Introduction
Invasive species have led to extinctions, extirpations, and population declines of numerous island endemic species through predation, competition, and disease (Atkinson Reference Atkinson and Moors1985, King Reference King and Moors1985, Wilcove et al. Reference Wilcove, Rothstein, Dubow, Phillips and Losos1998, Courchamp et al. Reference Courchamp, Chapuis and Pascal2003, Jones et al. Reference Jones, Tershy, Zavaleta, Croll, Keitt, Finkelstein and Howald2008). Non-native species, particularly mammals, and habitat loss are primary threats to seabirds (Towns et al. Reference Towns, Atkinson and Daugherty2006, Howald et al. Reference Howald, Donlan, Galvan, Russell, Parkes, Samaniego, Wand, Veitch, Genovesi, Pascal, Saunders and Tershy2007). Rodents are the most pervasive of non-native mammals, and rats Rattus spp. have been observed on 80–90% of oceanic islands (Atkinson Reference Atkinson and Moors1985, Towns et al. Reference Towns, Atkinson and Daugherty2006, Caut et al. Reference Caut, Angulo and Courchamp2008). However, the immediate effects of rat invasions have been rarely documented (but see Thorsen et al. Reference Thorsen, Shorten, Lucking and Lucking2000) and many went undetected until species or ecosystems responded.
Rat eradication has been used as a conservation tool for more than two decades, during which projects were aimed at progressively larger islandscapes (Thomas and Taylor Reference Thomas, Taylor, Veitch and Clout2002, Clout and Russell Reference Clout, Russell, Koike, Clout, Kawamichi, De Poorter and Iwatsuki2006, Howald et al. Reference Howald, Donlan, Galvan, Russell, Parkes, Samaniego, Wand, Veitch, Genovesi, Pascal, Saunders and Tershy2007). Acute rodenticides and anticoagulant baits have proven effective at eliminating rats. Of these, brodifacoum, a second generation anticoagulant that is highly toxic to birds and mammals, has been the most widely-applied poison (Eason and Spurr Reference Eason and Spurr1995, Eason et al. Reference Eason, Murphy, Wright and Spurr2002, Howald et al. Reference Howald, Donlan, Galvan, Russell, Parkes, Samaniego, Wand, Veitch, Genovesi, Pascal, Saunders and Tershy2007). The poisons have been deployed through bait stations, hand-broadcasting, aerial broadcasting, or a combination of techniques (Thomas and Taylor Reference Thomas, Taylor, Veitch and Clout2002, Courchamp et al. Reference Courchamp, Chapuis and Pascal2003, Howald et al. Reference Howald, Donlan, Galvan, Russell, Parkes, Samaniego, Wand, Veitch, Genovesi, Pascal, Saunders and Tershy2007). The synthesis of poison type, deployment technique, and application timing influences susceptibility of both target and non-target species (Savarie et al. Reference Savarie, Johns, Gaddis, Borrecco and Marsh1992).
Pre-eradication assessments of pellet palatability, bait uptake rates, bait application methods, focal species movement and behaviour, and movement of toxins within the ecosystem are critical to predicting success of eradication efforts and evaluating the impacts of poison bait on non-target species (Courchamp et al. Reference Courchamp, Chapuis and Pascal2002, Clout and Russell Reference Clout, Russell, Koike, Clout, Kawamichi, De Poorter and Iwatsuki2006, Rodríguez et al. Reference Rodríguez, Torres and Drummond2006). Biomarker studies are a type of pre-eradication assessment that employs non-toxic pellets containing a dye that fluoresces under ultraviolet (UV) light. Captured individuals, dead organisms, or faecal samples are examined under UV light to visually detect pellet consumption. The abundance and location of the fluorescence can be appraised to address questions about uptake frequency, animal movement, and dosage levels (Savarie et al. Reference Savarie, Johns, Gaddis, Borrecco and Marsh1992, Eason et al. Reference Eason, Murphy, Wright and Spurr2002).
