INTRODUCTION
Epidemiological evidence indicates that aerosols produced by cooling towers are one of the main causes of large community-wide outbreaks of Legionnaires' disease (LD) [Reference Dondero1–Reference Keller13], however, prevention of these kinds of outbreaks continues to pose considerable difficulties. The concentration of the population in the cooling tower area of influence and the opportune implementation of control measures are some of the factors that determine the variability and duration of such outbreaks and the number of affected people. The case-fatality rate sometimes exceeds 10% [Reference Nguyen8, Reference Watson12], and only rarely is <1% [Reference Brown4].
An outbreak of LD occurred in June 2006 in Pamplona (195 983 inhabitants), capital city of the region of Navarre, Spain. The outbreak was first detected on 1 June, when an increase in the number of cases of community-acquired pneumonia was reported in relation to district 2 of Pamplona (22 012 inhabitants), which is a commercial, financial and administrative area of the city and is visited daily by a large number of people who live in other neighbourhoods. Legionella pneumophila antigen in urine (Binax Now; Binax Inc., Scarborough, ME, USA) was detected in some of these cases [Reference Barricarte14]. Hence, the search for the source of infection was directed mainly to these and other nearby districts. The active search resulted in the detection of 146 cases of LD by 12 June. The epidemiological, microbiological and environmental investigations of this outbreak are described below.
METHODS
Case finding
From the time the outbreak was detected on 1 June 2006, an active prospective and retrospective search for cases clinically compatible with Legionella pneumonia in emergency services, hospitals and primary health-care centres was implemented, together with a protocol for radiological and microbiological confirmation and appropriate treatment of all cases. The alert was transmitted to the Spanish and European epidemiological surveillance networks [Reference Barricarte14], which resulted in two cases being detected associated with the outbreak diagnosed in other regions (Aragón and Asturias).
A case was defined as a patient with clinical symptoms of pneumonia, a compatible chest radiology study and microbiological confirmation of L. pneumophila antigen in urine (Binax Now). For several days before the outbreak, an elevated incidence of pneumonia in persons with a urine antigen test positive for Streptococcus pneumoniae and negative for Legionella had been observed. For this reason, only cases of pneumonia with a positive urine antigen test for Legionella were taken into account.
Epidemiological study
Clinical reports were consulted to evaluate whether they met the case definition and to determine the date of diagnosis, and all cases were followed until the patient recovered or was discharged from hospital. All cases were interviewed either personally during hospital admission or by telephone at home, to collect information about sex, age, symptom onset, residence, work, places visited during the incubation period (2–10 days before symptom onset) [Reference Heymann15] and other risk exposures for Legionella infection. The interviews took place on the same day the diagnosis was confirmed or the days immediately following. Given the obvious cluster of cases in district 2 of Pamplona, we investigated how long the cases had stayed in this or a neighbouring district, either because they lived or worked there, or had visited the area during the incubation period. When more than one of these circumstances coincided, residence in district 2 was given priority. The outbreak occurred in a financial, administrative and commercial area, which is also a popular walking area in the city, making it difficult to document the route taken and places visited. For this reason, the epidemiological analysis to locate the source was based on the attack rates for the city of Pamplona according to district and section (an administrative unit of about 1000 inhabitants) of residence. The resident population by sex, age and administrative unit distribution was obtained from a national register updated to January 2006. The total population of the city of Pamplona was taken as the reference population to calculate the rates adjusted for age and sex. Poisson regression was used to model the incidence rates of district 2 residents by sex, age and section.
Environmental investigation
On 2 June, all 31 cooling towers and seven ornamental fountains in district 2 were studied. In all cooling towers, the concentration of biocides authorized by the Spanish Ministry of Health was found to exceed the minimum level recommended by the manufacturer. Although rapid tests (Binax Now) have not been validated for environmental samples, given the emergency situation, they were performed to detect L. pneumophila antigen in the water of the cooling towers in situ and were repeated in the laboratory after 100 times concentration. At this first inspection, a sample of water from each cooling tower and ornamental fountain was taken to isolate Legionella in the laboratory. The existence of unregistered cooling towers or other installations that might pose a risk was ruled out after air inspection by helicopter.
