. More accessible parts of the forest (e.g. Boca do Inferno) have lost almost their entire snail population and areas deep inside the forest, where snails were once abundant, now have markedly smaller populations (e.g. Pico do Príncipe and A Mesa). For other species seasonal changes in detectability can make comparing survey results from different times of the year problematic. Recent scientific expeditions to Pico do Príncipe took place in September (Baillie, Reference Baillie1999) and May (California Academy of Sciences, 2001), whereas our surveys were in December. Although it remains possible that A. bicarinata undergoes seasonal changes either in abundance or in detectability associated with its annual life cycle, it is unlikely that these could be responsible for the dramatic decline reported here.
Two species of snail are routinely collected for food on São Tomé and Príncipe. The introduced A. marginata is common throughout the agricultural areas of the island and never encountered in the forest, whereas A. bicarinata is restricted to primary forest. Formerly, however, when the extent of forest was greater, A. bicarinata may have occurred across a larger portion of the islands (Gascoigne, Reference Gascoigne1994b). A. marginata is a recent arrival on Príncipe (A. Gascoigne, pers. comm.), as it was not present in the early 1990s (Gascoigne, Reference Gascoigne1994a,Reference Gascoigneb), and has spread rapidly throughout the human-modified environments within the past 20 years. It is unknown what impact this introduction may have had on the distribution of A. bicarinata, and it is possible that A. marginata may have brought over an infectious disease or parasite to which A. bicarinata has no resistance (Gascoigne, Reference Gascoigne1994b).
In common with many terrestrial molluscs in island systems A. bicarinata is severely threatened on Príncipe. Although no formal surveys have been carried out on São Tomé, fieldworkers have reported a similar situation there and hence it is thus possible that A. bicarinata could rapidly become extinct. Even if the population collapse were limited to Príncipe, immediate action is required if the species is not to disappear from the island.
Príncipe currently retains a relatively large area of primary rainforest. The recent legal designation of this as a protected area could help secure A. bicarinata and, although management resources are limited, the forest covers a substantial region within which the conservation of the remaining populations can be prioritized. However, we recommend, firstly, that harvesting of snails from protected areas on both Príncipe and São Tomé is immediately banned (although enforcing the ban is likely to be difficult and would need to be carried out in conjunction with an awareness campaign). Secondly, the commercial collection and sale of A. bicarinata should be made illegal. Thirdly, harvesting for subsistence purposes should be limited to large individuals only; the precise measurements of such snails would need to be clarified by further research. If populations recover, some relaxation of these restrictions may be possible but this should only be done within the context of a good understanding of population dynamics and sustainable harvesting rates, possibly by implementing an adaptive management approach (McCarthy & Possingham, Reference McCarthy and Possingham2006). Fourthly, we appeal to scientists, ornithologists and tourists visiting these islands not to eat this endemic species and to express their concerns to guides and tour operators if they observe that snails are being collected for human consumption.
Following our survey the Department of Protected Areas for São Tomé and Príncipe has taken some action to promote the conservation of A. bicarinata. As part of the management plan for the protected areas currently being drafted by the government and ECOFAC (Programme de Conservation et Utilisation Rationale des Ecosystemes Forestiers en Afrique Centrale; a European Union-funded conservation programme for the forests of central Africa), A. bicarinata was chosen as one of a suite of indicator species that will be regularly surveyed to assess the effectiveness of the protected areas for biodiversity conservation. In addition, the NGO Associação Monte Pico will start work on Príncipe with snail harvesters, with the aim of retraining them as guides and support staff for the protected area.
Acknowledgements
We thank Eng. Salvador Sousa Pontes, Director of Protected Areas for São Tomé and Príncipe for arranging permits. On Príncipe, we are grateful to A. and K. Salvaterra, R. Delport and P. Bosman of the Bom-Bom Island Resort and the Society for Conservation and Development. Bikegila and Sátiro were indispensable guides in the forests. N. Collar and R. Prys-Jones supported our grant applications. M. Parnell extracted digital data. A. Gascoigne provided useful discussions. Z.G. Davies, D.C. Raheem, R.H. Cowie and an anonymous reviewer commented on an earlier version of this article. The fieldwork was funded by the University of Edinburgh’s Davis Expedition Fund, the British Ecological Society and the British Ornithologists’ Union.
Biographical sketches
Martin Dallimer is a conservation ecologist, working primarily in the UK and Africa. He has had a research interest in São Tomé and Príncipe for the past 8 years. Martim Melo has been working on the evolution and conservation of the endemic birds of the Gulf of Guinea islands since 1998.