Pest control and eradication programmes have documented primary (direct consumption) and secondary (consuming a poisoned individual) poisoning of non-target species (Eason and Spurr Reference Eason and Spurr1995, Eason et al. Reference Eason, Murphy, Wright and Spurr2002). Experimental studies have focused on predatory birds, scavengers, and non-target mammals (Eason et al. Reference Eason, Murphy, Wright and Spurr2002). However, despite more than 284 insular eradication efforts (Howald et al. Reference Howald, Donlan, Galvan, Russell, Parkes, Samaniego, Wand, Veitch, Genovesi, Pascal, Saunders and Tershy2007), to our knowledge published information on mortality of non-target seabirds is almost entirely limited to opportunistic observations during and after eradication projects (e.g. Eason and Spurr Reference Eason and Spurr1995, Eason et al. Reference Eason, Murphy, Wright and Spurr2002). One exception was a study of birds (Larus heermanni, Sula leucogaster brewsteri, and S. nebouxii) interacting with 10 pieces of placebo bait placed inside 50 m2 plots for 30–60 minutes; no consumption was recorded (Samaniego-Herrera et al. Reference Samaniego-Herrera, Aguirre-Muñoz, Howald, Félix-Lizárraga, Valdez-Villavicencio, González-Gómez, Méndez-Sánchez, Torres-García, Rodríguez-Malagón, Tershy, Damiani and Garcelon2009). The second was a report from the Rat Island rat eradication programme, which used non-target species mortality collections, toxicology, and necropsies to assess cause of death (The Ornithological Council 2010). The lack of risk assessment information may relate to seabird absence during eradications or an assumption that some seabirds do not interact with bait. However, behaviours such as manipulating inedible objects and dropped food items, e.g. Sooty Terns (Feare Reference Feare1975) and Brown Boobies Sula leucogaster (Nelson Reference Nelson1978) may cause some seabirds to interact with poison bait pellets and increase the risk of ingestion. This is a concern because rodenticide use in active seabird colonies has been occasionally proposed when birds are present year-round (U.S. Air Force 2007, U.S. Fish and Wildlife Service 2007).
Our study provides an evaluation of the risk of rodenticide bait to adult and hatch-year seabirds. We used direct observations and camera-based monitoring to study bird consumption of placebo fluorescent biomarker bait to assess the potential risk of rodenticide bait to Sooty Terns on Wake Atoll Complex. During the onset of this study, previously unreported green and red environmentally-based fluorescence was observed and methods were adjusted to account for potential false positives.
Materials and methods
Study site and species
We assessed the risk of rodenticide bait to nesting Sooty Terns on Wake Atoll Complex, located in the central Pacific Ocean (19º18’55”N, 166º38’21”E). The atoll complex is comprised of three islets, including Wilkes, Wake, and Peale, with a total area of 739 ha. Rat eradication has been planned to eliminate two invasive rodents, R. exulans and R. tanezumi, likely in 2012 (U.S. Air Force 2008, Helm pers. comm. 2010).
Sooty Terns were selected as the focal species. The Sooty Tern is an abundant pan-tropical species that nests on many islands with introduced rodents (Woodward Reference Woodward1972, Feare Reference Feare1976, Harrison et al. Reference Harrison, Hida and Seki1983, Ratcliffe et al. Reference Ratcliffe, Hughes and Roberts1999, Schreiber et al. Reference Schreiber, Feare, Harrington, Murray, Robertson, Robertson, Woolfenden and Poole2002). Sooty Terns nest in large colonies with ample population sizes that are suitable for a control treatment design. Adults and chicks also exhibit behaviours, such as moving dropped food items and inedible objects (Feare Reference Feare1975 pers. obs. 2008, 2009), which may increase their risk of consuming bait. Thus, we studied bait interactions during the chick rearing period in order to assess the risks posed to both age classes. Moreover, Sooty Terns may be present on Wake Atoll Complex during proposed eradication dates as Sooty Tern breeding periodicity is uncertain.