Records of chlorine levels in the public water network of Pamplona showed that they remained between 0·6 and 0·8 parts per million. This network supplies all homes in the city and is used to fill all the cooling towers.
Records from the weather station located in district 2 of Pamplona were reviewed.
Microbiological analysis
Environmental samples were processed for Legionella culture in the Public Health Laboratory of Navarre according to ISO 11731/1998 and following an accredited method. At least five typical colonies were taken for identification from each positive cooling tower, and two colonies were sent to the reference laboratory in the National Centre of Microbiology for typing. Clinical and environmental L. pneumophila isolates were identified by immunofluorescence with rabbit antisera against L. pneumophila (14 serogroups) and eight other Legionella species, as has previously been described [Reference Pelaz, García and Martín-Bourgon16]. L. pneumophila sg 1 was typed by monoclonal antibodies (mAb) with International and Dresden mAb panels [Reference Joly17, Reference Helbig18] by immunofluorescence, and compared by two molecular methods.
(a) Amplified fragment length polymorphism (AFLP) was performed according to the standardized European Working Group on Legionella Infections (EWGLI) protocol for the epidemiological typing of L. pneumophila sg 1 [Reference Fry19]. Molecular Analyst software (Bio-Rad Laboratories, Hercules, CA, USA), the Dice coefficient and the unweighted pair group method with averages (UPGMA) were used for gel analysis and clustering. AFLP patterns were compared with 42 previously defined representative strains included in the Spanish AFLP type collection [Reference Baladrón and Pelaz20].
(b) Pulsed field gel electrophoresis (PFGE) was performed using SfiI as restriction enzyme [Reference Lück21]. Electrophoresis was carried out in a CHEF DR II (Bio-Rad) system with a constant voltage of 200 V for 22 h using a linear switch time ramp of 0·5–75 s.
RESULTS
A total of 146 cases of pneumonia confirmed by L. pneumophila antigen in urine were included in the analysis. The median age was 61 years (range 21–97), and 72 cases (49%) occurred in men. Slightly more than half of the cases (52%) required hospitalization, and seven (5%) received intensive care, but no deaths were reported. In five hospitalized patients Legionella was isolated in the sputum, and all were identified as L. pneumophila sg 1 Pontiac (Allentown/France) mAb subgroup, AFLP type CNM 037 and PFGE type A.
Temporal analysis
Three at first apparently unrelated cases of LD were diagnosed on 30 and 31 May, but it was not until the afternoon of 1 June when several new cases of pneumonia in a primary health- care centre alerted us of a possible outbreak. By night of the same day, 10 confirmed cases had been detected, all of which were found to be related to district 2 of Pamplona. Twenty-four hours after the alert was activated 61 cases had been confirmed, and a total of 146 confirmed cases had been detected by 12 June, after which no new cases were seen.
The median time between symptom onset and diagnosis was 3 days, and in 95% of cases this time was less than 7 days. The first case started to show symptoms on 27 May and the last one on 9 June. The epicurve showed two peaks of incidence, on 30 May and 1 June. Two approximations were used to determine the probable period of the spread of Legionella (Fig. 1):
(a) Eight cases had been in the presumed risk area only once during the incubation period:
The dates of the single exposure of these cases were distributed between 23 May and 30 May.
(b) As a function of the dates of symptom onset and the incubation period:
date of the first case (27 May) – minimum incubation (2 days)=25 May;
date of the last case (9 June) – maximum incubation (10 days)=30 May.
Combining these two methods, the period of Legionella spread could be delimited to the time between 23 May and 30 May. Although we cannot rule out transmission in the days immediately before or after, the probability decreases as we move further away from these dates. For all cases of LD detected in Navarre before 30 May or after 12 June, the epidemiological nexus with the outbreak was ruled out.
