Obesity, defined as excess body fatness( Reference Dietz and Bellizzi 1 ), is a major public health problem among adolescents in many countries, including New Zealand. The prevalence of overweight and obesity in New Zealand adolescents aged 15–18 years has risen from 26 % in 1997 to 37 % in 2009( Reference Russell, Parnell and Wilson 2 , 3 ). Obesity predisposes adolescents to physical and psychological consequences, which together link to increased co-morbidities( Reference Reilly, Methven and McDowell 4 ) and reduced quality of life( Reference de Beer, Hofsteenge and Koot 5 , Reference Keating, Moodie and Swinburn 6 ). Since obesity in adolescence is likely to persist into adulthood( Reference The, Suchindran and North 7 , Reference Craigie, Matthews and Rugg-Gunn 8 ), it poses a significant threat to adolescents’ current and future health.
Adolescents have been shown to have unsatisfactory dietary status as marked by suboptimal intakes of certain nutrients and food intakes that do not meet dietary recommendations( 3 , Reference Kobe, Stimec and Ribic 9 , Reference Savige, Ball and Worsley 10 ). Food intakes departing from a healthy eating pattern have been hypothesized to contribute to adolescent obesity( Reference Savige, Ball and Worsley 10 , Reference Newby 11 ). However, existing research investigating diet in relation to obesity has produced inconsistent results possibly due to the focus on the roles of individual foods, food groups and nutrients( Reference Rodriguez and Moreno 12 ). As foods are not eaten in isolation, consideration of overall dietary patterns has emerged as a more intuitive approach to better understand the role of diet in obesity development( Reference Rodriguez and Moreno 12 , Reference Nicklas, Baranowski and Cullen 13 ). A diet index generates a summary score representing overall diet quality and is used as one of two common approaches to describe dietary patterns( Reference Waijers, Feskens and Ocke 14 ).
Some studies have examined diet quality by means of diet indices in relation to body composition in populations that include adolescents aged 14–18 years( Reference Acar Tek, Yildiran and Akbulut 15 – Reference Mirmiran, Azadbakht and Esmaillzadeh 24 ). The majority use diet indices originally developed using adult guidelines, which may differ from paediatric guidelines. These are predominantly based on adherence to the US dietary recommendations( Reference Acar Tek, Yildiran and Akbulut 15 , Reference de Andrade, de Azevedo Barros and Carandina 17 , Reference Feskanich, Rockett and Colditz 18 , Reference Hurley, Oberlander and Merry 20 ) and the Mediterranean diet( Reference Farajian, Risvas and Karasouli 25 , Reference Jennings, Welch and van Sluijs 26 ) which may not be applicable to other countries. There is also a scarcity of studies that employ measurements other than BMI and waist circumference, such as dual-energy X-ray absorptiometry (DXA)( Reference Hurley, Oberlander and Merry 20 ) and bioelectrical impedance analysis (BIA)( Reference Jennings, Welch and van Sluijs 26 ), to assess the diet–obesity relationship in paediatric populations. To date, only one study has examined the association between diet quality and DXA-measured body composition in adolescents. However it focused on relatively young African American urban adolescents aged 11–16 years( Reference Hurley, Oberlander and Merry 20 ).
Two knowledge gaps are identified here: (i) adolescent- and country-specific diet indices have seldom been used to describe diet quality in relation to body composition; and (ii) associations with multiple measures of body composition have not been examined in older adolescents. Therefore, the present study aimed to examine potential associations between diet quality, assessed by a New Zealand-specific diet quality index, and different measures of body composition in a sample of New Zealand adolescents aged 14–18 years. We hypothesized that higher diet quality is associated with a more favourable body composition.
