The successful rearing of fish larvae depends on optimised first-feeding regimens and the nutritional quality of starter diets. Lipid has important roles in larval nutrition, as a source of metabolic energy, essential fatty acids that serve as precursors of important signalling molecules and as structural components of biological membranes(Reference Watanabe, Kitajima and Fujita1–Reference Izquierdo, Socorro and Arantzamendi3). Marine fish larvae that are fed diets containing TAG as the main lipid source show extensive lipid vacuolisation in the intestinal mucosa and liver, indicating that the transport of lipids from the intestine or liver into the blood is inhibited, reducing the availability and utilisation of lipids(Reference Fontagne, Geurden and Escaffre4–Reference Cahu, Zambonino Infante and Barbosa6). Supplementing diets with phospholipid (PL) alleviates these problems, indicating that fish larvae have limitations in synthesising PL required for the production of lipoproteins for the transport of lipids from the intestine(Reference Cahu, Zambonino Infante and Barbosa6–Reference Teshima, Kanazawa and Horinouchi9).
During digestion, dietary TAG is partially hydrolysed to NEFA and monoacylglycerol (MAG) or completely hydrolysed to NEFA and glycerol mainly by pancreatic bile-activated lipase in the gut lumen of fish(Reference Tocher and Sargent10–Reference Koven, Henderson and Sargent13). Pancreatic phospholipase A2 (PLA2) digests PL by catalysing the hydrolysis of the fatty acid ester bond at the SN2 position of PL, with the production of a NEFA and a lysophospholipid (lysoPL)(Reference Izquierdo, Socorro and Arantzamendi3). Also, the activity of PLA2 depends on the presence of bile salts(Reference Nordskog, Phan and Nutting14). The digested products of bile-activated lipase and PLA2, e.g. MAG, NEFA, glycerol and lysoPL, are absorbed across the brush-border membrane and into the enterocytes, mainly by passive diffusion(Reference Johnsen15, Reference Tocher, Bendiksen and Campbell16). The majority of the lysoPL and MAG are re-esterified with activated NEFA in the microsomes of the enterocytes before export to the circulatory system(Reference Kanazawa, Teshima and Inamori17–Reference Olsen, Henderson and Pedersen20). De novo synthesis of PL and MAG also occurs in the enterocyte of fish, but it is uncertain to what extent this process is active in marine fish larvae.
Most studies have reported that marine fish larvae have lipase activity at the onset of first feeding, and that the activity and gene expression do not increase as the larvae develop(Reference Hoehne-Reitan, Kjørsvik and Gjellesvik21–Reference Pérez-Casanova, Murray and Gallant25). This indicates that fish larvae have sufficient capacity in lipid digestion. However, most of these studies have focused mainly on the early development, until maximum 43 d post-hatch, while there are indications that the digestive system in cod continues to develop at least until 100 d post hatch. Sæle et al. (Reference Sæle, Nordgreen and Olsvik26, Reference Sæle, Nordgreen and Olsvik27) showed that the gene expression of bile-activated lipase and PLA2 in cod increased steeply from approximately 50 to 80 d post hatch, while the enzyme activities did not show the same increase.
A recent study in Atlantic halibut larvae by Mollan et al. (Reference Mollan, Tonheim and Hamre28) demonstrated that hydrolysed lipids were more efficiently absorbed and utilised than intact lipids when fed directly to larvae by tube feeding. The larvae increased the absorption of lipids in the order TAG < diacylglycerol (DAG) = phosphatidylcholine (PC) < MAG, indicating that pre-hydrolysis improves absorption of neutral lipids in the larvae of this species. Morais et al. (Reference Morais, Koven and Rønnestad29–Reference Morais, Rojas-Garcia and Conceicao31) found conflicting results when feeding Senegalese sole and herring larvae with different lipid classes.
The hypothesis of the present study was that cod larvae have limited capacity for lipid digestion, and that supplementation of pre-digested dietary lipid would increase absorption. To investigate this, cod larvae were fed a single meal with diets containing hydrolysed TAG (hTAG), hydrolysed phosphatidylcholine (hPC), intact TAG (iTAG) or intact phosphatidylcholine (iPC), and the fatty acids in the studied lipid fraction contained a radioactive 14C tracer. After feeding, the larvae were incubated in individual chambers for 10 h with aeration and collection of CO2(Reference Rønnestad, Rojas-Garcia and Tonheim32). A metabolic budget was constructed based on the mass balance of the tracer evacuated into the water, present in the intestine, retained in the body carcass and catabolised to CO2.
Materials and methods
Preparation of the basic diet
The basic diet was prepared at the Institute of Marine Research, Austevoll, Norway, using an agglomeration technique. The ingredients of the non-lipid portion of the diet are given in Table 1. The dry components were mixed (Spar Mixer Fuji Paudal Company Limited, Osaka, Japan) for about 5 min. Then, 20 % of distilled water and 80 % of dry mixture were combined. The mixture was transferred to the extruder (Multi Gran, Fuji Paudal Company Limited) with a 0·5 mm screen and screws turning at 20 rpm. The diet strings were transferred to a Marumeriser (Fuji Paudal Company Limited) and spun at 300 rpm for 15–20 s to make the strings shorter and spherical in shape. The small pellets of 1–3 mm collected from the Marumeriser were frozen in liquid N2, transferred to a Centrifugal Mill (R-etsch-ZM 200; RETSCH Norge AS, Sunnfjord, Norway) and centrifuged at 18 000 rpm, using a 1000 μm ring sieve. The pellets were then passed through different sieves (200, 315, 400 and 560 μm) and freeze-dried. The pellet size used for this experiment was 200–315 μm.
Table 1 Composition of the experimental diets (g/kg dry weight)*

* Lipids added at the end.
† Codrico, BAMA, Oslo, Norway.
‡ Rieber fish powder GVP (Rieber & Søn ASA, Bergen, Norway).
§ Rieber squid meal (Rieber & Søn ASA, Bergen, Norway).
∥ As recommended by NRC (1993). The vitamins and astaxanthin are from Hoffman la Roche (Basel, Switzerland) and the minerals are from Merck (Darmstadt, Germany).