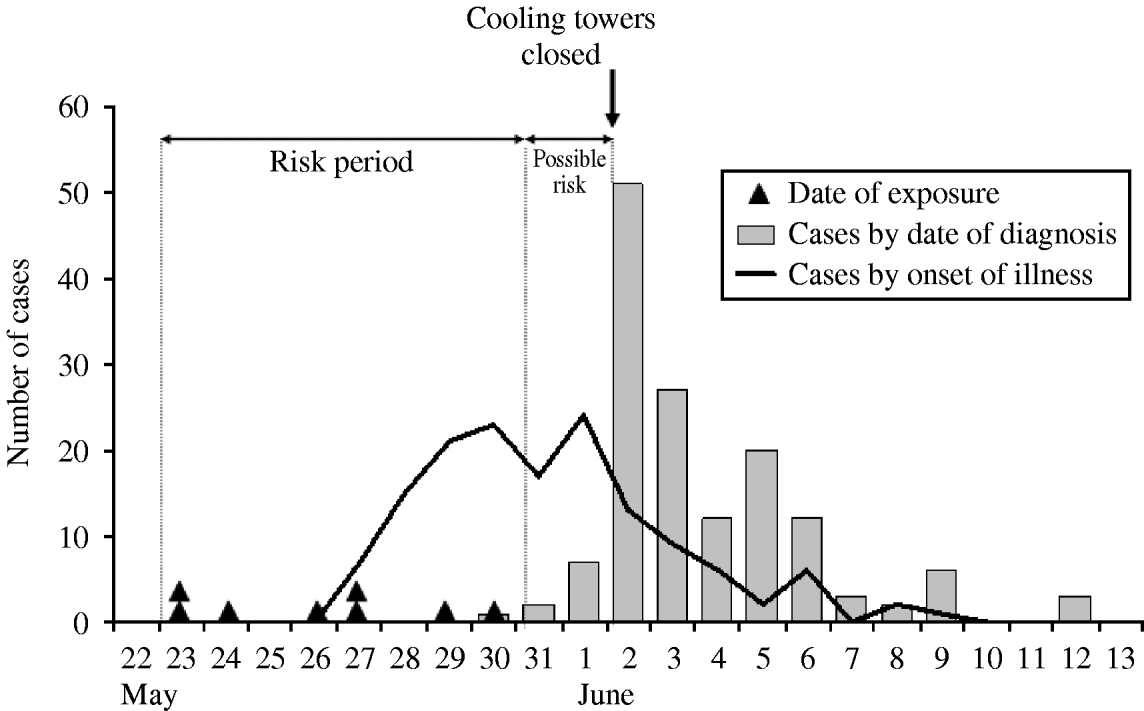
Fig. 1. Number of Legionnaires' disease cases by date of onset of illness and date of diagnosis in the outbreak of Pamplona, Spain, 2006. Black triangles indicate visits to district 2 of Pamplona by persons visiting the area a single time during the incubation period.
Epidemiological study
Of the 146 cases, 65 (45%) resided in district 2, and another 73 cases (50%) visited this district for work or other reasons during the incubation period. Eight cases (5%) were not in district 2 during the incubation period, but resided in other neighbouring districts. On 4 June, an additional case of LD was diagnosed in Navarre in a person who had not visited Pamplona during the entire incubation period, but who had other possible risk exposures for Legionella. This case was not considered linked to the outbreak.
Two of the cases confirmed by culture lived in district 2, and two other cases had visited the district during the incubation period. The fifth case was a woman who lived in a neighbouring district 1048 m from the source, and she reported not having left her house during the incubation period.
All 123 cases residing in Pamplona were used to calculate the Legionella pneumonia attack rates per administrative unit. The incidence rates did not show significant differences according to sex, but increased with age (Table 1).