We conducted our investigation in the Sooty Tern colony on Wilkes Island in 2010, where habitat consisted of varying combinations of bare ground, grass Ipomoea spp., and puncture vine Tribulus terrestris. We mapped the colony with global positioning systems (Garmin Ltd, Olathe, KS) and estimated colony size (5.2 ha) in the global information system (ArcView 3.3, ESRI, Redlands, CA; Sztukowski Reference Sztukowski2011). By combining area and a mean nest density of 4.67 nests m-2 (see Sztukowski Reference Sztukowski2011), we estimated the colony size to be 242,840 nests (52,000 m-2 * 4.67 nests m-2).
Study design
Six 10 m x 10 m plots were marked in areas with newly hatched Sooty Tern chicks. Plot locations were selected to be separated by the greatest distances possible, and all were at least 20 m apart. Chicks frequently travel from their hatch site to nearby shade but longer movements are limited by aggressive adults. Consequently, hatch year individuals were unlikely to travel between plots. The plots were randomly assigned as three treatment and three control sites. Control plots did not receive biomarker bait (Bell Laboratories, Inc., Madison, WI). Treatment plots and a 5 m surrounding buffer received two applications of non-toxic biomarker pellets, which were applied nine days apart. Pellets were applied on 28 April and 7 May 2010 using hand broadcast protocols (U.S. Air Force 2007). Eighteen kg ha-1 of biomarker bait were dispersed within the treatment plots during the first application, followed by 9 kg ha-1 during the second application. Biomarker pellets were formulated to resemble conservation brodifacoum rodenticide. The pellets fluoresced green under UV light and lacked poisonous ingredients. Dead chicks are common in Sooty Tern colonies, and all plots were cleared of dead chicks prior to initial treatment.
Camera-based observations
We used camera-based observations to assess the potential risk of rodenticide to adults and hatch year Sooty Terns. Within each of the three treatment plots, we placed a Reconyx PC900 camera (Reconyx, Inc., Holmen, WI). Each camera was mounted on a 60 cm tripod at a 45º angle and aimed at four bait presentation plates constructed of 21 cm x 21 cm off-white cloths with raised sides. The bait presentation plates were used to demarcate a defined area in the photos, and raised sides reduced the likelihood that pellets would be kicked out of the photographed area. The colour of presentation plates was similar to ground cover, and plates did not appear to affect tern behaviour. Each presentation plate was stocked with four biomarker pellets, for a total of 16 pellets at each camera station during each deployment. Pellet size (c.16 mm in length by 9 mm diameter) was similar to rodenticide bait, and pellets were smaller than forage items seen within the colony (pers. obs). Photographs were recorded at 10 second intervals for three days, or until camera batteries failed. After downloading photographs, camera stations were redeployed to previously unphotographed locations within the plots on 28 April, 2 May, 4 May, 7 May, 10 May and 13 May 2010.
We scrutinised a subsample of photographs collected during our study to identify the likelihood of pellet consumption by adult and hatch-year Sooty Terns. We used a random number generator to select 10 10-minute observation periods from each 3-day survey interval from 28 April 2010 through 17 May 2010. In total, we identified 60 10-minute observation periods from each of the three treatment plots. Of the total 449,804 photographs, we retrieved 10,800 photographs recorded during the 10-minute observation periods and scrutinised each for signs of pellet consumption by Sooty Terns. We recorded consumption if a bait pellet disappeared after a Sooty Tern was observed handling the pellet with the bill in the previous photograph. We also noted when an individual pecked at, moved, or picked up a pellet. Photographs also encompassed surrounding area, so we noted when pellets were flicked from presentation plates.
We also scanned the entire set of 449,804 photographs for consumption and pellet handling by adults and hatch-year Sooty Terns. Each set of photographs was converted to video (10 frames/second) and then videos were reviewed for pellet movement. When pellet movement was identified, we examined the photographs immediately prior to and after movement to pinpoint the cause. When the cause of pellet movement could be determined, it was classified as one of the following: (1) adult consumed a pellet, which was defined as an individual seen with a pellet in its bill or pecking at a pellet that disappeared in the subsequent photograph; (2) chick consumed a pellet; (3) chick moved or picked up a pellet; (4) adult moved or picked up a pellet; (5) adult pecked at a pellet; and (6) chick pecked at a pellet. Pecking behaviour may be underestimated in our scans of the entire set of 449,804 photographs as pecking may not produce noticeable pellet movement and hence reduced detectability. We excluded instances of birds apparently kicking or stepping on pellets.