¶ Radiolabelled lipids (ARC, St Louis, MO, USA); cold lipids (Sigma-Aldrich, Steinheim, Germany).
Preparation of lipids and addition to the basic diet
To the four experimental diets was added 15 % lipid, of which 40 % was polar (PC) and 60 % was neutral lipid (TAG) and contained different combinations of intact and hydrolysed TAG and PC with an added 14C tracer (Table 2). Radiolabelled TAG (triolein [carboxyl-14C]) and PC [dioleoyl-1-14C] were obtained from ARC (St Louis, MO, USA). The labelled lipids were mixed with unlabelled lipids (triolein: C57H104O6 18:1; Sigma-Aldrich, Steinheim, Germany and PC: lipoid 18:1; Lipoid GmbH, Ludwigshafen, Germany). All lipids added as oil to the diets, both labelled and unlabelled, contained only oleic acid. The labelled lipids added constituted an insignificant part of the total dietary lipid.
Table 2 Composition of lipids in the experimental diets (%)
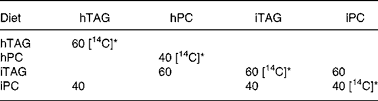
hTAG, hydrolysed TAG; hPC, hydrolysed phosphatidylcholine; iTAG, intact TAG; iPC, intact phosphatidylcholine.
* [14C] indicates lipids where the fatty acids are labelled with 14C in the acid group.
For the diet containing hTAG, labelled triolein [carboxyl-14C] (0·3 MBq/g diet) and unlabelled triolein, which added up to 0·9 g, were hydrolysed with 5 μl lipozyme (Lipozyme TL, Novozymes, A/S, Bagsvaerd, Denmark) in 160 μl distilled water at 55°C for 6 h in a water-bath. Thereafter, 0·6 g cold PC and 200 μl ethyl acetate were added, and the solution was mixed until PC was dissolved. Finally, 200 μl sodium acetate buffer (50 mm, pH 5·6) were added.
The diet containing hPC contained a total of 0·6 g labelled (0·3 MBq/g diet; PC [dioleoyl-1-14C]) and unlabelled PC. A minimum amount of ethyl acetate was used to dissolve PC, and an equal amount of sodium acetate buffer (50 mm, pH 5·6) was added. PC was then hydrolysed with 3 μl PLA2 (Sigma-Aldrich, St Louis, MO, USA) at 30°C for 6 h. Finally, 0·9 g TAG was added with 200 μl ethyl acetate and 200 μl sodium acetate buffer.
In the diet with iTAG, 0·9 g labelled (0·3 MBq/g diet; triolein [carboxyl-14C]) and unlabelled TAG were mixed with 0·6 g unlabelled PC (lipoid 18:1), 200μl ethyl acetate and 200 μl sodium acetate buffer (50 mm, pH 5·6).
In the diet with iPC, 0·6 g labelled (0·3 MBq/g diet; PC [dioleoyl-1-14C]) and unlabelled PC were mixed with 0·9 g TAG (triolein 18:1) in 200 μl ethyl acetate until dissolved. Finally, 200 μl sodium acetate buffer (50 mm, pH 5·6) were added.
Lipid (1·5 g) dissolved in ethyl acetate and tocopherol (200 mg/kg) added as an antioxidant were mixed with 8·5 g of freeze-dried pellets in a beaker. The pellets were left to absorb the lipid for 20 min, and excess ethyl acetate was evaporated using N2 gas. The feed was then freeze-dried and stored at − 20°C until it was fed to cod larvae.
Fish larvae
The experiment was conducted in accordance with the Animal Welfare Act of 12 December 1974, nr 73, §§22 and 30, amended 17 November 1998. Atlantic cod larvae, 40 d post-hatch, with a wet weight of 4·53 (sd 1·02) mg and a length of 9·75 (sd 0·63) mm (n 10) were obtained from the Institute of Marine Research (Austevoll, Norway). The larvae were transported (2 h) to the laboratory 2 d before the trial, in oxygenated water and at a constant temperature of 11°C. The whole experiment was performed in a cold room at 10°C. After transport, the larvae were gently transferred to four buckets, containing 5 litres of aerated seawater, collected 1 d before the larvae were obtained, filtered through a 100 μm sieve and kept in the cold room. To each bucket, sixty larvae were transferred with some water. From 2 h after the transfer and for the next 2 d, the larvae were fed a diet with iTAG and PC but with no tracer being added. The feeding was stopped one night before the experiment. Siphoning and replacement of 50 % seawater were done twice a day. On the day of the experiment, cod larvae in the four buckets were fed one of the four different radiolabelled diets, hTAG, hPC, iTAG or iPC, respectively, for 30 min. Using a pipette of 5 ml volume with a tip cut at the top, ten larvae were collected from each bucket. The fish larvae were rinsed by immersing them twice in 500 ml clean seawater before transfer to the incubation chambers (one larva per chamber), which contained 5 ml seawater and were supplied with aeration and a CO2 trap according to Rønnestad et al. (Reference Rønnestad, Rojas-Garcia and Tonheim32). All the labelled diets were then siphoned out of the buckets, and 75 % of the water was replaced with clean seawater. The remaining larvae were kept in the buckets for 10 h. After 10 h, the larvae were removed from the incubation chambers using forceps. The larvae were killed with an overdose of metacain, and the gastrointestinal tract without liver and the remaining carcass including liver were separated by dissection under a light microscope. The gastrointestine and the carcass, including liver, were transferred to two 15 ml glass vials, dissolved in 1 and 3 ml of Solvable (PerkinElmer Life and Analytical Sciences, Boston, MA, USA), respectively, and kept for 1 d at room temperature. The incubation water and the water in the CO2 trap were collected as described by Rønnestad et al. (Reference Rønnestad, Rojas-Garcia and Tonheim32). At the same time, twenty larvae (two pooled samples of ten larvae) were collected from each bucket for the analysis of lipid classes using high-performance TLC.