Table 1. Legionella pneumonia cases and incidence rates per 1000 inhabitants by sex and age in resident populations of Pamplona and district 2, May–June 2006

Table 2 shows the rates for Legionella pneumonia in the districts of Pamplona. Only district 2 presented a rate significantly higher than the city average (P<0·0001). Section 2 of district 2, with 16 cases and an adjusted rate of 12·3 cases/1000 inhabitants, had more than double the rate of all other sections (P<0·0001) (Fig. 2). Another four sections of district 2, all of them south of section 2, presented an adjusted incidence rate of >3 cases/1000 inhabitants, significantly higher than the average district rates (P<0·05), but significantly lower than the rate in section 2 (P<0·0001).

Fig. 2. Sex- and age-adjusted Legionnaires' disease incidence rates in Pamplona, Spain, by sections. White square represents the cooling tower related to the outbreak.
Table 2. Incidence of Legionella pneumonia by district of Pamplona and by section of district 2, Pamplona, May–June 2006
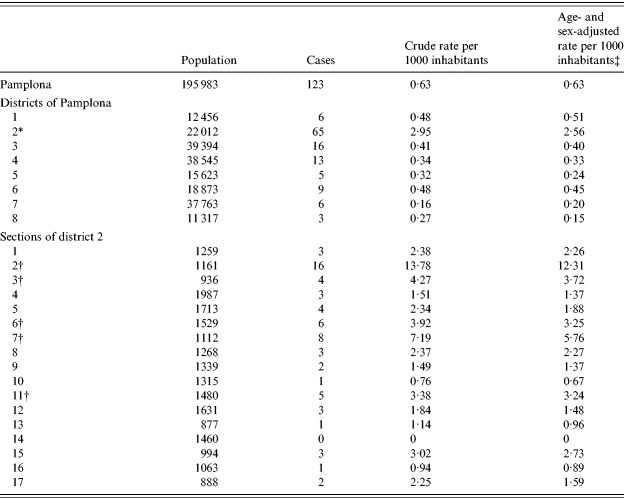
* District 2 is the only one that differs significantly (P<0·0001) from the average rate for Pamplona.
† Adjusted incidence rates significantly higher than the average rate for district 2.
‡ Taking Pamplona as the reference population.
The lack of significant differences by sex in the Legionella pneumonia attack rate remained in the multivariate analysis of the resident population of district 2. Taking those aged 20–29 years as the reference group, the incidence rate was found to be eight times higher among those aged 40–59 years and about 20 times higher in those aged ⩾60 years. The previously described pattern of spatial distribution by sections was maintained in the multivariate analysis (Table 3).
Table 3. Association between Legionnaire's disease and sex, age and section of residence. Results of multivariate Poisson regression analysis of population resident in district 2 of Pamplona

* Sections with adjusted incidence rate lower than 1/1000 inhabitants.
Seventy-six patients (52%) required hospitalization. This proportion was higher among patients aged ⩾60 years (62%, P=0·009), but there were no statistically significant differences by sex, reason for being in district 2, or district of residence.
Environmental inspection and microbiological study
In four of the 31 cooling towers, L. pneumophila antigen was detected by the rapid test, and on 2 June these towers were temporarily closed as a preventive measure. Legionella grew in culture from three of these four cooling towers [⩾104 colony-forming units per litre (c.f.u./l) of sample] and from another cooling tower (103 c.f.u./l) that had not tested positive with the rapid test; this tower was also closed. Five typical colonies from each positive culture were processed and all of them were identified as L. pneumophila sg 1. Table 4 shows a comparison of the results obtained by rapid test and culture. In the present outbreak, rapid tests showed a 75% sensitivity and 96% specificity in the screening of cooling towers. In the four cooling towers where Legionella was isolated only non-oxidant biocides had been used, and in all cases the concentration was higher than the recommended minimum.
Table 4. Comparative results obtained by Legionella pneumophila antigen rapid tests (Binax Now) and cultures of samples from the 31 cooling towers studied

Sensitivity=3/4=0·75→75%.
Specificity=26/27=0·96→96%.
