CLINICIAN’S CAPSULE
What is known about the topic?
Advanced care paramedics (ACPs) in our emergency department (ED) successfully perform procedural sedation and analgesia (PSA) for several procedures, including orthopedic manipulations.
What did this study ask?
How does the novel practice of ACP-led ED PSA for upper gastrointestinal (UGI) endoscopy compare to that for orthopedic procedures?
What did this study find?
Adverse events occurred more frequently during UGI endoscopy sedations than orthopedic sedations (41.0% v. 17.5%), with hypotension occurring most often.
Why does this study matter to clinicians?
ACP-led ED PSA may be expanded to include UGI endoscopy when the risk of hemodynamic compromise is taken into account.
INTRODUCTION
In the emergency department (ED), patients routinely undergo procedural sedation and analgesia (PSA).Reference Campbell, Magee and Kovacs 1 Because medications used for procedural sedation often carry a risk of hypotension and respiratory depression, it is important that qualified personnel closely monitor patients. In most centres, the emergency physician directs ED PSA while performing the procedure. Nurses, respiratory therapists, and, at times, a second physician may assist. However, PSA can be time-consuming and prevent the ED physician from performing other important tasks, leading to problems with flow. This may be particularly difficult in community EDs where physician resources are scarce.Reference Bawden, Villa-Roel and Singh 2 To streamline the PSA process, an expanded role for paramedics has been introduced at our institution, the QEII Health Sciences Centre in Halifax.
In our ED, approximately 1000 PSAs are performed per year by specially trained advanced care paramedics (ACPs) and critical care paramedics (CCPs) who are responsible for drug administration and monitoring under the oversight of a physician. ACPs and CCPs are particularly suited for this role because their scope of practice includes monitoring unstable patients and providing advanced airway management. To become paramedic sedationists, they receive additional training in PSA pharmacology, complications, and appropriate patient selection, in keeping with the American Society of Anesthesiologists’ (ASA) guidelines for sedation and analgesia by non-anesthesiologists.Reference Campbell, Magee and Kovacs 1 , Reference Campbell, Petrie and MacKinley 3 , 4 Although both ACPs and CCPs perform sedations in our ED, we will refer only to ACPs because this is the minimum level of training required to perform PSA.
For more than a decade, ACPs at our institution have delivered ED PSA for procedures such as cardioversion, orthopedic reductions, and abscess drainage.Reference Campbell, Magee and Kovacs 1 , Reference Bawden, Villa-Roel and Singh 2 Recently, their role was expanded to include sedation for urgent upper gastrointestinal (UGI) endoscopies in the ED. Previously, gastroenterologists, assisted by gastrointestinal (GI) nurses, performed both ED endoscopy and sedation. New PSA guidelines at our institution now mandate, however, that the person monitoring sedation should not be involved in the procedure itself. 5 Therefore, a decision was made to broaden the scope of ACP sedationists to include PSA for this more complex procedure as well.
UGI endoscopy differs from many other procedures requiring sedation in that the patient’s oropharynx is manipulated, and the sedationist is relatively removed from the patient’s head, presenting challenges to airway management. The patient is also placed in the lateral decubitus position so that one arm cannot be easily used for IV access, blood pressure, and pulse oximetry monitoring. It is therefore preferable, in any case, that providers uninvolved in the procedure perform PSA for UGI endoscopy. The use of ACPs in this role is, to our knowledge, a novel practice unique to our institution. This study aims to provide a benchmark of outcomes for this practice. In a retrospective analysis of prospectively gathered data, we compare ACP-led ED PSA for UGI endoscopy and orthopedic procedures in terms of adverse events, airway intervention, and medication use.
STUDY DESIGN AND METHODS
Objectives and outcomes
The study objective was to characterize how ACP-mediated ED PSA for UGI endoscopy differs from ED PSA performed by ACPs for orthopedic indications. The primary outcome was to determine the rate of adverse events and rate of interventions for patients undergoing PSA for emergent UGI endoscopy in the QEII Health Sciences Centre ED, as compared to patients undergoing PSA for orthopedic procedures as a control group matched by age, gender, and ASA class. ASA classification subjectively assesses patients’ preoperative status from I (healthy), II (mild systemic disease), III (severe systemic disease), IV (incapacitating disease), to V (moribund).Reference Dripps 6
Adverse events were defined as hypotension (systolic blood pressure<100 mm Hg or a 15% from baseline if pre-procedure systolic blood pressure<100 mmHg), hypoxia (oxygen saturation<90%), apnea (lasting 30 seconds), vomiting, arrhythmia, and death in the ED. Interventions studied were airway intervention (airway repositioning, oral or nasal airway, assisted ventilation, and endotracheal intubation) and the need for vasopressor therapy. The secondary outcomes of interest were the type of medication and doses used for PSA.
