INTRODUCTION
The dynamic nature of influenza virus is such that an individual will be exposed to several different viral strains throughout their life [Reference Carrat1]. The immune response to influenza is known to be defined by an individual's first exposure, which probably occurs during childhood [Reference Francis2]. Upon infection or vaccination, individuals will produce virus-specific antibodies that may offer some protection against future infection by similar strains via cross-reactive antibodies. The haemagglutination inhibition (HI) titre is associated with the level of protection [Reference Hobson3, Reference Coudeville4] against a given strain. However, as influenza epidemiological studies adopt a ‘life course view’ to the immune response, it has become apparent that interpretation of HI titre should be done within the context of the timing, order, and antigenic similarity of the viral strains to which an individual is exposed [Reference Fonville5–Reference Li8].
Often lacking detailed influenza infection history, epidemiological studies have historically used age as a proxy for exposure; very early studies [Reference Francis2, Reference Davenport, Hennessy and Francis9] and several cohorts [Reference Fox10–Reference Monto, Koopman and Longini12] have repeatedly shown a varied antibody repertoire according to age. One recent study identified age-related patterns in neutralization titre to A(H3N2) viral strains that circulated during childhood in particular [Reference Lessler7]. To further explore the effects of repeated exposure to influenza on antibody titre, we used serological data from a longitudinal cohort against seasonal [1956 A(H1N1), 1977 A(H1N1), 2007 A(H1N1)] and pandemic [A(H1N1)pdm09] influenza strains. Age-related differences in HI titre, and independent correlations between strain-specific HI titres, were explored. Finally, we investigated age-dependent patterns in HI titres to seasonal and pandemic influenza A(H1N1) strains.
METHODS
Subject recruitment/participation
This analysis relied on data collected from a French cohort designed to study recurrent influenza infection. Subject recruitment has been described previously [Reference Lemaitre13]. Briefly, 36 general practitioners (GPs) in metropolitan France recruited subjects during the 2007–2008 and 2008–2009 influenza seasons. Subjects were stratified by age (10-year age groups) and reason for medical visit [influenza-like illness (ILI) or an illness unrelated to ILI]. An ILI was defined as a sudden onset of fever (38 °C) in conjunction with muscle aches and cough. Study data (including medical history, socio-demographic information, ILI-related illnesses, influenza vaccinations) were entered by GPs on electronic case report forms at inclusion and updated at all study visits. Enrolled subjects performed a maximum of three annual study visits post-inclusion and, in the event of ILI, a dedicated study visit was performed. Blood serum was collected at inclusion for ILI subjects and at all annual study and post-inclusion ILI visits. Influenza infection was diagnosed from nasal swabs collected during ILI study visits at inclusion or during study follow-up (see Supplementary material for more details).
This study received approval by both the French ethics committee [Comité de Protection des Personnes Ile-de-France V (no. 07715)], and the Data Protection Authority (no. 1261460). All study participants provided written informed consent.
Laboratory procedures
HI titre
Serum samples were tested for antibodies against seasonal influenza A strains [2007 A(H1N1), 1977 A(H1N1), 1956 A(H1N1)] and the recent pandemic strain A(H1N1)pdm09 using A/Paris/6/2007 (H1N1)-like, A/Arkhangelsk/1/77, A/Nederland/1/1956, and OPYFLU-1 (isolated in Marseille in early May 2009) strains, respectively. Serum HI titre was determined following the HI technique assay; specific procedures have been previously described [Reference Delabre14]. Twofold serial dilutions (1:10–1:1280) of heat-inactivated sera were used; HI titre was determined to be the reciprocal of the highest serial dilution that prevents haemagglutination upon two independent readings [Reference Wood15].
Statistical methods
This analysis was restricted to study samples, regardless of study visit type, for which influenza vaccination data was complete; vaccinated subjects at inclusion were excluded to avoid confounding, follow-up of subjects reporting seasonal and/or pandemic vaccination during the study period was censored at the given visit date. Age-dependent patterns of antibody titre were investigated according to three defined cohorts depending on year of birth (1977–1991, 1956–1976, 1930–1955), age at the time of study inclusion and/or age at initial strain circulation (subject's age at the time the strain was first isolated).