To interpret and understand the mechanisms underlying the tracer data, a compartmental analysis was used(Reference Rønnestad, Rojas-Garcia and Tonheim32–Reference Conceicao, Ribeiro and Engrola34). This method provides a useful framework for investigating digestibility, gut absorption rates, catabolism and retention (assimilation) of lipids in the present experiment. The approach (Fig. 1) consists of a mass balance method that follows the compartmental distribution of the radioactive tracer that was fed to a larva as a single meal and that cod larvae were allowed to ingest for a period that is shorter than the gut transit time. The sum of the tracer in all compartments represents the ingested amount (I; Fig. 1). The larvae were sampled when the gut was emptied of visible contents, and the tracer in the incubation water thus represents unabsorbed lipid (evacuated, E). The label in the gut (G; Fig. 1) is most probably absorbed and incorporated into the intestinal tissue(Reference Rønnestad, Kamisaka and Conceição33). Using a conservative approach, the sum of the tracer metabolised (CO2, C) and retained in the carcass (R) was taken to represent the absorbed amount (A; Fig. 1).

Fig. 1 A compartmental model for describing the fate of ingested (I) dietary lipids added as a tracer in Atlantic cod larvae. The model is based on scintillation counting of the dissected larva (gut (G) and carcass) fed 14C-labelled lipids in combination with analysis of incubation water (E) and metabolic trap (C). See text for further information.
Analytical methods
The larval and water samples collected from the incubation studies were analysed by a liquid scintillation analyser (TRI-CARB, Pacard Model 2300TR; Meriden, CT, USA), as described by Rønnestad et al. (Reference Rønnestad, Rojas-Garcia and Tonheim32).
Dietary lipid content was determined gravimetrically as the sum of free and bound fat. Free or loosely bound fat was extracted with petroleum ether and dried at 103 ± 1°C. The samples were thereafter hydrolysed with HCl in a Tecator Soxtec Hydrolysing unit to release the bound fat, which was extracted with petroleum ether and dried at 103 ± 1°C. The reproducibility of the method has been determined as 2 sd = 3 % of mean, corresponding to 4·5 mg/g.
Lipid class composition in the diets was analysed using high-performance TLC, as described by Bell et al. (Reference Bell, Dick and McVicar35) and Jordal et al. (Reference Jordal, Lie and Torstensen36). The plates (20 × 10 cm) were developed at 5 cm in methyl acetate–isopropanol–chloroform–methanol–0·25 % (w/v) aqueous KCl (25:25:25:10:9, by vol.) to separate PL classes from neutral lipids running at the solvent front(Reference Vitello and Zanetta37). After drying, the plates were developed fully in isohexane–diethyl ether–acetic acid (80:20:1·5, by vol.) to separate neutral lipids and cholesterol. Lipid classes were visualised by charring at 160°C for 15 min after spraying with 3 % copper acetate (w/v) in 8 % (v/v) phosphoric acid and identified by comparison with commercially available standards. Lipid classes were quantified by scanning densitometry using a CAMAG TLC Scanner 3 and calculated using an integrator (WinCATS-Planar Chromatography, version 1.2.0).
To describe the incorporation of the different dietary lipids into body tissues, lipid from the diets and the whole larvae sampled from the bucket after 10 h was extracted using 4 ml chloroform–methanol (2:1, v/v)(Reference Folch, Lees and Stanley38). The solvent was evaporated, and the lipids were resuspended to 5 mg/ml in chloroform. Extracted lipid (50 μl) from the diets and the whole body of larvae and 10 μl standard (lysophosphatidylcholine (LPC), sphingomyelin, PC, phosphatidylinositol, phosphatidylethanolamine, NEFA, TAG, MAG and DAG from Sigma, cardiolipin and cholesteryl esters from Avanti) were loaded in lanes of 3 cm in pre-dried silica plates (10 × 20 cm; Merck KGaA, Darmstadt, Germany). The plates were developed for the separation of the polar lipids as described for the diets. To separate the neutral lipids, the same plate was developed in isohexane–diethyl ether–acetic acid (85:15:1, by vol.). The silica plate was then dried with N2, and distinct bands representing different lipid classes were visualised using I (I-3380; Sigma-Aldrich, Steinheim, Germany). Each band was carefully scraped off and transferred into 20 ml scintillation vials with 8 ml scintillation cocktail (Lumasafe, Lumac-LSC B.V. Groningen, The Netherlands) and counted in the liquid scintillation analyser.
Calculations and statistics
For the metabolic study, each larva was incubated, dissected and analysed individually, and each larva constitutes one replicate for the study of the distribution of a radioisotope in different compartments. The sum of radioactivity found in all four compartments (E, G, R and C; Fig. 1) was used as a measure of the total ingested label (I), and the sum of radioactivity in C and R was taken as a measure of the absorbed diet (A). Based on the analysis of weight-specific radioactivity for the lipid in each diet, the compartmental model is calculated in μg and in percentage of the total ingested (E and G) or absorbed (R and C) diet.
The software Statistica version 9 (StatSoft, Inc., Tusla, OK, USA) was used for statistical treatments. The raw data were first tested for outliers. Data on feed intake and weight-specific radioactivity in the diets were analysed with a one-way ANOVA, and data on incorporation of radioactivity in body lipid classes were analysed with a nested ANOVA using triplicate analyses nested in the two extracted samples and Tukey's post hoc test. Data used in the ANOVA analyses were tested for homogeneous variances using Levene's test and log-transformed in the cases of significant results. In Figs. 5 and 6, 100 % absorption and catabolism, respectively, are given as y = 100 when calculated in % and as y = x when calculated in μg. The data at low feed intakes were approximated to these equations. The data at high feed intakes were subjected to linear regression, and the breakpoints between these regressions and the equations y = 100 and y = x were calculated from the regression equations. The breakpoints indicate the limits of capacity for the absorption and catabolism of the different lipids.