Three cooling towers contained L. pneumophila sg 1 OLDA, while the forth one contained L. pneumophila sg 1 Pontiac (Allentown/France). Three towers in which Legionella was isolated were located in section 2 of district 2; the fourth one was located in section 7 of the same district. L. pneumophila isolates from a cooling tower situated in section 2 of district 2 matched the five clinical strains by all typing methods used (Fig. 3). L. pneumophila isolates from the other three cooling towers were different by all methods (Table 5).
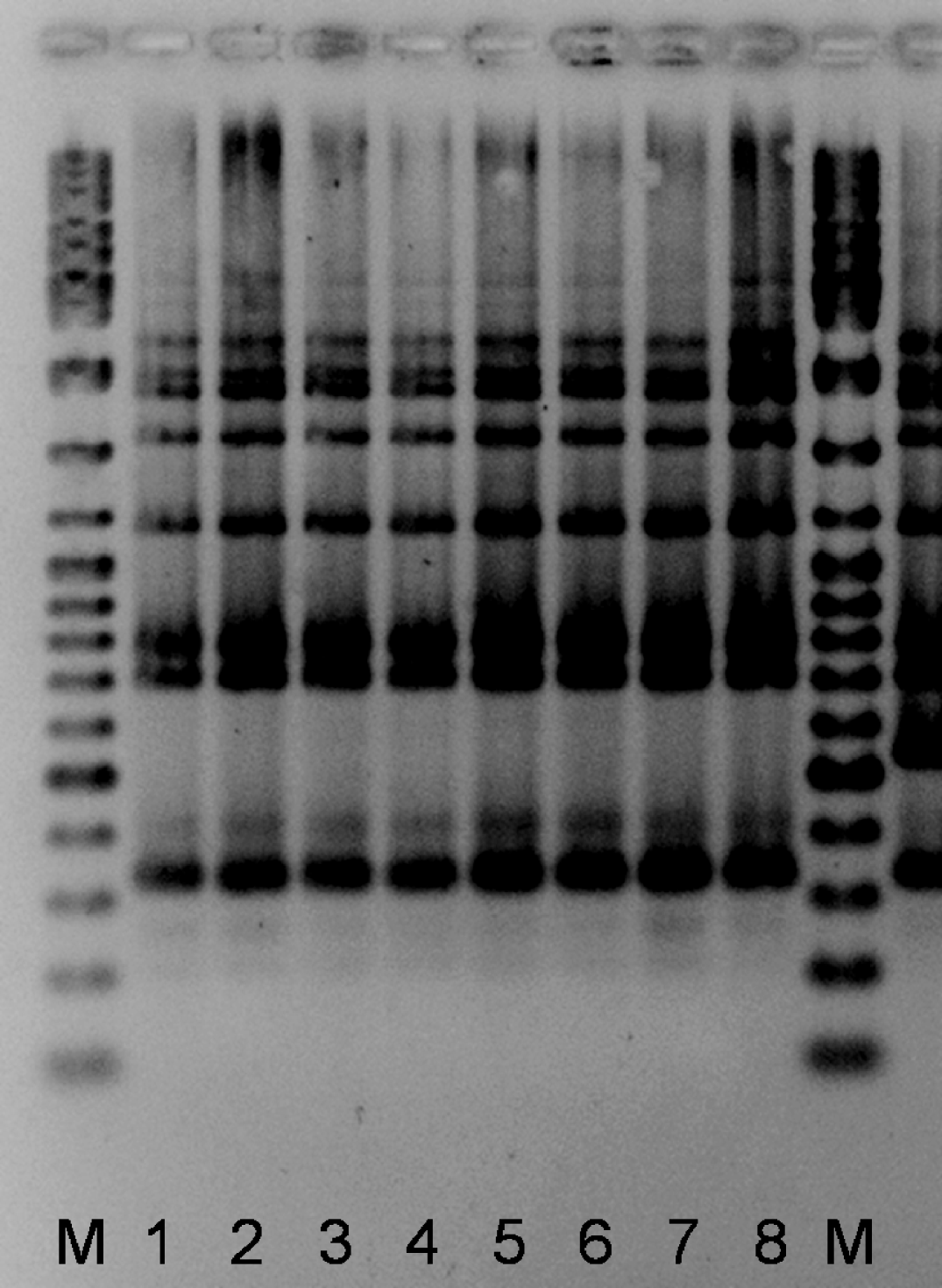
Fig. 3. Amplified fragment length polymorphism gel containing human and environmental L. pneumophila sg 1 (Allentown/France, mAb type) isolates. M, Molecular weight marker (Ladder Mix, MBI Fermentas, Vilnius, Lithuania). Lanes 1–4, patients 1–4, respectively. Lanes 5 and 6, patient 5. Lanes 7 and 8, two colonies from cooling tower 4 (probably responsible for the outbreak).