Methods
We performed a chart review of ED PSA records for emergent UGI endoscopy from December 1, 2014 to October 31, 2015. These data were gathered prospectively on a standardized ED PSA form (Appendix A) and entered into an existing quality management database in the Dalhousie University Department of Emergency Medicine. This database houses clinical details of all procedural sedations performed by ACPs in the QEII Health Sciences Centre ED. Patient consent was obtained prior to entry into the database. All endoscopy PSA records are fully audited by our paramedic lead educator for quality control purposes; approximately 20% of PSA records for other indications, including orthopedic procedures, undergo review. The following variables were extracted from the registry: patient age and gender, ASA category, weight, indication for ED PSA, medications and doses, adverse events, and interventions. Each subject was given a unique identifier.
Because historical data for PSA performed by gastroenterologists and GI nurses for emergent UGI endoscopies at the QEII Health Sciences Centre ED are unavailable, we were unable to compare PSA performed by GI team members to PSA performed by ACPs. Instead, we used existing data for orthopedic procedural sedations stored in the ED PSA database as a matched comparison group for UGI endoscopy sedations. Orthopedic sedations were chosen as a control group because they represent the most common indication for ED PSA and have been performed safely and effectively by ACPs at our institution for many years.Reference Campbell, Magee and Kovacs 1 , Reference Campbell, Petrie and MacKinley 3 Propensity scores matching was done by age, gender, and ASA grade, with three orthopedic patients matched for each endoscopy patient. This ratio was chosen because it reflects the natural ratio of orthopedic to UGI endoscopy patients that occurred during the study period.
Analysis
A descriptive statistical analysis of the data was performed. Continuous variables were tested for normality. If normal, they were described and tested for differences using t-statistics. Results were described using means, standard deviations, counts, and percentages. Effect sizes for the outcomes were described using OR with 95% confidence bounds. All confidence tests were done at a confidence level of 0.05. Matching was performed using the TriMatch package within the R statistical language, matching on age, gender, and ASA class. An analysis of our outcomes was done using conditional logistic regression to stratify our matching variables. All analyses were done using R (v.3.4.1, “Single Candles”) and the RStudio GUI (v.0.99). Data manipulation was done using MySQL (v.5.6).
Ethics approval
This study received ethics approval from the Nova Scotia Health Authority Research Ethics Board.
RESULTS
During the study period, 61 UGI endoscopies with ED PSA were performed on 59 patients, with 1 patient undergoing three ED endoscopies. The 61 UGI endoscopy cases (mean age 56.9 years, 73.8% male) were matched using propensity scores by age, gender, and ASA class to 183 orthopedic cases (mean age 57.5 years, 72.1% male) in a 1:3 ratio. Patient demographics are displayed in Table 1. There were no differences in gender, ASA, and age due to the proportional matching scheme.
Table 1. Study demographics

ASA=American Society of Anesthesiologists; SD=standard deviation.
As shown in Table 2, the most common indication for UGI endoscopy was UGI bleeding (28/59 cases), followed by esophageal obstruction (27), foreign body (3), and nausea and vomiting (1). The most frequent endoscopic diagnosis in patients with UGI bleeding was peptic ulcer disease (12/28) with relatively fewer cases of variceal bleeding (3/28), Mallory-Weiss syndrome (2/28), and other conditions (6/28). In 5/28 cases, bleeding was not localized. The majority of patients with esophageal obstruction required endoscopy for food bolus impaction (22/27).
Table 2. Indications and endoscopic diagnoses for emergent upper GI endoscopy during the study period

* Normal exam or undetermined diagnosis.
† 4/22 patients also diagnosed with eosinophilic esophagitis.
Adverse events
Adverse events were documented for 32/183 (17.5%) orthopedic sedations and 25/61 (41.0%) UGI endoscopy sedations (Table 3). Hypotension was the most frequent adverse event, occurring in 36.1% of endoscopy cases and 12.0% of orthopedic cases (22/61 v. 22/183 cases, OR=4.11, CI: 2.05-8.22). Other adverse events were rare in both groups. Hypoxia was recorded in 10 orthopedic cases (5.5%) and no endoscopy cases. Arrhythmia occurred infrequently among endoscopy patients (1.6%) and was not observed in orthopedic patients. No episodes of apnea or vomiting were noted in either group. There were no patient deaths.