Results
The analysis shows that TAG in the hTAG diet was almost totally hydrolysed to glycerol and NEFA, since TAG and DAG were not detectable and the concentration of NEFA was more than 30 % of the total lipid (Fig. 2(A)). MAG is not detected by the analytical method applied, since it elutes with the solvent front. The enzyme seems also to have hydrolysed PC, since PC in the hTAG diet was less than half of that in the iTAG and iPC diets and LPC was present at a similar level as in the hPC diet. Hydrolysis of PC in the hPC diet were more successful, with a dramatic reduction in PC, a corresponding increase in LPC and NEFA and a limited effect on TAG (Fig. 2(A)). In the iTAG and iPC diets, the lipid was mainly present as iTAG and PC; however, the planned ratio of 40 % polar lipid and 60 % neutral lipid was not achieved (Fig. 2(B)). Rather, the ratio was approximately 60:40 in all the diets except the hPC diet, where PC was hydrolysed to LPC and NEFA, and the latter was categorised as a neutral lipid. The weight-specific content of the tracer in each of the four diets was (cpm/mg) 4453 (sd 32) (hTAG), 4570 (sd 340) (hPC), 3507 (sd 60) (iTAG) and 4076 (sd 18) (iPC). The activity was similar in the hTAG and hPC diets, while the iTAG and iPC diets had activities different from all the other diets (ANOVA; P < 0·03). The dietary lipid levels (mg/g) were 160 (hTAG), 132 (hPC), 149 (iTAG) and 148 (iPC).

Fig. 2 (A) Lipid class distribution of the dietary lipid (weight % of the total lipid). (B) Neutral and polar lipid (weight % of the dietary lipid) in the experimental diets. (A) LPC, lysophospholipid; SM, sphingomyelin; PC, phosphatidylcholine; PS, phosphatidylserine; PI, phosphatidylinositol; PE, phosphatidylethanolamine; DAG, diacylglycerol; CHOL, cholesterol; hTAG, 14C in hydrolysed TAG (![]() ); hPC, 14C in hydrolysed phosphatidylcholine (
); hPC, 14C in hydrolysed phosphatidylcholine (![]() ); iTAG, 14C in intact TAG (
); iTAG, 14C in intact TAG (![]() ); iPC, 14C in intact phosphatidylcholine (
); iPC, 14C in intact phosphatidylcholine (![]() ). (B)
). (B) ![]() , Sum phospholipids;
, Sum phospholipids; ![]() , sum neutral lipids (NL).
, sum neutral lipids (NL).
Each cod larva ingested 16–29 μg diet, equivalent to 3·4–5·2 % of their dry body weight. The larvae fed the iTAG diet consumed a significantly higher amount of diet than those that received the hydrolysed diets (Fig. 3), hTAG (P = 0·001) and hPC (P = 0·004). The larvae fed the diet with iPC consumed an intermediate amount of diet (Fig. 3(A)). Also, the consumption of diet in percentage of larval dry body weight was higher in the larvae fed the iTAG diet, than that in the larvae fed the hTAG diet (P = 0·05). The larvae fed the hPC and iPC diets had intermediate feed intake when calculated as percentage of body weight (Fig. 3(B)).

Fig. 3 Food ingestion in the larvae fed the four diets. (A) Diet eaten per larva (μg). (B) Diet eaten in percentage of larval dry body weight. a,b Values with unlike letters were significantly different (P < 0·05). hTAG, 14C in hydrolysed TAG; hPC, 14C in hydrolysed phosphatidylcholine; iTAG, 14C in intact TAG; iPC, 14C in intact phosphatidylcholine.
The amount of lipid evacuated was small and ranged from 0 to 0·45 μg, representing from 0 to 16 % of the dietary lipid ingested by the larvae (Fig. 4). There was no significant relationship between evacuation and the amount of lipid eaten per larvae, and no differences in evacuation were observed between larvae fed the different diets.

Fig. 4 Lipid evacuated (μg) as a function of the amount of lipid ingested per larva (μg). Cod larvae were fed labelled lipid and incubated for 10 h. hTAG, 14C in hydrolysed TAG (●); hPC, 14C in hydrolysed phosphatidylcholine (![]() ); iTAG, 14C in intact TAG (
); iTAG, 14C in intact TAG (![]() ); iPC, 14C in intact phosphatidylcholine (
); iPC, 14C in intact phosphatidylcholine (![]() ).
).
Absorption calculated as percentage of lipid ingested was more than 85 % in all larvae fed the hTAG diet (Fig. 5(A)). For the other diets, the larvae that ate less than 1·9–2·1 μg lipid (Table 3) absorbed all (99–100 %) the ingested lipid, while above these feed intakes there was reduced absorption described as a linear relationship between lipid intake and percentage of absorption. The slopes of the equations indicate that a larger fraction of iTAG was absorbed than that of iPC and hPC (Table 3). All larvae absorbed 55 % or more of the dietary lipid. Absorption of 100 % of the dietary lipid would infer that the absorbed lipid is equal to the ingested lipid (y = x). This relationship is given in Fig. 5(B) and is an approximation of the situation at low feed intakes. When the larvae ate more than 2·4–2·7 μg lipid (Table 3, breakpoint), there was still a linear relationship between the absorbed and ingested lipid, but the slopes were < 1, with the larvae fed the iTAG diet having a steeper slope than the larvae fed the hPC and iPC diets (Fig. 5(B); Table 3). The majority of the lipid not absorbed to the carcass of the larvae was retained in the gut tissue, since evacuation accounted for only a maximum of 16 % of the ingested lipid and since visual observation indicated that no food was left in the gut lumen after 10 h incubation. This means that iPC and hPC were retained in the gut tissue to a greater extent than iTAG at high feed intakes (Fig. 5(A) and (B)).

Fig. 5 Absorbed lipid (retained+metabolised to carbon dioxide as a function of the amount of lipid ingested per larva (μg). Absorbed lipid in % (A) and μg (B) of lipid eaten per larva. Fitted equations are given in Table 3. Cod larvae were fed radioactively labelled lipid and incubated for 10 h. hTAG, 14C in hydrolysed TAG (![]() ); hPC, 14C in hydrolysed phosphatidylcholine (
); hPC, 14C in hydrolysed phosphatidylcholine (![]() ); iTAG, 14C in intact TAG (
); iTAG, 14C in intact TAG (![]() ); iPC, 14C in intact phosphatidylcholine (
); iPC, 14C in intact phosphatidylcholine (![]() ).
).