Experimental methods
Study design and sample
The Otago School Students Lifestyle Survey Two (OSSLS2) is a cross-sectional survey undertaken to assess diet and associated behaviours in adolescents from Otago, New Zealand. Invitations to participate in the survey were extended to eighteen out of twenty-four secondary schools in Otago. Six schools were not invited to participate as they had a total Year 11–13 roll of less than forty and were more than an hour travel time away from Dunedin. From the eleven schools that agreed to participate, Year 11–13 students (aged 14–18 years) from one class from each school year in smaller schools, up to four classes in larger schools, were invited to take part in the study. In the week before data collection, each student received an invitation package that included a study information sheet for participants and their parents, a participant consent form and a parental opt-out form. Students who were present at school on the day of data collection and returned the signed consent forms were eligible to participate in the study.
Measures
On the school visit day, an online OSSLS2 Questionnaire was administered in school computer rooms during a class period under the supervision of a team of three or more trained research assistants. All questions used were taken from previously validated questions used in other surveys in this age group and were pre-tested in students of the relevant age before use in OSSLS2. Anthropometry assessment was conducted in the same class period in a private, separate room or screened-off area. Data collection was completed across two school terms from February until June 2011. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Otago Human Ethics Committee.
Demographic measures
Participants reported information including age, sex, date of birth, ethnicity and residential address in the first section of the OSSLS2 Questionnaire. Ethnicity was classified into three main categories: ‘Māori’, ‘Pacific’ and ‘New Zealand European or Others’ (NZEO) using a hierarchical approach( 27 ). NZEO refers to the group of New Zealand Europeans and ethnic groups other than Māori and Pacific. Socio-economic status was determined using two indicators: (i) the New Zealand Deprivation Index 2006 (NZDep2006); and (ii) the School Decile Rating. The NZDep2006 is a neighbourhood-based index of deprivation based on the census meshblocks( 27 ) and was calculated based on the self-reported residential address of participants. Quintile 1 represents the least deprived area; while quintile 5 represents the most deprived area( Reference Salmond, Crampton and King 28 ). As NZDep2006 data were not available for every participant, this information was used only for description of the participants’ background. School Decile Rating is a school-based index that reflects the family socio-economic status of students at participating schools. Decile 1 schools are the 10 % of schools with the highest proportion of students from low socio-economic backgrounds; while decile 10 schools are the 10 % of schools with the lowest proportion of these students( 29 ). As all the schools participating in the present study were from decile 5 and above, we further collapsed the decile rating into ‘medium’ for deciles 5 to 8 and ‘high’ for deciles 9 and 10.
Diet quality assessment
Five summary questions that had been used in National Nutrition Surveys documented intake of recommended food groups( Reference Russell, Parnell and Wilson 2 , 3 ), followed by a validated non-quantitative FFQ( Reference Wong, Parnell and Black 30 ). Participants were asked to indicate their frequency of intake from one of seven frequency categories, ranging from ‘none’ to ‘more than once a day’. Both summary and food frequency data were used to calculate a score of diet quality, namely the New Zealand Diet Quality Index for Adolescents (NZDQI-A). The composition and scoring system of the NZDQI-A are summarized in Table 1. In brief, this food-based diet quality index assesses diet variety and adequacy based on adherence to the serving recommendations for five major food groups as depicted in the New Zealand Food and Nutrition Guidelines for Healthy Adolescents( 31 ). The validity of the NZDQI-A had been examined in a pilot sample of the OSSLS2. It had been shown to be associated with more desirable nutrient intakes independent of energy and valid for ranking diet quality against 4 d estimated food records( Reference Wong, Parnell and Howe 32 ).
Table 1 Components and scoring of the New Zealand Diet Quality Index for Adolescents (NZDQI-A)Footnote *

* Adapted from Wong et al. ( Reference Wong, Parnell and Howe 32 ).
† Ratio calculated as the different subgroups (v) consumed at least once in a week divided by the total subgroups (V) in a food group.
‡ Based on achievement of the recommended daily servings as suggested by the Ministry of Health( 31 ).
§ For each component, a score is calculated by multiplying v/V by A. The possible score range is 0 to 20. For example, for a person who consumes at least three daily servings of vegetables from three varieties in a week, Vegetables score=(3/6)×20=10.