Table 5. Characterization of the strains of Legionella isolated from humans and cooling towers in the outbreak of Pamplona, 2006
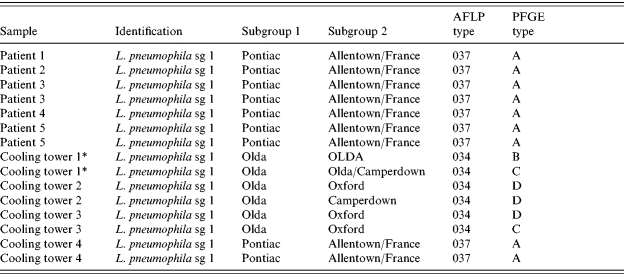
* Cooling tower 1 was negative for Legionella pneumophila antigen in rapid test.
Eight days after the first towers were closed, no new cases related with the outbreak were detected.
Weather and environmental conditions
During the period of the probable spread of Legionella (23–31 May), there was no rain, and northerly mild winds predominated. On 21 May, for the first time in the season, a maximum temperature of 28°C was reached, with a second peak of 32°C on 27 May and a return to 20°C after 29 May. Major construction projects were underway in district 2, with frequent movements of construction materials within 200 m of the towers where Legionella was isolated.
DISCUSSION
We have described an explosive community outbreak with 146 confirmed cases of Legionella pneumonia in 13 days. The outbreak was detected quickly, and the immediate systematic measures taken – alerting physicians and emergency personnel and rapid diagnosis of suspected cases – made it unlikely that any cases of Legionella pneumonia were missed. The urine antigen test was systematically performed only in pneumonia patients, but non-pneumonia profiles could have gone undetected. At least four non-pneumonia profiles with positive L. pneumophila antigen in urine were detected. Varying percentages of non-pneumonia cases have been described in other outbreaks [Reference Barrufet-Barque7, Reference Bursed22].
The absence of case fatality, despite the large number of cases, is in contrast with other community outbreaks [Reference Barrufet-Barque7, Reference Addis23, Reference Den Boer24]. The explosive quality and early detection of the outbreak not only led patients to quickly seek assistance at hospital emergency units, but also allowed us to alert and coordinate health-care services and emergency units to perform an accurate diagnosis and to initiate antibiotic treatment immediately; these factors have been reported as linked to low case fatality [Reference García-Fulgueiras2, Reference Heath, Grove and Looke25, Reference Benin, Benson and Besser26].
In contrast to other outbreaks [Reference García-Fulgueiras2, Reference Benin, Benson and Besser26], we did not find a higher incidence in men than women, however, our findings were consistent with other studies in detecting an increasing incidence with age.
The fact that the strains isolated in the cases were identical to those obtained in one cooling tower suggests that this may have been the source of infection. Epidemiological analysis also supports this hypothesis, since the highest attack rate was observed in the administrative unit where this tower is located, and the rest of the most affected sections were located in the direction of the predominating winds. Moreover, 77 cases (52%) lived within a radius of 1 km from this tower, and all the cases had visited or lived within a radius of <2 km, reflecting a greater risk of infection associated with proximity to the source [Reference García-Fulgueiras2, Reference Heymann15].