Table 3 Regression analyses of lipid absorbed into the carcass (y) in relation to lipid ingested (x) and lipid catabolised to carbon dioxide (y) in relation to lipid absorbed (x)*
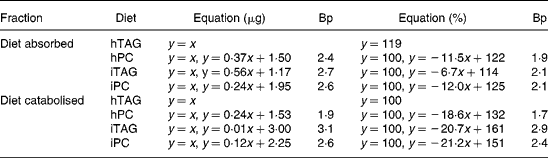
Bp, breakpoint where the regression line for high feed intakes crosses the line where all the lipid is absorbed or catabolised (y = x or y = 100, approximation for low feed intakes); hTAG, hydrolysed TAG; hPC, hydrolysed phosphatidylcholine; iTAG, intact TAG; iPC, intact phosphatidylcholine.
* The absorbed or catabolised fraction is expressed in μg lipid or in percentage of eaten or absorbed lipid. See figure legends for further information.
At low amounts of the absorbed lipid, almost 100 % of the absorbed lipid was catabolised to CO2. With a higher lipid absorption (above the breakpoints), the percentage of lipid catabolised decreased in parallel for the three diets with a corresponding increase in the percentage of lipid retained in the larval body (Fig. 6(A); Table 3). Breakpoints were 1·7–1·9 for hPC, 2·9–3·1 for iTAG and 2·4–2·6 for iPC, dependent on whether the calculation was done in μg or %, while 91–100 % of hTAG was catabolised due to a low feed intake (Table 3). The minimum fraction of the absorbed lipid that was catabolised was 60 %. Retention increased at lower lipid intakes when the larvae were fed hPC, compared with iPC, and when the larvae fed iPC, compared with iTAG, as also indicated by the differences in breakpoints (Fig. 6(B); Table 3). When 100 % of the absorbed lipid is catabolised, the amount of the catabolised lipid equals the amount of the absorbed lipid (y = x). This was the case when the amount of the absorbed lipid was below the breakpoints for the different diets (Fig. 6(B)). Above the breakpoints, catabolism of the lipid showed a minor or no increase, and the rest of the absorbed lipid was retained in the body carcass. hPC was retained to a greater extent than iPC, which was retained to a greater extent than iTAG (Fig. 6(A) and (B); Table 3).

Fig. 6 Lipid metabolised to carbon dioxide as a function of the absorbed lipid (μg). The data are given as μg lipid (A) and as percentage of absorbed lipid (B), respectively. Fitted equations are given in Table 3. Cod larvae were fed radioactively labelled lipid and incubated for 10 h. hTAG, 14C in hydrolysed TAG (![]() ); hPC, 14C in hydrolysed phosphatidylcholine (
); hPC, 14C in hydrolysed phosphatidylcholine (![]() ); iTAG, 14C in intact TAG (
); iTAG, 14C in intact TAG (![]() ); iPC, 14C in intact phosphatidylcholine (
); iPC, 14C in intact phosphatidylcholine (![]() ).
).
The polar lipids in the larval body were pooled into four different groups before the radioactivity was counted: LPC/sphingomyelin; PC; phosphatidylserine/phosphatidylinositol/cardiolipin; phosphatidylethanolamine. Between 10 and 40 % of the labelled body lipids were in PC, which was the polar lipid class containing the highest level of tracer. The larvae fed the iPC diet had a significantly higher level of label in PC than those fed the other diets (Fig. 7(C); P < 0·005). The larvae fed the hPC diet also had more isotope in PC than the larvae fed the iTAG and hTAG diets (P < 0·01). There were no significant differences in the level of the tracer in LPC/sphingomyelin, phosphatidylserine/phosphatidylinositol/cardiolipin or phosphatidylethanolamine among the larvae fed the four diets (Fig. 7(A), (B) and (D)).