Table 3. Adverse events and interventions occurring during ED PSA
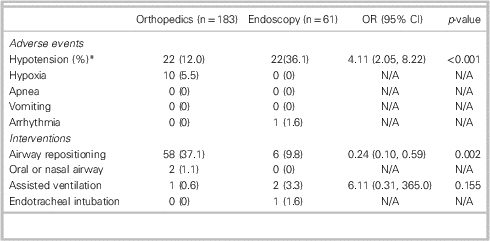
*Hypotension is defined as systolic BP<100 mm Hg, or a 15% drop if the baseline systolic BP is<100 mm Hg
N/A=Hypothesis not testable
Airway repositioning was the most common intervention in both groups and occurred less frequently in patients undergoing UGI endoscopy than in orthopedic patients (6/61 v. 58/183 cases, OR=0.24, CI: 0.10-0.59). Other interventions were rare. Two orthopedic patients (1.1%) had an oral or nasal airway placed. Bag valve mask ventilation was used in one orthopedic and two endoscopy cases (0.6% and 3.3%, respectively). One endoscopy patient required endotracheal intubation during the procedure. In terms of vasopressor therapy, four endoscopy patients (6.6%) received phenylephrine compared to none in the orthopedic group (Table 4).
Table 4. Medications used and dosing for ED PSA
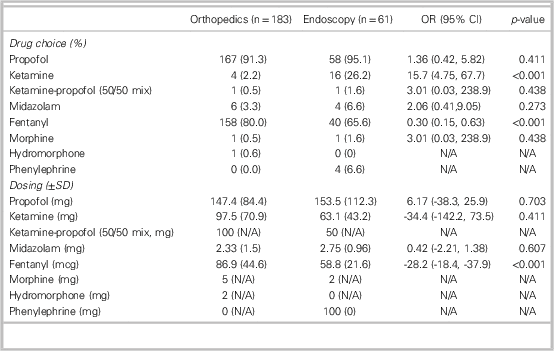
CI=confidence interval; N/A=hypothesis not testable; OR=odds ratio; SD=standard deviation.
Medication use
Propofol was the most frequently used sedative agent, given in 91.3% of orthopedic cases and 95.1% of UGI endoscopy cases (see Table 4). Ketamine was used more frequently in the endoscopy group than in the orthopedic group (16/61 v. 4/183 cases, OR=15.7, CI: 4.75-67.7). Midazolam administration was infrequent, used in 3.3% of orthopedic cases and 6.6% of endoscopy cases. Fentanyl was used in 80.0% of orthopedic cases and 65.6% of endoscopy cases. Compared to the orthopedic group, fentanyl was given less frequently in the endoscopy group (40/61 v. 158/183 cases, OR=0.3, CI: 0.15-0.63). When fentanyl was administered, endoscopy patients received an average of 28.2 mcg less than orthopedic patients. There were no other significant differences in medication use between the groups.
DISCUSSION
This study examined adverse events and medication use in patients undergoing ACP-led PSA for UGI endoscopy and orthopedic procedures in a Canadian ED setting. As mentioned earlier, using ACPs for ED PSA during UGI endoscopy is unique to our institution. Our investigation provides the first analysis of outcomes with this model. To our knowledge, sedation-related adverse events for UGI endoscopy in an ED environment have not been previously studied. There are, however, several studies reporting on adverse events during urgent UGI endoscopy performed in dedicated endoscopy suites.Reference Agostoni, Fanti and Gemma 7 - Reference Tohda, Higashi and Sakumoto 10
In our study, the rate of complications for endoscopy was higher than for orthopedic procedures, with hypotension occurring most frequently. Vasopressor use with phenylephrine was exclusive to endoscopy cases. This is not entirely unexpected because the most common indication for endoscopy in our study population was UGI bleeding. Our endoscopy patients were therefore more likely to be hypovolemic and hemodynamically unstable prior to sedation and intervention than patients undergoing sedation and orthopedic manipulation for fractures and dislocations. Vagal stimulation occurring during esophageal intubation can further contribute to peri-procedural hypotension.Reference Amornyotin 11 , 12 Patient positioning in the lateral decubitus position during UGI endoscopy is also more likely to lead to hypotension than the standard semi-Fowler’s position used for other procedures, with the patient supine and head of bed elevated.Reference Becker and Haas 13 , Reference Klare, Huth and Haller 14
In the literature on urgent endoscopy for UGI bleeding under sedation, reported rates of hypotension vary from 3.5% to 12.5%.Reference Agostoni, Fanti and Gemma 7 - Reference Tohda, Higashi and Sakumoto 10 Because these studies excluded patients with hemodynamic instability, it is not surprising that our incidence of hypotension was comparatively higher. ED patients requiring UGI endoscopy are often inherently hemodynamically compromised. Our results may also be due to our conservative definition of hypotension as a systolic blood pressure less than 100 mm Hg or a 15% decrease from baseline if the pre-procedure systolic blood pressure was less than 100 mm Hg. Other authors used decidedly lower blood pressure cut-offs to define hypotension.Reference Agostoni, Fanti and Gemma 7 - Reference Tohda, Higashi and Sakumoto 10 Further factors contributing to hypotension in our endoscopy cohort may have been inadequate pre-procedure volume resuscitation and medication use. Importantly, the clinical significance of transient hypotension in the peri-procedure ED PSA setting is unclear and warrants further evaluation.