Anthropometry and body composition assessment
Standing height, weight, waist circumference, fat mass and fat-free mass were collected by trained research assistants using standardized procedures. Height was measured without shoes to the nearest 0·1 cm using a portable stadiometer. Body weight was measured in light clothing and no footwear to the nearest 0·1 kg. BMI (kg/m2) was calculated and age- and sex-specific BMI Z-scores were determined using the WHO reference 2007 method( Reference de Onis, Onyango and Borghi 33 ).
Waist circumference was measured at the midpoint between the iliac crest and lowest palpable rib( 34 ) over bare skin using a calibrated steel tape (Lufkin SECA, Germany). A third measurement was taken if the discrepancy between the first two measurements exceeded 0·5 cm. Waist-to-height ratio was calculated and a cut-off value of more than 0·5 was used to define central obesity as this had been reported to correctly discriminate between New Zealand adolescents with low and high levels of DXA-measured regional body fat( Reference Taylor, Williams and Grant 35 ).
Body composition (fat mass and fat-free mass) was measured using the segmental BIA technique (BC418, Tanita, Tokyo, Japan). This BIA machine produces reliable estimates of fat mass and fat-free mass with CV of 0·74 % and 0·18 %, respectively (PML Skidmore, unpublished results). Fat-to-lean mass ratio, fat mass index (FMI) and fat-free mass index (FFMI) were also calculated to compare fat mass and fat-free mass in individuals of differing height( Reference Schutz, Kyle and Pichard 36 ). The following formulas were used: fat-to-lean mass ratio=fat mass (kg)/fat-free mass (kg); FMI=fat mass (kg)/height2 (m2); FFMI=fat-free mass (kg)/height2 (m2).
Statistical analysis
All statistical analyses were performed using the statistical software package Stata version 11·1 at 5 % significance levels. Sex differences in demographic characteristics and body composition were examined using the χ 2 test for categorical variables and the Mann–Whitney U test for continuous variables with non-normal distribution. To examine associations between diet quality and body composition, generalized estimating equations were employed, taking into account data clustering by school. NZDQI-A scores were included as the independent variable while BMI Z-score, waist circumference, waist-to-height ratio, body fat percentage, fat-to-lean mass ratio, FMI and FFMI were the outcome variables. All regression models were assessed for normality by examination of the model residuals plotted against their normal scores. Three regression models were generated and were adjusted for potential variables known to be associated with dietary patterns and body composition in adolescents, including sex, age, ethnicity and socio-economic status( Reference Golley, Hendrie and McNaughton 19 , Reference Aounallah-Skhiri, Traissac and El Ati 37 , Reference Kontogianni, Farmaki and Vidra 38 ). Specifically, Model 1 was adjusted for sex while the Model 2 was additionally adjusted for age. The last model (Model 3) was adjusted for sex, age, ethnicity and School Decile Rating. In a secondary analysis, Model 3 was repeated using NZDep2006 to replace School Decile Rating. However, as this different marker of socio-economic status made no difference to the effect sizes and data were not available for all participants, School Decile Rating was used as the indicator of socio-economic status in the presented data analyses. Regression beta coefficients (β) were expressed as the percentage change in geometric mean of an outcome variable for a unit change in the NZDQI-A score. For BMI Z-score, regression coefficients were expressed as the change in BMI Z-score for a unit change in the NZDQI-A score.
Results
Sample characteristics
Of the 730 eligible participants who took part in OSSLS2, 681 (93 %) had complete anthropometry, demographic and food intake data and were included in the present study. The study sample was representative of the overall OSSLS2 study population with regard to age, school year and BMI (data not shown). Table 2 presents the demographic characteristics of the participants included in the present study. Except for age and school year distribution, there were no significant sex differences with regard to the distribution of other demographic variables. The overall mean NZDQI-A score was 52·4 (sd 15·4) and ranged from 9·0 to 90·5. There were no significant differences in NZDQI-A scores between males and females or by weight status (normal v. overweight or obese; data not shown).
Table 2 Sample characteristics of the Otago School Students Lifestyle Survey Two (OSSLS2)Footnote *

IQR, interquartile range; NZDQI-A, New Zealand Diet Quality Index for Adolescents; NZEO, New Zealand European or Others; NZDep2006, New Zealand Deprivation Index 2006.