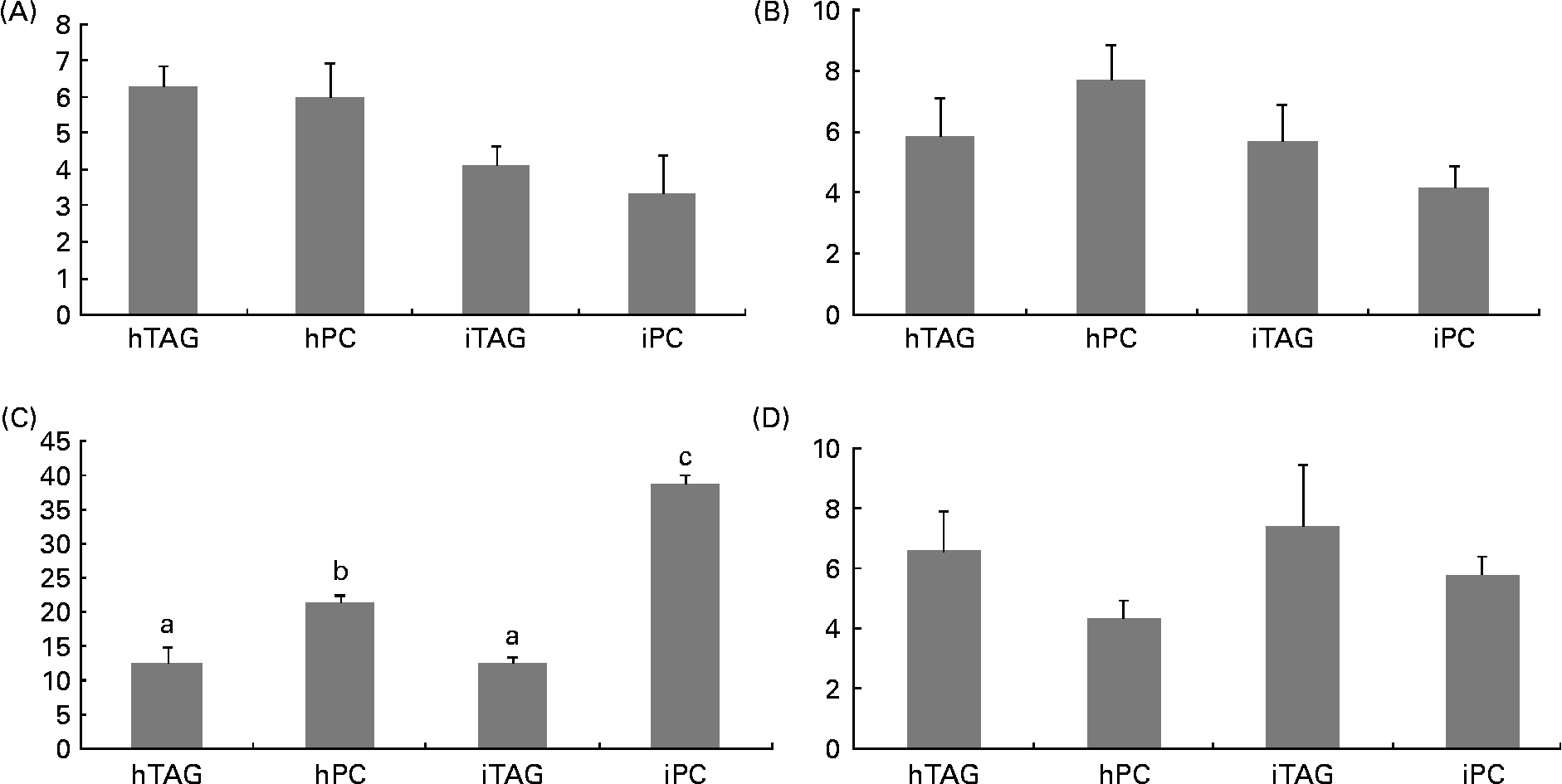
Fig. 7 Radioactivity in the polar lipid classes (% of total lipid radioactivity) in the whole body of cod larvae fed the four different diets and incubated for 10 h. (A) Phosphatidylserine, phosphatidylinositol, cardiolipin (PS/PI/CL). (B) Lysophosphatidylcholine and sphingomyelin (LPC/SM). (C) Phosphatidylcholine (PC). (D) Phosphatidylethanolamine (PE). Values are means of two pooled samples of ten larvae each with two technical replicates. The results were analysed using a nested ANOVA. a,b Mean values with unlike letters were significantly different (P < 0·05). hTAG, 14C in hydrolysed TAG; hPC, 14C in hydrolysed phosphatidylcholine; iTAG, 14C in intact TAG; iPC, 14C in intact phosphatidylcholine.
Five different classes of neutral lipids from the larval body were separated and analysed: MAG; DAG/cholesterol; TAG; NEFA; cholesteryl esters. The reason that MAG is detected here and not in the diet analyses is the difference in detection methods, e.g. colour density and radioactivity. TAG and NEFA contained most of the label in the neutral lipids ranging from 10 to 35 % and from 10 to 45 % of the label in total lipids, respectively (Fig. 8(C) and (D)). The larvae fed the iTAG diet had a higher level of labelled TAG in the body lipids than the larvae fed the hTAG and iPC diets (P < 0·005), but similar to the larvae fed the hPC diet. The larvae fed the hPC diet also had a higher level of labelled TAG than the larvae fed the hTAG diet (P = 0·005). Compensating for the low levels of labelled TAG, the larvae fed the hTAG diet had high levels of labelled NEFA, dramatically higher than in the larvae fed the other diets (45 and 10–20 %, respectively; P < 0·001). The larvae fed the iTAG diet also had higher levels of marker in NEFA than the larvae fed the iPC diet (P = 0·04). There were no differences in labelling of the whole-body MAG, DAG and cholesteryl esters between the larvae fed the different diets (Fig. 8(A), (B) and (E)).
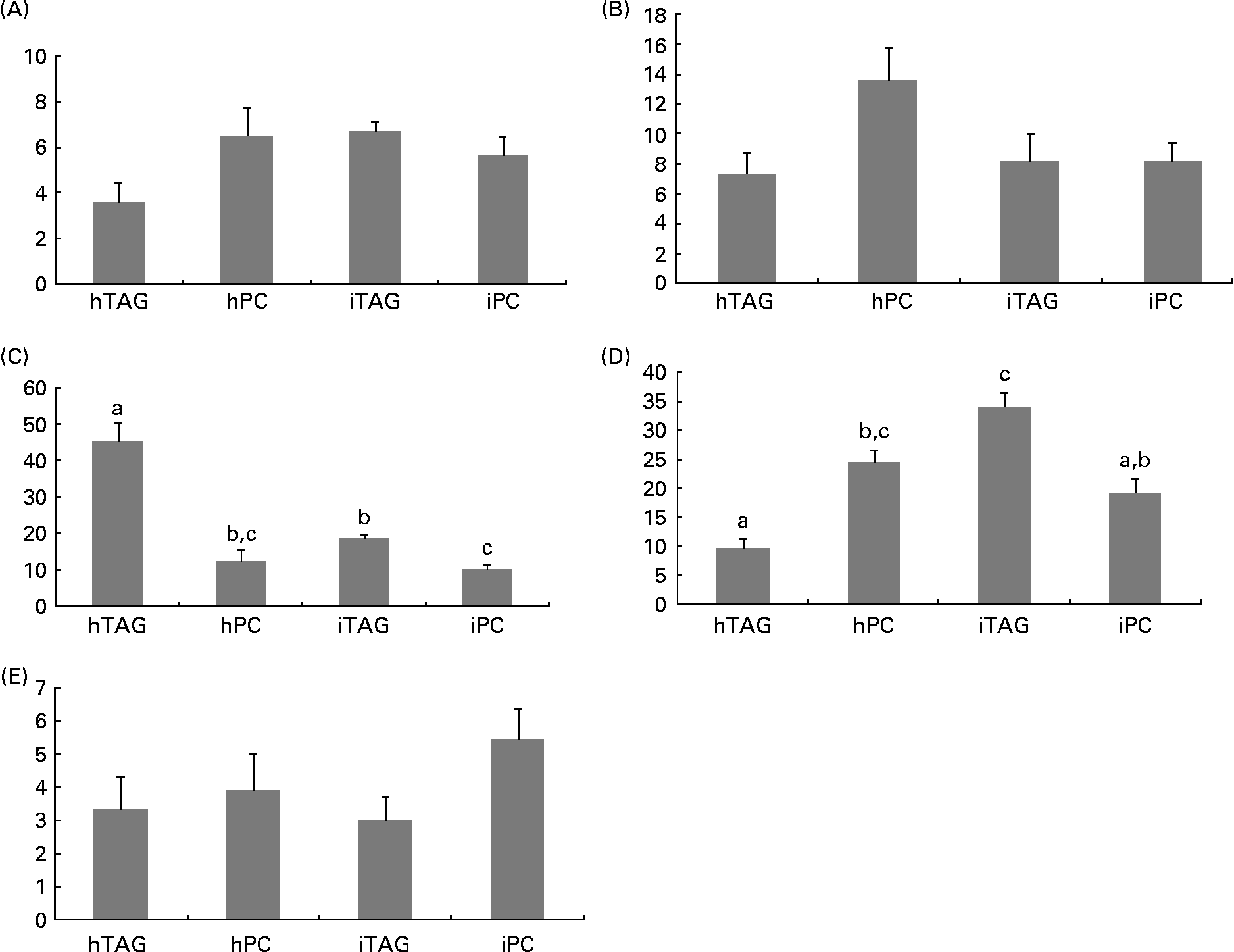
Fig. 8 Radioactivity in the neutral lipid classes (% of total lipid radioactivity) in the whole body of cod larvae fed the four different diets and incubated for 10 h. (A) Monoacylglycerol (MAG); (B) diacylglycerol (DAG); (C) NEFA; (D) TAG; (E) cholesteryl esters (CE). Values are means of two pooled samples of ten larvae each with two technical replicates. The results were analysed using a nested ANOVA. a,b,c Mean values with unlike letters were significantly different (P < 0·05). hTAG, 14C in hydrolysed TAG; hPC, 14C in hydrolysed phosphatidylcholine; iTAG, 14C in intact TAG; iPC, 14C in intact phosphatidylcholine.