Faecal samples
We collected faecal samples from Sooty Terns as a second means of assessing bait consumption by both adult and hatch-year birds. We deployed eight 14 cm x 26 cm faecal collection plates composed of off-white fabric within each plot. Faecal collection plates were placed after an observer walked seven steps within a plot. The observer entered plots at night with minimal light and thus the destination for each faecal collection plate was concealed at the onset of the seven steps. Faecal collection plates were retrieved at six survey intervals (total survey period was 19 days; Table 1). Both treatment and control plots were surveyed every third night (henceforth referred to as a survey interval) from 28 April 2010 through 17 May 2010. If we failed to find a faecal collection plate during the next survey interval, that sample was excluded from analyses. New faecal collection plates were distributed using the same protocol. Each faecal collection plate (n = 241) was examined under UV light to determine the presence, absence, and colour of UV fluorescence in faeces.
Table 1. Survey results for postmortem and live chick inspections of Sooty Terns on Wake Island in 2010 during a biomarker bait trial. Specimens with green fluorescence indicated biomarker bait contact or consumption, or accumulation of fluorescing material from environmental sources.
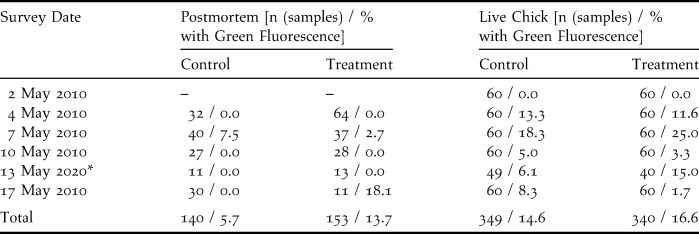
* Two plots collected during the day on 15 May 2010. Surveys were delayed due to prolonged precipitation.
Live chick inspections
We captured and examined the first 20 hatch-year Sooty Tern chicks encountered within each plot during each of six survey intervals. Rain prevented the completion of live chick inspections in two plots on 13 May, thus reducing the sample size (n = 689 chicks examined). Cloaca, feet, ventral feathers, and the external surface of the bill of each live chick were examined under UV light for fluorescence traces before each chick was released. Mouth lining of live chicks was viewed opportunistically to reduce stress and handling time. Fluorescence found on the cloaca and bill may indicate bait handling and consumption, whereas fluorescence on the feet or ventral feathers implied exposure to bait. The rate of consumption and exposure (proportion of individuals upon which fluorescence was detected) were compared between treatment and control plots using a two-tailed Fisher’s exact test (Fisher Reference Fisher1958).
Post-mortem inspections
We inspected all dead chicks retrieved from each plot during each survey interval (n = 293 chicks collected from six plots). We used a UV light to detect fluorescent reflectivity on the inspected bill, mouth lining, feet, cloaca, body cavity, stomach lining, and stomach contents of dead chicks. The entire external surface of desiccated individuals was inspected because dissection of the dried and flattened corpses was impossible. Individuals with liquefied organs were excluded from analyses. Presence or absence of fluorescence was recorded for each individual. Sample size for each plot ranged between one and 59 individuals with an average of nine samples in each survey interval. The proportion of individuals with green fluorescence was compared between treatment and control plots using a two-tailed Fisher’s exact test (Fisher Reference Fisher1958). Throughout the study we considered differences significant at α < 0.05.
Results
Sooty Terns moved, pecked at, and consumed biomarker bait. We identified one occurrence of a tern moving a pellet on a bait presentation plate within the scrutinised subsample of 10,800 photographs (Figure 1). Within the entire collection of 449,804 photographs, we observed one occasion when a Sooty Tern chick consumed a biomarker bait pellet, 34 pellet movement events by chicks, 5 pellet movement events by adults, 21 pellet pecking events by chicks and 5 occasions in which adults pecked at a pellets (Figure 1). One to four Sooty Tern adults and chicks were in close proximity to bait presentation plates in most photographs, so the contacts were relatively rare.