* Continuous variables are presented as median and interquartile range; categorical variables are presented as frequency and percentage.
† P value of a Mann–Whitney U test for age and NZDQI-A, or of a χ 2 test for other variables.
‡ Values represented as median and IQR.
§ Defined using the age- and sex-specific BMI cut-offs by Cole et al. ( Reference Cole, Flegal and Nicholls 50 , Reference Cole, Bellizzi and Flegal 51 ).
|| Defined as waist-to-height ratio greater than or equal to 0·5.
Association with body composition measures
The associations between NZDQI-A scores and body composition measures were examined using regression models as presented in Table 3. Higher NZDQI-A scores were significantly associated with lower body fat percentage and fat-to-lean mass ratio. Significant negative associations between NZDQI-A scores and body fat percentage and fat-to-lean mass ratio were seen even after adjustment for sex, age, ethnicity and School Decile Rating using three regression models. As shown in the multivariate model (Model 3), each 10-point increase in NZDQI-A score was associated with a 1·9 % decrease in geometric mean of body fat percentage and a 2·6 % decrease in geometric mean of fat-to-lean mass ratio. In addition, a higher NZDQI-A score was significantly associated with a lower FMI after multivariate adjustment.
Table 3 Association between New Zealand Diet Quality Index for Adolescents (NZDQI-A) and body composition measures in the Otago School Students Lifestyle Survey Two (OSSLS2)
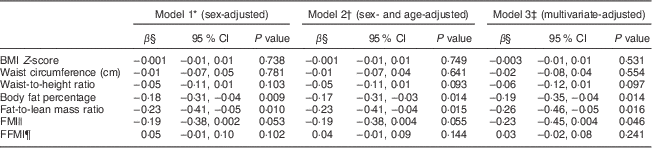
β, unstandardized beta coefficient; FMI, fat mass index; FFMI, fat-free mass index.
* Regression models adjusted for sex.
† Regression models adjusted for sex and age.
‡ Regression models adjusted for sex, age, ethnicity and socio-economic status (assessed by School Decile Rating).
§ Regression coefficients expressed as the percentage change in geometric mean of the outcome variable for a unit change in the NZDQI-A score. For BMI Z-score, regression coefficient is expressed as the change in BMI Z-score for a unit change in the NZDQI-A score.
|| Calculated by dividing fat mass (kg) by the square of height (m2).
¶ Calculated by dividing fat-free mass (kg) by the square of height (m2).
Discussion
The aim of the present study was to examine associations between diet quality and body composition in a sample of New Zealand adolescents. As well as being the first in New Zealand, to our knowledge, the present study is the first one in adolescents that examines the diet quality–body composition association by measuring body composition using BIA and employing a food-based diet index approach. Confirming our hypothesis, the study demonstrated that a higher NZDQI-A score is associated with a lower body fat percentage, fat-to-lean mass ratio and FMI after adjustment for sociodemographic confounders.