Discussion
The present study showed that 40-d-old cod larvae digested and absorbed both intact and hydrolysed dietary lipids well. The larvae absorbed 55–100 % of the dietary lipids ingested, and at low feed intakes the absorption was at or close to 100 %. At higher feed intakes, there were slight differences between the diets, where the iTAG diet appeared to be absorbed with a higher efficiency than the iPC and hPC diets; conversely, there were slightly more lipids still present in the gut tissue of the larvae fed the iPC and hPC diets than in the larvae fed the iTAG diet. However, the most important determinant of the fraction of diet absorbed was definitely the feed intake. The larvae fed the hTAG diet had a low feed intake (16 μg/larva, 3·5 % of body dry weight) and approximately 100 % of this diet was absorbed in all cases. The results are different from those observed in Atlantic halibut larvae by Mollan et al. (Reference Mollan, Tonheim and Hamre28), where MAG and DAG were absorbed to a much greater extent than TAG. The TAG was absorbed at only approximately 20 %, which is very low compared with the absorption rates in the present study. In the studies by Morais et al. (Reference Morais, Koven and Rønnestad29–Reference Morais, Rojas-Garcia and Conceicao31), the variation in lipid absorption was probably caused by tube feeding of pure oil, instead of giving the lipid as an emulsion or in a complete diet. This may have caused reduced digestion and absorption due to a very low surface:volume ratio of the dietary lipid.
In the hTAG diet, TAG was completely hydrolysed to glycerol and NEFA, instead of remaining as MAG and NEFA. The neutral lipase employed for the hydrolysis of TAG also hydrolysed PC, resulting in lowered iPC and increased LPC in this diet. The hPC diet contained LPC, NEFA and iTAG, while the iPC and iTAG diets contained iTAG and iPC. The polar:neutral lipid ratios were higher than formulated, probably due to the contribution of PL from the white fishmeal. However, this apparent error was the same for all diets and will have had little effect on the results of the experiment.
The contents of NEFA in the hydrolysed diets, and especially in the hTAG diet, were different from the diet compositions in Mollan et al. (Reference Mollan, Tonheim and Hamre28), where the radioactive MAG and DAG were collected from a TLC plate after separation of the lipid classes resulting from hydrolysis of the labelled oil. In the study by Mollan et al. (Reference Mollan, Tonheim and Hamre28), labelled MAG, DAG and TAG were administered to Atlantic halibut larvae in an emulsified TAG vector, serving the larvae with a large portion of oil in addition to the traced lipid type. In the present study, the lipid was fed as part of a complete diet, possibly providing more normal conditions for digestion, absorption and metabolism of the labelled lipid. Another difference between the two experiments is of course the difference in fish species.
The feed intake appeared higher in the larvae fed the intact diets compared with that in the larvae fed the hydrolysed diets, although there was a significant difference only between the hTAG and iTAG diets. It is possible that NEFA provide a poor taste and odour to the diet, and that this resulted in reduced feed intake with the hydrolysed diets. NEFA may also have toxic effects on cells(Reference Adas, Berthou and Salaun39, Reference Feldstein, Werneburg and Canbay40), and their addition to the diets may have negatively affected the larval condition (Ø. Sæle, unpublished results), which possibly could also reduce the feed intake. The low feed intake in the larvae fed the hTAG diet led to the absence of saturation of the digestive, absorption and metabolic systems handling lipids in the larvae fed this diet, as opposed to the larvae that ate the largest amounts of the other diets.
The maximum evacuated amount (E; Fig. 1) for any diet was 16 % of the ingested amount, and most of the larvae evacuated less than 8 % of the ingested lipid. Furthermore, E was similar in the larvae fed the different diets, and there was no relationship between the feed intake and evacuation. Not much is known about the regulation of evacuation in larval fish, but when the fish are fed a single meal with unlimited time for processing the feed, both secretion of enzymes and bile, digestion and motility can in theory be optimised to maximise the absorption of nutrients. Previous studies(Reference Tonheim, Espe and Hamre41) have demonstrated that intestinal loading will reduce the processing efficiency of digestible feedstuffs, and in some cases the chyme is evacuated before the digestion is complete. Further studies should be conducted to explore the mechanisms that regulate motility and evacuation in fish larvae.