Figure 1. Photographic series illustrating Sooty Tern chicks physically contacting (A and B) and consuming (C) biomarker bait on Wake Island in 2010. (A and B) Chick handled the pellet, indicated by the arrow, but then dropped the pellet. (C) The pellet indicated by the arrows was grasped and then ingested, which was demonstrated in the subsequent photographs. Note that bait appears fluorescent green in actual colour photographs, which enhanced our ability to detect handling and consumption.
We observed fluorescence that appeared to be derived from sources other than our biomarker bait, because we observed red and green fluorescence during live chick inspections and in faecal samples in both treatment and control plots. Biomarker fluorescence used in our study reflected green, which was similar to the environmentally-based fluorescence observed in control plots. We believe that green fluorescence detected in control plots, and a similar proportion observed in treatment plots, may have been introduced to the colony in marine food items brought by adult birds. Thus, we verified the presence of environmentally-based fluorescence by capturing 30 hatch-year individuals at night more than 95 m from the nearest study plot, to ensure that chick movement between treated plots and the test area was unlikely. Chicks were arbitrarily selected from a 4 m- wide transect. We examined the cloaca, feet, ventral feathers, and surface of the bill of each live chick under UV light for red and green fluorescence traces before the chick was released. The presence of green fluorescence more than 95 m from the study plots was observed in three of 30 individuals, and red fluorescence was documented in one of 30 individuals outside our plots. These results provided strong evidence of an alternative source of fluorescence and thus indicated that some fluorescence was likely to occur in both treatment and control plots, and may not indicate bait contact. We assumed that sources and detectability of environmentally-based fluorescence were similar among plots.
Biomarker pellet consumption was not evident in faecal samples and live chick inspections. Green fluorescence was absent from the faecal samples on the 241 faecal collection plates examined. Green fluorescence was encountered on 61 of 689 live Sooty Tern chicks in both treatment and control plots (Table 1). Placement of the fluorescence was primarily on the cloacae, implying consumption of fluorescent food items. Additionally, a single chick was observed with a small piece of biomarker bait adhering to its ventral feathers, so there was conclusive evidence of exposure to biomarker bait. We did not detect differences in the proportions of individuals with green fluorescence in treatment and control plots (P = 0.894; Table 1). Thus we believe that the majority of detections were caused by environmentally-based fluorescence, rather than from chick consumption of biomarker bait.
Post-mortem inspections did not indicate bait consumption by Sooty Terns. A total of 293 dead chicks were included in the analysis, yielding six individuals with green fluorescence, three from control plots and three from treatment plots (Table 1). The proportions of dead individuals with green fluorescence did not differ among treatment and control plots (P = 0.686, Table 1).
Discussion
We recorded photographs of Sooty Tern adults and chicks on Wake Atoll Complex contacting biomarker bait pellets, and one series of photographs documented a bait consumption event. Further, a live chick was observed with a small piece of biomarker bait attached to its ventral feathers, which likely occurred when the chick sat on a bait pellet. Traces of dry biomarker were not detected on the other live chicks, despite photographic records of birds lying and standing on biomarker bait. Although simple contact with poison pellets seems unlikely to harm birds, direct ingestion of brodifacoum could be lethal. Birds are somewhat tolerant to brodifacoum when compared to rodents. Among birds, doses of brodifacoum needed for a 50% mortality rate (LD50) range from < 0.75 to > 20.0 mg kg-1 of body weight, whereas LD50 doses for Rattus norvegicus (0.27 mg kg-1) and Mus domesticus (0.4 mg kg-1) are much lower (Eason and Spurr Reference Eason and Spurr1995). Nonetheless, previous reports documented avian mortalities from brodifacoum, with specific observations of Petroica sp. that were found dead after eating crumbs of brodifacoum bait left by rodents (Eason and Spurr Reference Eason and Spurr1995, Stone et al. Reference Stone, Okoniewski and Stedelin1999).