BMI, being simple and practical to measure( Reference Pietrobelli, Faith and Allison 39 ), has been used as the indicator of body composition in the majority of studies that examined diet quality and body composition among adolescents( Reference Acar Tek, Yildiran and Akbulut 15 – Reference Mariscal-Arcas, Romaguera and Rivas 23 ). Four studies found significant associations between diet quality and BMI( Reference Chiplonkar and Tupe 16 , Reference Feskanich, Rockett and Colditz 18 , Reference Golley, Hendrie and McNaughton 19 , Reference Kosti, Panagiotakos and Mariolis 22 ); however, only two of these found an inverse association between diet quality and BMI( Reference Feskanich, Rockett and Colditz 18 , Reference Kosti, Panagiotakos and Mariolis 22 ). The other two studies found positive associations after adjustment for multiple confounders such as age, household education and income( Reference Chiplonkar and Tupe 16 , Reference Golley, Hendrie and McNaughton 19 ). The use of different types of diet index and inconsistent adjustment for confounders may contribute to these contradictory results. Better diet quality assessed using the Youth Healthy Eating Index (HEI) and HEI was found to be inversely associated with BMI in a large cohort of older children and adolescents aged 9–14 years (n 16 771) in the USA( Reference Feskanich, Rockett and Colditz 18 ). The associations with BMI were, however, weak (r=−0·08 and r=−0·12, respectively) and unadjusted for potential confounders. In another study by Kosti et al. ( Reference Kosti, Panagiotakos and Mariolis 22 ), a higher Diet-Lifestyle Index score was associated with lower odds of being overweight or obese among Greek adolescents aged 12–17 years. However, as the composite index incorporated dietary and lifestyle behaviours, the significant BMI association may not necessarily be linked only to diet quality( Reference Kosti, Panagiotakos and Mariolis 22 ). Using multivariate regression models, our study revealed that NZDQI-A scores were not associated with BMI Z-scores. One possible explanation for this is that diet quality affects specific aspects of body composition (i.e. fat mass) rather than total body weight. BMI is a proxy measure of body fatness because it measures excess weight rather than excess body fat( Reference Sweeting 40 ). BMI performs well as an indicator of excess fatness among overweight adolescents but not normal-weight adolescents, whose BMI differences may be largely due to differences in amounts of fat-free mass( Reference Freedman, Wang and Maynard 41 ).
Only two studies had examined the association between diet quality and waist circumference in adolescents using the HEI-2005( Reference Acar Tek, Yildiran and Akbulut 15 ) and an Australian dietary guideline index( Reference Golley, Hendrie and McNaughton 19 ). However, both studies showed no( Reference Acar Tek, Yildiran and Akbulut 15 ) or unexpected positive associations( Reference Golley, Hendrie and McNaughton 19 ). Our study showed that increased diet quality was related neither to waist circumference nor its height-adjusted derivative (i.e. waist-to-height ratio). This lack of association may be related to the lack of variability in the waist circumference data due to our relatively ethnically homogeneous sample. Besides BMI and waist circumference, we used additional body composition measures to examine any potential associations with diet quality. NZDQI-A scores were associated with body fat percentage, fat-to-lean mass ratio and FMI independent of sex, age, ethnicity and socio-economic status (Table 3). In our study, every 10-point increase in NZDQI-A score was associated with decrease of about 2 % in total body fat percentage. That is to say, that those in the lowest third of NZDQI-A (median score=36) would have approximately 6 % more body fat than those in the highest third of NZDQI-A (median score=68). A large study in American children and adolescents showed that the odd ratios of being in the most adverse cardiovascular risk factor category increased significantly with every 5 % increase in percentage body fat( Reference Going, Lohman and Cussler 42 ). While currently there is no definitive definition of a clinically meaningful body fat change, these small body fat differences may be important in reducing the risk of chronic diseases in the long term or at the population level.
The present study produced results that corroborate the findings of Hurley et al. ( Reference Hurley, Oberlander and Merry 20 ), the only study to date which had examined diet–body composition associations among adolescents using a country-specific diet index and multiple measures of body composition. In their study, HEI was significantly associated with lower percentage body fat and abdominal fat as measured by DXA, but not with BMI, among 196 African American adolescents aged less than 16 years( Reference Hurley, Oberlander and Merry 20 ). Therefore, our findings provide support for the conceptual premise that total body fatness, rather than body weight or central fatness, may have a stronger association with diet quality. This can be seen by the significant associations of diet quality with all variables that measure overall body fat (body fat percentage, fat-to-lean mass ratio, FMI), but not with BMI, waist circumference or waist-to-height ratio. These measures should be used in combination with a more direct measure of body fatness, such as BIA whenever possible, in investigating the diet–body composition relationships.