UGI endoscopy carries procedure-related risk factors for hypoxia, including difficulty intubating the esophagus, prolonged procedure time, and patient positioning.Reference Ben-Menachem, Decker and Early 15 It is therefore somewhat unexpected that hypoxia, although infrequent, occurred more often in our orthopedic group. Analgesic use may explain this result. Orthopedic patients received fentanyl more often and at an average higher cumulative dose than endoscopy patients. Some orthopedic patients may therefore have experienced opioid-related respiratory depression. Overall, the 5.5% incidence of hypoxia in our orthopedic group is similar to that reported in a meta-analysis of 42 studies of 7116 ED PSAs of 40.2 hypoxic events per 1000 sedations (95% CI: 32.5-47.9).Reference Bellolio, Gilani and Barrionuevo 16 This suggests that the risk of hypoxia for ACP-guided sedation is comparable to ED sedation by other providers.
Airway repositioning and nasal/oral airway device use was less frequent among endoscopy patients, likely because airway maneuvers are inherently more challenging due to endoscope placement in the patient’s mouth and the distance of the patient’s head from the sedationist. One hypoxic episode occurred in the endoscopy group, which unfortunately could not be included in our database because it was not documented as an adverse event on the ED PSA record. This patient with active UGI bleeding developed hypoxia during endoscopy and required intubation by the paramedic. Generally, intubation in ED PSA is exceedingly rare. Bellolio et al. reported a rate of 1.6 intubations per 1000 sedations (95% CI: 0.3-2.9) in a meta-analysis of 3636 PSAs.Reference Bellolio, Gilani and Barrionuevo 16 However, in one study of 50 patients with active UGI bleeding undergoing emergent ED endoscopy without sedation, two intubations (4%) were reported.Reference Yen, Hu and Chen 17 It seems likely that our patient’s condition, namely active GI bleeding rather than the sedation itself, was the risk factor for intubation.