Biomarker bait was not detected in faecal samples, post-mortem inspections, or live chick observations. The conflicting results may be attributable to the rarity of contacts and the number of hand-inspected chicks. Our photographic dataset likely included observations of many tens of thousands of birds, as two or three individuals were almost always present in each photograph. Samples included in our other hand inspection data numbered in the hundreds, however. Further, bait contact observations were rare in the photographic dataset, and we did not observe bait residue on the bills or feet of Sooty Terns in photos, so only the single consumption event would have yielded a condition that might be detected in both the photos and our other hand inspection methods.
Our observations of red and green fluorescence throughout the Sooty Tern colony suggested a source of fluorescence that was environmentally based. We suspect that the source of fluorescence was in marine food items brought to chicks by adult Sooty Terns. Sooty Terns primarily forage on small fish and squid, some of which have been documented to luminesce (Ashmole and Ashmole Reference Ashmole and Ashmole1967, Feare Reference Feare1976, Harrison et al. Reference Harrison, Hida and Seki1983, Herring Reference Herring1983, Nealson et al. Reference Nealson, Haygood, Tebo, Roman, Miller and McCosker1984, Hensley and Hensley Reference Hensley and Hensley1995). Although naturally present, we expected higher proportions of green fluorescence in treatment plots if biomarker bait was ingested at high rates, assuming that sources and detectability of marine-based fluorescence are similar among plots. Thus, by nearly every measure, bait contact events were rare.
Environmentally-based fluorescence has the potential to bias bait consumption rates predicted by biomarker trials, and to our knowledge, the presence of environmentally-based fluorescence on islands has not been previously considered. Both the environmentally-based fluorescence and some biomarker dyes, such as the one used in this study, glow green under UV light (Savarie et al. Reference Savarie, Johns, Gaddis, Borrecco and Marsh1992). Therefore, without testing the prevalence of environmentally-based fluorescence, previous studies may have underestimated dosage level of poison needed to eradicate the target species and overestimated bait palatability. Additionally, environmentally-based fluorescence may interfere with studies aimed at tracking toxin movement within the ecosystem. We recommend that future studies begin with an evaluation of environmentally-based fluorescence before formal biomarker studies are conducted.
In summary, though faecal samples, post-mortem inspections, and live chick observations did not provide evidence of bait pellet ingestion, camera-based data documented bird-bait interactions for adult and hatch-year Sooty Terns. The bait presentation plates used in our photo survey covered 0.176 m2, which represented 3.39 x 10-6 of the total colony area. We cannot use area ratios to directly extrapolate the photographed bait consumption and predict what might happen to the entire colony in an eradication programme, because the density of bait on our presentation plates was higher than a standard conservation application. Nonetheless, our results indicate that there are substantial risks associated with distributing brodifacoum across an entire Sooty Tern colony. Thus, care should be taken when designing rodent eradications and conservationists should exercise care in evaluating the risks of rat poison posed to seabirds. A cautions approach would include conducting rat eradications using poison bait only when nesting seabirds are not rearing chicks. In addition, pre-eradication studies should include evaluations of environmentally-based fluorescence prior to formal biomarker studies, as false positives could contribute to current eradication failure rates.
Acknowledgements
We appreciate funding from Chugach Support Services, Inc.; U.S. Air Force, 15th Airlift Wing; U.S. Department of Defense Legacy Resource Management Project, and University of Missouri. K. Ramirez, S. Suwantha, S. Kaewputtan, P. Torres, T. Suttawat, and P. Natsupa and Base Operations personnel provided invaluable assistance in the field. We would also like to thank J. Faaborg, M. Gompper, the Avian Ecology Lab, D. Jachowski, M. Weijht, L. Sakaguchi, and all those that provided comments on manuscript drafts. We very much appreciate the helpful comments made by anonymous reviewers. The view of the authors does not necessarily reflect positions of Chugach Support Services, Inc., the U.S. Government, the U.S. Department of Defense, the U.S. Air Force, or any of the contributors.