The mechanisms explaining the association between diet quality and body composition are not well understood. As previously shown in the validation study of the NZDQI-A( Reference Wong, Parnell and Howe 32 ), higher scores were associated with lower total fat intake independent of total energy intake. Therefore, it is likely that higher NZDQI-A scores reflect healthier food choices, which may in turn lower excess energy intakes from high-fat foods and therefore provide a less obesity-promoting diet. The NZDQI-A used in the present study focused primarily on meeting the New Zealand Food and Nutrition Guidelines for recommended servings of food groups and variety within them( 31 ) rather than energy balance. While not designed to be an obesity-specific index, diet variety and adequacy of food groups have previously been shown to be associated with adiposity among adolescents( Reference Azadbakht and Esmaillzadeh 43 , Reference Bradlee, Singer and Qureshi 44 ). It may be likely that participants who scored high in the NZDQI-A are adolescents who presented with more healthful lifestyle behaviours( Reference Kontogianni, Farmaki and Vidra 38 , Reference de Moraes, Adami and Falcao 45 , Reference Ottevaere, Huybrechts and Benser 46 ) leading to more favourable energy balance.
Strengths and limitations
The main strength of our study is the relatively large sample of school-aged adolescents with detailed body composition data. The use of various methods for body composition assessment, including body fat, lean body mass and waist circumference in addition to BMI, has enabled comprehensive examination of diet quality in relation to obesity and allowed comparison with previous studies. Another merit lies in the ability of the simple, food-based NZDQI-A to associate with body composition. NZDQI-A has the advantage of simplicity over previously published nutrient-based indices given that estimation of energy and nutrient intakes is not required for its score calculation.
BIA was used in the present study because it is simple to use, non-invasive and relatively inexpensive, making it a feasible field method to measure body composition in large samples( Reference McCarthy, Cole and Fry 47 ). The estimates of fat mass, percentage body fat and fat-free mass from the BIA machine used in the present study were highly correlated with measurements by DXA (r=0·93, 0·92 and 0·96, respectively) in a multi-ethnic adolescent sample in New Zealand( Reference Sluyter, Schaaf and Scragg 48 ). However, we are cautious that the machine tends to underestimate absolute fat mass and percentage body fat in fatter adolescents( Reference Sluyter, Schaaf and Scragg 48 ); therefore the body fatness of some participants in our study may be underestimated.
It was not possible to measure energy intake or dietary misreporting in the present study. Food quantity was not directly measured, but was estimated using summary questions in the OSSLS2 Questionnaire. The use of summary questions might mitigate concerns about over-reporting of fruits and vegetables( Reference McNaughton, Ball and Crawford 49 ); however, we are uncertain about how the summary questions affected reporting of other food groups. Therefore, the chance that a higher NZDQI-A score represents a higher energy intake rather than a better diet quality per se cannot be entirely ruled out, although the earlier validation study in an adolescent sample similar to the present study with respect to age, sex and location showed that the index scores are independent of energy intake( Reference Wong, Parnell and Howe 32 ). Because of the cross-sectional nature of the study, no conclusions on causality can be made and the results should be considered sample-specific.
Conclusion
In conclusion, diet quality as measured by the NZDQI-A was associated with measures of body fat in adolescents aged 14–18 years from Otago, New Zealand. The absence of associations between diet quality scores and surrogate measures of body fat including BMI and waist circumference suggest the possibility that these associations are mainly driven by total body fat in our adolescent population. As the health consequences of obesity are related to excess adiposity, the present study also adds support to the need for a more direct measurement of body fatness, beyond BMI and waist circumference, for more accurate ascertainment of body composition in examining diet–body fat relationships in this age group.
Acknowledgements
Financial support: This work was supported by grants from the National Heart Foundation of New Zealand, Lottery Health Research New Zealand and the University of Otago. The funders had no role in the design, analysis or writing of this article. J.E.W. is a recipient of a University of Otago Postgraduate Publishing Bursary. Conflict of interest: None. Authorship: P.M.L.S. and K.E.B. (principal investigators) obtained funding, conceived and designed the study. J.E.W., A.S.H. and A.C.L. were involved in the study design and data collection. P.M.L.S., W.R.P. and J.E.W. were responsible for the conception of this manuscript. J.E.W. analysed the data and wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Otago Human Ethics Committee.