Geometric mean titres (GMT) were estimated, and compared between age groups, using regression models for interval-censored data accounting for repeat measurements [Reference Nauta16, Reference Siev17]. Regression models were also used to determine the correlation between two strain-specific titres while removing the effect of the other titres (partial correlation), using Spearman's correlation and random effects at the subject level. Generalized additive mixed models were fit to log-transformed HI estimates (defined as the GMT of the lower and upper bound of the interval-censored HI measures) to study the relationship between HI titre and age (age at inclusion and/or age at initial strain circulation). Models used by Lessler et al. [Reference Lessler7] were adapted to longitudinal data; HI titre was fitted as a function of: model A (age at the time of initial strain circulation and age at inclusion); model B (age at inclusion); model C (age at the time of initial strain circulation). All models were fitted with random effects at the subject level and for the tested strain as well as with and without strain dependence for the variation of HI titre as a function of age variables; complete details of the models can be found in the Supplementary material. Models were compared using Bayesian information criteria (BIC), the model with the lowest BIC was preferred. P value threshold significance was <0·05.
A sensitivity analysis was performed to examine the effect of A(H1N1)-infected subjects on the partial correlation tests and models of age and HI titre. Detailed information regarding corresponding procedures and methods is given in the Supplementary material.
Statistical analyses were performed with the R statistical program v. 3.1.1 (https://cran.r-project.org/bin/windows/base/old/3.1.1); the mgcv package was used to fit generalized additive mixed models.
RESULTS
A total of 592 subjects were recruited to the cohort from January 2008 to December 2009 and 555 subjects went on to complete at least one study visit for which a serological sample was available. Subjects indicating seasonal (n = 154), pandemic (n = 1) or missing vaccination status (n = 2) at inclusion were excluded from the analysis. Subjects with missing or confirmed seasonal and/or pandemic vaccination during study follow-up (n = 54), were censored. This analysis relied on a total of 1053 serological samples collected from January 2008 to November 2010 from 398 subjects; 338 (85%) study subjects provided at least two blood samples, 226 (57%) provided at least three blood samples, 81 (20%) provided at least four blood samples and 10 (3%) provided five blood samples. In the subjects included in this analysis, a total of 46 subjects had laboratory-confirmed A(H1N1) infection with seasonal 2007 A(H1N1) (n = 44) or A(H1N1)pdm09 (n = 2) viral strains.
Subjects’ ages at inclusion ranged from 18 to 79 years (median 44, interquartile range 32–54 years); subjects were divided into three age groups based on year of birth: 1977–1991 (n = 104), 1976–1956 (n = 182), and 1930–1955 (n = 112). Age at the time of initial strain circulation ranged from 35 years before birth [for A(H1N1) 1956] to 79 years [for A(H1N1)pdm09].
We noted differences in GMT according to birth cohort (Table 1). Younger subjects (subjects born after 1976) had significantly lower 1977 A(H1N1) GMT [40·0, 95% confidence interval (CI) 37·5–42·7] compared to subjects born between 1956 and 1976 (47·9 95% CI 44·9–51·0, P < 0·0001) and before 1956 (46·9, 95% CI 42·8–51·4, P = 0·0051) but higher 2007 A(H1N1) GMT (42·5, 95% CI 39·2–46·2) vs. 38·5 (95% CI 36·7–40·5, P = 0·0405) and 38·2 (95% CI 35·8–40·8, P = 0·0393). The oldest birth cohort had significantly higher 1956 A(H1N1) GMT compared to both younger birth cohorts (P < 0·0001 for both); no significant differences were noted in the birth cohorts with regard to A(H1N1)pdm09 GMT.
Table 1. Strain-specific GMT by birth cohort and 95% confidence intervals (CI)

GMT, Geometric mean titre.
P value refers to comparison with the youngest birth cohort.
Partial correlations between A(H1N1)pdm09 and 2007 A(H1N1) and between 1956 A(H1N1) and 1977 A(H1N1) were significant across all age groups (Fig. 1). Titres against 1977 A(H1N1) were correlated with all other tested strains in younger subjects (born between 1977 and 1991), where 1977 A(H1N1) and 1956 A(H1N1) titres had the strongest partial correlation (partial rank correlation 0·2550, P < 0·0001). In subjects born between 1956 and 1976, partial correlations were significant for all tested titres against A(H1N1)pdm09 and 1956 A(H1N1); only 2007 A(H1N1) and 1977 A(H1N1) titres were not significantly associated (partial rank correlation 0·0853, P = 0·0583). Partial correlations between 1977 A(H1N1) and 2007 A(H1N1) were also significant in the older birth cohort.