In the present outbreak an identical strain was found in the cooling tower and in a patient who lived 1 km from the tower. Another three cases, confirmed only by urine antigen test, lived between 1·5 and 2 km from the source and reported not having visited areas near the source. In a study of an outbreak of LD in France, some cases were found to be associated with a source situated at a distance of >6 km [Reference Nguyen8].
The estimated period of spread followed the first hot days of the season. This could facilitate the multiplication of Legionella and probably resulted in increased cooling tower activity. A mild wind could increase the distance over which these aerosols are disseminated [Reference Addis23]. The presence of construction works has been related with the origin of other outbreaks [Reference Cayla27, Reference Storch28]. Although all the evidence points to the aerosols produced by one cooling tower as the cause of the outbreak, environmental dust produced by the construction could have facilitated the proliferation of Legionella in the area of the neighbouring cooling towers.
Since 2003, Spain has had legislation requiring that all cooling towers and evaporation condensers be registered, and that these installations have maintenance plans establishing the procedures and frequencies of cleaning and disinfection [29, Reference Ordóñez-Iriarte30]. All the towers inspected in this outbreak met these requirements, suggesting that these measures may not always be enough to ensure they will not be a danger to health. The experience acquired with this outbreak led the Regional Health Council of Navarre to promulgate stricter regulations, including the following requirements: express permission of the Health Authority for the installation of new towers; filtration, control of conductivity and use of biodispersants in the water circulating in the tower; preferential use of oxidant biocides and, in the case of non-oxidant biocides, use of two different products with continuous dosage; more frequent cleaning in conditions of severe environmental contamination or proximity to construction or demolition projects; and, in outbreak situations, shutdown, disinfection and inspection of all suspect towers [31].
The manufacturer of the Binax Now Legionella urinary antigen test recommends that it not be used to test environmental samples (i.e. potable water), and we do not know of any validation study for this use. However, in the emergency situation of a LD outbreak, rapid testing was shown be useful for the screening of potential environmental sources of L. pneumophila and for the early application of control measures. In our case, rapid testing made it possible to close down preventively the four cooling towers a few hours after the outbreak was detected. One of these towers was subsequently shown to be the probable cause of the outbreak. All the evidence seems to indicate that, from that time on, no new infections occurred, as the last case related to the outbreak had onset of symptoms 8 days later. Nevertheless, the epidemiological evidence is equally compatible with the possibility that transmission could have ceased any time between 30 May and 2 June. Thus, we cannot be certain that the closure of the tower was the determining factor in ending the outbreak.
In this study we have described an explosive outbreak of Legionella associated with a cooling tower situated in a central and frequently visited area of the city of Pamplona, Spain. Efficient coordination between public health officials and the health-care services permitted early detection of the outbreak, which probably contributed to the null case fatality and to early control of the sources. Existing regulations for the inspection and control of installations where there is a risk of Legionella spread were shown to be insufficient to protect public health, thus they were reviewed and modified following the outbreak.
ACKNOWLEDGEMENTS
We thank Agurtzane Zabala for administrative assistance; Fátima Irisarri, Francisco Guillén and Javier Gost for collaborating in the survey of patients; Javier Iribarren, Jacinto Irisarri, José María Barricarte, Beatriz Agudo, Marta García and José Luis Rodrigo for their help in inspecting the cooling towers; Josetxo Herrero and Marina Nuñez of the Instituto de Estadística de Navarra for providing population and geographical information; the health professionals of the emergency wards of the Hospital de Navarra, Hospital Virgen del Camino and Ambulatorio General Solchaga; the doctors of the II Ensanche Primary Health Care Centre for their useful contribution to the early detection of the outbreak; and Kathy Fitch for editorial assistance. This study was supported in part by the Spanish Fund for Health Research (FIS PI061346) and by the Carlos III Institute for Health (CB 06/02/0074).
DECLARATION OF INTEREST
None.