The slope of the absorbed (Fig. 5(B)) to ingested lipid at high feed intakes was steeper for the larvae fed the iTAG diet than that for the larvae fed the hPC and iPC diets, but the breakpoint of the functions with the function y = x seemed similar for the three diets. This indicates that the higher the feed intake, the larger the fraction of lipid present in the gut, and that more lipid was retained and most probably incorporated into the gut tissue (G; Fig. 1) of the larvae fed the iPC and hPC diets than in the larvae fed iTAG. Mollan et al. (Reference Mollan, Tonheim and Hamre28) also found that dietary PC to a greater extent than neutral lipids accumulated in the gut of Atlantic halibut larvae, and suggested that PC is used directly in the intestine for the formation of chylomicrons and for the delivery of membrane lipids for the cells in the gut epithelium, which are believed to have a very high turnover(Reference Krogdahl, Waagbo, Espe, Hamre and Lie42). The reason that the breakpoints were slightly different when absorption was calculated in μg compared with % is unknown. All the diets had a similar lipid class composition, but the label was situated in different lipid classes in the different diets. The fact that there was such a similarity between the breakpoints for the larvae fed the different diets therefore suggests that lipid digestion and absorption occurs as a well-coordinated process where the different lipid classes are processed in concert, so that when the capacity for processing the polar lipids is filled, neutral lipid processing is also reduced.
The amount of the catabolised lipid increased linearly up to breakpoints of 1·7–1·9, 2·4–2·6 and 2·9–3·1 μg lipid absorbed for the hPC, iPC and iTAG diets, respectively, dependent on the calculation in % or μg. Here there was only a minor difference in the breakpoint between the calculation methods, but a clear difference between the larvae fed the different diets was observed. At lower lipid absorptions, almost 100 % of the absorbed lipid was converted to CO2, while at lipid absorptions above the breakpoints, additional lipid was almost exclusively retained in the carcass. Catabolism levelled off at a higher level for iTAG than for iPC and higher for iPC than for hPC. Conversely, more hPC than iPC and more iPC than iTAG were retained in the larval body. Cod larvae probably first cover their energy requirement for maintenance. Keeping in mind that the larvae were fed a complete diet where only the studied fraction was labelled, one can speculate that the larvae fed the hPC diet, containing the labelled fatty acid in lysoPL, would use all the labelled fat for the synthesis of membranes. The larvae fed the iPC diet would have one labelled fatty acid available for energy production for each lysoPL used for membrane building. This could explain the higher catabolism of the label in the larvae fed the iPC than in those fed the hPC diet, but would have as a premise that NEFA were lost from the hPC diet during diet production or during absorption of the lipid by the larvae. TAG is the best energy substrate with three fatty acids available for energy production, explaining the increased catabolism of the label in the larvae fed the iTAG diet. Morais et al. (Reference Morais, Koven and Rønnestad29) also found that in Senegalese sole, oleic acid present in TAG was metabolised to a greater extent than when present in PC or as a NEFA, while retention in the tissues was higher for PC- and oleic acid-oleate than for TAG-oleate.
The hypothesis that there is a direct transfer of lipids from the diet to peripheral larval tissues is supported by the higher level of marked PC in the lipid classes of the larvae fed the iPC and hPC diets. Furthermore, the highest level of labelled TAG was found in the lipid classes of the larvae fed the iTAG diet, and the highest level of labelled NEFA was found in the larvae fed the hTAG diet, which contained 95 % NEFA. Direct transfer is in agreement with previous findings in zebrafish larvae, where Farber et al. (Reference Farber, Pack and Ho43) proposed that lipid uptake occurred through pinocytosis. There was a tendency of a higher inclusion of the tracer in the PC fraction in the larva fed the hPC diet as well. If LPC is absorbed intact, it may be resynthesised to PC by 1-acylglycerophosphocholine acyltransferase, a part of the Lands cycle, a shortcut from de novo synthesis(Reference Lands44, Reference Holub, Nilsson and Piekarski45). More work is required to investigate the mode of dietary lipid absorption and resynthesis in fish larvae.
An interesting detail is the higher level of NEFA in the larvae fed the iTAG diet than in those fed the iPC diet and the similar level of labelled NEFA in the larvae fed the hPC diet to that in the larvae fed the iPC and iTAG diets, even though the hPC diet contained five and seven times the amount of NEFA, respectively. The first result may be connected to the need to hydrolyse at least the fatty acids in the SN1 and SN3 positions, but perhaps all three fatty acids of TAG before absorption, liberating two to three NEFA as opposed to the liberation of one NEFA during digestion of PC. The other result may correspond to the possible presence of head groups of PC in the absorbed material from the hPC diet and direct re-esterification of the lipids inside the enterocytes, which could utilise most of the NEFA present in the diet.
Conclusion
Cod larvae, 40-d-old, have a high capacity for the absorption and metabolism of lipids, since 100 % of the lipids present at 15 % of diet DM in the present study were processed within 10 h when the feed intake was less than 14·5–19·5 μg (corresponding to 3·2–4·3 % of larval dry body weight). There were only small differences in lipid processing between the larvae fed the different diets, and hydrolysis of the dietary lipids did not seem to increase absorption. This shows that cod larvae have a high capacity for processing dietary lipids. The high incorporation of iPC and hPC into the body PC may indicate the absorption of iPC and LPC and the resynthesis of PC through the Lands cycle. When the capacity for processing of lipid became compromised at high feed intakes, lipid accumulated in the digestive tract of the larvae was higher for the polar than for the neutral lipid. Furthermore, approximately 100 % of the absorbed lipid was catabolised to CO2 at low feed intakes, which demonstrates that lipids are important energy substrates in cod larvae. At higher feed intakes, label retained in the body carcass was increased in the order iTAG < iPC < hPC. Conversely, iTAG appeared to be used for energy production to a greater extent than the polar lipids.
Acknowledgements
The present study was financed by the Research Council of Norway (project no. 179016/S40). I. M. L. was in Norway on a personal grant (project no. 190632), funded by the Research Council of Norway under the Culture exchange programme between India and Norway. The contribution of the authors to the present paper was as follows: K. H. developed the principal design of the study and was the main author during the preparation of the manuscript. Ø. S. contributed to the more detailed planning and follow-up during the practical part of the study. I. M. L. performed the practical part of the study and participated in the data analyses. I. R. provided inputs on the trial design and methodology. A. N. designed and supervised the production of the experimental diets. All the authors had inputs during the writing of the manuscript. There are no conflicts of interest.