Fig. 1. Partial rank correlations (Spearman's rho) between A(H1N1) viral strains by birth cohort group: 1977–1991 (n = 104), 1956–1976 (n = 182), 1930–1955 (n = 112). Significance: *P < 0·05, **P < 0·01, ***P < 0·001.
With the exception of the model depending only on age at inclusion (model B2), models including strain dependence generally resulted in slightly lower BICs than models without (complete results are given in Supplementary Table S1). Comparing the models with the lowest BIC of each type of model tested: the model describing HI titre as a function of age at the time of strain circulation (model C1) had the lowest BIC compared to the model depending only on age at inclusion (model B1) or compared to model A3 which included both age at inclusion and age at circulation – although the difference in BIC criteria between models A3 and C1 was low. Subjects that were exposed to the tested strains during childhood showed higher HI titres than those who were not; titres peaked in subjects aged 8 years for circulation of 1956 A(H1N1) and subjects aged 7 years for circulation of 1977 A(H1N1) (Fig. 2). A second peak was observed against 1977 A(H1N1), corresponding to subjects aged ~34 years at initial strain circulation. Regarding the more recently circulating viral strains, a slight peak in titres against 2007 A(H1N1) was observed at age 27 years, whereas titres against A(H1N1)pdm09 remained stable.
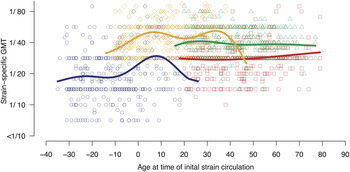
Fig. 2. Strain-specific geometric mean titre (GMT) as a function of age at initial strain circulation (lines) and observed GMT per subject (points) where A(H1N1)pdm09 is represented in red, seasonal 2007 A(H1N1) is in green, 1977 A(H1N1) in orange, and 1956 A(H1N1) in dark blue.
The sensitivity analysis excluding all laboratory-confirmed A(H1N1)-infected subjects relied on 912 serological samples from 352 subjects. Titres against 2007 A(H1N1) and A(H1N1)pdm09 were significantly correlated across all age groups as were correlations between 1977 A(H1N1) and 1956 A(H1N1) (Supplementary Fig. S1). The primary model of HI titre as a function of age produced comparable results to our main results (Supplementary Fig. S2): HI titre peaked in subjects aged 8 years for circulation of 1956 A(H1N1) and aged 10 years for circulation of 1977 A(H1N1).
DISCUSSION
We identified age-dependent patterns in A(H1N1) viral strains using longitudinal data collected from a French cohort investigating recurrent influenza infection. Older subjects (born before 1956) had significantly higher HI titre against 1956 A(H1N1) and 1977 A(H1N1) strains compared to younger subjects (born between 1977 and 1991). We found that HI titres against seasonal 2007 A(H1N1) were independently correlated with A(H1N1)pdm09 across all age groups, suggesting that recent exposures may have offered protection against A(H1N1)pdm09 infection. Finally, our analysis shows that age at the time of A(H1N1) strain isolation is highly predictive of HI titre, confirming results from a cross-sectional study investigating only A(H3N2) strains [Reference Lessler7].
A lifetime of exposure to the influenza virus makes interpretation of HI titres extremely challenging as the immune response to prior infection and cross-reactive antibodies are difficult to differentiate [Reference Lessler7]. Moreover, influenza infections may be poorly documented, or go undiagnosed [Reference Hayward18]. Using age as a proxy for exposure, studies have identified age-related differences in susceptibility to influenza [Reference Davenport, Hennessy and Francis9–Reference Monto, Koopman and Longini12] and this was further underscored during the 2009 A(H1N1) pandemic [Reference Miller19, Reference Hancock20]. Not surprisingly, our study identified significant differences in GMT according to birth cohort group. Compared to the youngest group, older subjects in our study (born before 1956) had significantly higher HI titres against 1956 A(H1N1) and 1977 A(H1N1). The youngest group (born between 1977 and 1991) had significantly higher HI titres against 2007 A(H1N1) compared to both older groups. While HI titres against 1956 A(H1N1) and A(H1N1)pdm09 in older subjects were significantly correlated using standard tests (Spearman's rank correlation rho 0·1462, P = 0·0132), this correlation was no longer significant when taking into account the effects of the other A(H1N1) strains. The results of this study suggest that exposure to recent seasonal viruses may have played an important role in protecting subjects against A(H1N1)pdm09 infection, supporting the results of another study based on this cohort [Reference Lemaitre13], which found a cross-reactive antibody response between the 2007 A(H1N1) and A(H1N1)pmd09 strains. Other studies have also found evidence that recent seasonal exposure was protective during the 2009 A(H1N1) pandemic [Reference Carter21, Reference Couch22]; however, it is also important to note that exposure to 1918-like viruses may have provided protection in older age groups [Reference Hancock20, Reference Xu23].