The transition from gastroenterologist-led to ACP-led sedation for UGI endoscopy in our ED prompted a change in medication selection. Gastroenterologists routinely use midazolam for sedation with fentanyl for analgesia – probably the most well-known drug combination for PSA.Reference Amornyotin 11 , Reference Bahn and Holt 18 - Reference Vargo, Cohen, Rex and Kwo 22 In the ACP-led sedations we studied, propofol was the most commonly used sedative, whereas midazolam was used infrequently. Propofol’s advantages over midazolam are its more rapid onset (30-40 sec v. 1-2 min) and considerably shorter duration of action (4-5 min v. 15-80 min).Reference Igea, Casellas and González-Huix 20 However, propofol may cause significant hypotension in patients with hypovolemia or serious comorbidities.Reference Miner and Burton 23 Propofol use is increasing for UGI endoscopy sedation.Reference Triantafillidis, Merikas, Nikolakis and Papalois 21 , Reference Vargo, Cohen, Rex and Kwo 22 , Reference Lichtenstein, Jagannath and Baron 24 , Reference Vargo 25 In stable endoscopy patients, the rate of complications with propofol, when administered and monitored appropriately, was the same or lower than with traditional sedation.Reference Igea, Casellas and González-Huix 20 Evidence also supports propofol’s safety in high-risk and bleeding UGI endoscopy patients.Reference Ljubičić, Supanc, Roić and Sharma 9 , Reference Tohda, Higashi and Sakumoto 10 , Reference Heuss, Schnieper and Drewe 26 , Reference Ooi and Thomson 27
Ketamine, a dissociative agent with sedative, analgesic, and amnestic properties, has been used safely for ED PSA.Reference Strayer and Nelson 28 Its advantages are a rapid onset (30 sec) and relatively brief duration of action (10-20 min).Reference Bahn and Holt 18 , Reference Strayer and Nelson 28 Ketamine has been used successfully for pediatric UGI endoscopy patients, but literature on its use in adult endoscopies is scarce.Reference Amornyotin 11 , Reference Green, Klooster and Harris 29 In our study, ketamine alone or with propofol was chosen more often for endoscopy than for orthopedic patients (27.8% v. 2.7%), likely because of its favourable hemodynamic profile in potentially compromised patients. Its effect on blood pressure is minimal when compared to propofol alone, which can cause hypotension due to negative inotropic and vasodilatory effects.Reference Godwin, Burton and Gerardo 30 , Reference Tobias and Leder 31 There is some concern about an increased risk of laryngospasm with ketamine use for procedures that stimulate the posterior pharynx, such as endoscopy, although this may be less relevant in adults due to their larger airway.Reference Green, Klooster and Harris 29 , Reference Green, Roback, Kennedy and Krauss 32 In a retrospective case series of 548 pediatric ketamine sedations for UGI endoscopy, transient laryngospasm occurred in 8.2% but resolved spontaneously or was easily reversed with brief-assisted ventilation.Reference Green, Klooster and Harris 29 Although laryngospasm did not occur in our ketamine-sedated endoscopy cases, the extensive airway management skills of ACP sedationists make them ideally suited to manage this potential complication. Our study may add some preliminary evidence that ketamine can be successfully used for adult UGI endoscopy in an ED setting.
LIMITATIONS
This study has several limitations. Due to a lack of historical records, we were unable to compare the current practice of using ACPs to perform PSA for emergent UGI endoscopy directly to the previous practice of sedation by gastroenterologists. As an alternative, we selected orthopedic cases from our PSA database and matched patients by age, gender, and ASA class to UGI endoscopy cases. Proportional matching allowed for the detection of differences between the orthopedic and endoscopy groups but evidently could not yield information about the relative safety of PSA performed by ACPs as compared to gastroenterology staff.
The standardized PSA record used in our ED also has limitations. Patient weight was not consistently recorded. We therefore could not calculate weight-based medication doses. This limits our conclusions about medication dosing, because patients in the orthopedic and endoscopy groups may have differed in body habitus, despite matching for age, gender, and ASA class. Errors in the ED PSA record may have occurred. As mentioned previously, one case of hypoxia was missed. We also did not examine procedure time, recovery time, and in-hospital mortality.
This study was limited by a small sample size, short study period, and analysed data from a single tertiary care centre only. A potential confounding factor is the lack of specific training of ACPs for UGI endoscopy sedations, which may have influenced the adverse events observed. Finally, the use of ACPs in procedural sedation is not routine in Canadian EDs, and our results may not be generalizable to other institutions where emergency physicians perform PSA.
CONCLUSION
This study provides a benchmark of outcomes for ED sedations performed by ACPs for emergent UGI endoscopy as compared to orthopedic procedures. Adverse events occurred rarely in both groups with the notable exception of hypotension. Hypotension was more frequent in patients undergoing UGI endoscopy, and only these patients required vasopressor treatment. This underscores the importance of pre-procedural resuscitation in volume-depleted patients undergoing sedation for this procedure. A general shift in PSA providers’ practice patterns is also required. Although adequate pain control is the sedationist’s main task for most other ED procedures, including orthopedic manipulations, managing hemodynamic compromise must be the top priority during endoscopy. Furthermore, one endoscopy patient required intubation in our study, emphasizing the need for vigilance with respect to airway management in these high-risk patients as well. This study provides preliminary evidence that ACPs cannot only successfully manage ED PSA for orthopedic cases, but also for the inherently more compromised population undergoing emergent UGI endoscopy.
Acknowledgements
The authors wish to thank the ACPs and CCPs at the QEII Health Sciences Centre ED for their commitment to this project and Ms. Corinne DeMone for her assistance with this study.
Competing interests
None declared.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cem.2018.372