Interestingly, we found that for the two younger age groups, all tested A(H1N1) strains were independently associated with the A(H1N1) strain that circulated during the first year of the birth cohort group: 1977 A(H1N1) in the case of the youngest cohort and 1956 in the middle-aged cohort. This finding further supports the fact that age at the time of initial circulation of a given strain was the main factor explaining the level of HI titres against that strain and other related strains. Association between 1956 A(H1N1) and 1977 A(H1N1) across all age groups was not surprising given that these strains are antigenically similar [Reference Huang24, Reference Kilbourne25]. However, we note a weaker correlation between these strains in older subjects who would have been ‘primed’ by 1956 A(H1N1) or a similar virus [Reference Francis2] compared to the younger age groups.
We found the relationship between HI titre and age at initial circulation to be strain dependent, whereas Lessler et al. [Reference Lessler7] found the relationship between age and neutralization titres against A(H3N2) strains to be strain independent. Nevertheless, we observed that HI titres peaked in subjects aged 7–8 years at the time of initial A(H1N1) strain circulation, confirming Lessler et al.’s finding that individuals retained a high response to strains that circulated within the first 10 years of life [Reference Lessler7]. However, it should be noted that as our cohort was limited to adult subjects, we were unable to confirm this pattern against the more recent A(H1N1) viral strains [2007 A(H1N1) and A(H1N1)pdm09]. These findings recall the theory of original antigenic sin by which birth cohorts may be marked by the strains to which they were exposed during childhood [Reference Francis2, Reference Davenport, Hennessy and Francis9]. Although similar patterns have been reported [Reference Miller26], there exists evidence that higher HI titres may not always signal the first childhood infection [Reference Gagnon27]. The second peak in HI titres against 1977 A(H1N1), corresponding to subjects aged 34 years in 1977 according to our model, suggests a cross-reactive response with A(H1N1) viruses circulating in the 1940s [Reference Glezen11]; however, these results should be confirmed using isolates such as PR8 or FM1 in future studies.
The important immune responses to older A(H1N1) strains shown in our study, have also been well demonstrated in another longitudinal study [Reference Miller26], and may be due to boosting by continued exposure to influenza viruses [Reference Fonville5, Reference Glezen11]. Observation of higher serological titres to earlier strains may also be due to a diminished immune response with each subsequent infection [Reference Lessler7]. In a follow-up study to Lessler et al. [Reference Lessler7], a mechanistic model was used to explore the effects of prior exposures on the immune response; strain-specific boosting (following infection/vaccination), cross-reactive antibody responses and a reduced immune response due to antigenic seniority were found to explain the observed neutralization titres [Reference Kucharski6]. Research has shown the potential importance of the timing and order of infecting strains in the development of antibody responses [Reference Kucharski6, Reference Li8], signalling an increasing need to collect detailed influenza exposure histories to better understand the antibody patterns observed in this cohort and elsewhere.
CONCLUSION
Our study confirms previous findings that a significant immune response is retained against viral strains circulating within the first 10 years of an individual's life. As seroepidemiological studies investigating influenza move towards a ‘life course’ approach, future longitudinal studies collecting serological data against a variety of viral strains may lead to better understanding of the evolution of the immune response over time.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268816002156.
ACKNOWLEDGEMENTS
This study cohort was supported by a grant from the Agence Nationale de la Recherche and Region Ile-de-France. Additional funding for the cohort and this research study was provided by the Université Pierre et Marie Curie (Paris 6).
DECLARATION OF INTEREST
F. Carrat has received honoraria from Boiron, AstraZeneca and GSK for advising on influenza epidemiology. The remaining authors declare no competing interests.








