Introduction
The overweight/obesity status continues its global rise, Reference Afshin, Forouzanfar and Reitsma1 compromising women of reproductive age since the risk of developing gestational diabetes mellitus is approximately two, four, and eight times higher in overweight, obese, and severely obese women. Reference Chu, Callaghan and Kim2
The Developmental Origin of Health and Disease (DOHaD) hypothesis associates environmental conditions in early life, such as maternal nutritional status, with the offspring’s metabolic health in the long term. Reference Barker3,Reference Ong and Ozanne4 In addition, maternal obesity contributes to a raised risk of obesity and insulin resistance in the offspring in childhood, adolescence, and adult life. Reference Nicholas, Morrison, Rattanatray, Zhang, Ozanne and McMillen5 Indeed, maternal obesity during pregnancy and lactation in mice increased beta-cell mass due to beta-cell proliferation, Reference Gniuli, Calcagno and Caristo6 inducing adverse pancreatic changes in the progeny. Reference Bringhenti, Moraes-Teixeira, Cunha, Ornellas, Mandarim-de-Lacerda and Aguila7
Melatonin (N-acetyl-5-methoxy tryptamine), a pleiotropic hormone, is implicated in circadian rhythm and is involved in glucose homeostasis. Reference Karamitri and Jockers8 Melatonin is an endogenous indoleamine secreted by the pineal gland and shows bioactive anti-inflammatory properties in epigenetic regulation and fetal development. Reference Voiculescu, Zygouropoulos, Zahiu and Zagrean9,Reference Tain, Huang and Chan10 Its short-term use does not cause adverse effects, even at extreme doses. Reference Andersen, Gögenur, Rosenberg and Reiter11 In addition, melatonin has been studied as a reprogramming factor for diseases related to maternal metabolic programming. Reference Tain, Huang and Hsu12,Reference Feng, Zhang and Li13
Melatonin might protect against oxidative stress, Reference Xu, Liu and Zhao14 facilitating electron transfer antioxidant processes in the mitochondrial membrane. Reference Tan, Manchester, Qin and Reiter15 Also, melatonin induces the endogenous synthesis of superoxide dismutase (Sod), glutathione peroxidase (Gpx), and glutathione reductase, enzymes with antioxidant activities or stimulates enzymes that metabolize reactive species. Reference Tamura, Nakamura and Korkmaz16
The role of melatonin in pregnancy is emerging, but maternal melatonin supplementation’s long-term metabolic effects are not well-known. Reference Feng, Zhang and Li13 Also, melatonin signaling influences the placenta directly Reference Berbets, Davydenko, Barbe, Konkov, Albota and Yuzko17 and regulates the proliferation, apoptosis, and invasion of trophoblasts in preeclampsia by inhibiting endoplasmic reticulum (ER) stress. Reference Zhou, Ding, Yu, Nie and Yang18 In addition, we have demonstrated recently that melatonin supplementation in obese mothers can alleviate the development of nonalcoholic liver disease in their male offspring by decreasing lipogenesis and increasing beta-oxidation in the liver tissue. Reference Ajackson, Nagagata, Marcondes-de-Castro, Mandarim-de-Lacerda and Aguila19
These findings allow us to hypothesize that maternal melatonin supplementation during gestation and lactation in a known model of diet-induced obesity (DIO) in mice Reference Fraulob, Ogg-Diamantino, Santos, Aguila and Mandarim-de-Lacerda20,Reference Mandarim-de-Lacerda, Del Sol, Vazquez and Aguila21 would mitigate the development of altered glucose metabolism and insulin resistance, inflammation, ER stress, oxidative stress, and islet remodeling and beta-cell dysfunction in adult male offspring.
Material and methods
Animals and procedures
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals (National Academies Press, 8th Edition, Washington, DC, 2011) and ARRIVE guidelines. Reference du Sert, Hurst and Ahluwalia22 The local ethics committee approved animal experimentation (CEUA protocol n° 003/2021). Male and female mice of the C57BL/6 lineage at one month of age were maintained in ventilated cages under a controlled and enriched environment (NexGen system, Allentown Inc., PA, USA, 21 ± 2°C, 12 h/12 h dark/light cycle) with free access to water and food.
Females (mothers) were randomly assigned to one of two experimental diets for eight weeks before the mating: control diet (17 % kJ as fat, defined as C group, n = 20) or high-fat diet (49 % kJ as fat, defined as HF group, n = 20) to allow for the development of obesity. Males (fathers) received just the C diet. The Pragsoluções (Jau, SP, Brazil) manufactured the diets based on rodents’ AIN-93G recommendations Reference Reeves, Nielsen and Fahey23,Reference Aguila, Ornellas and Mandarim-de-Lacerda24 (Supplementary Table S1). Then, two weeks before mating, the C and HF female mice were again randomly allocated to one of two supplemented groups, Melatonin (Mel) or vehicle, forming four groups: C; CMel, HF; HFMel (Fig. 1).

Figure 1. Experimentation timeline. 4th-week-old female C57BL/6 (future mothers) were grouped according to the allocated diet control (C) or high fat (HF). Melatonin supplementation (Mel) started in the 10th week, and females were regrouped as C, CMel, HF, and HFMel (Mel extended for the preconception, pregnancy, and lactation periods). The animals were mating with nonconsanguineous males of the same age in the 12th week. Male offspring were randomly grouped at weaning, fed the C diet, and sacrificed at the 12th-week-old.
Melatonin (M5250 Sigma-Aldrich Co., St Louis, MO, USA) was daily subcutaneously administrated at 10 mg/Kg one hour after starting the dark vivarium cycle. Reference Baydas, Nedzvetsky, Nerush, Kirichenko, Demchenko and Reiter25 The melatonin supplementation lasted eight weeks during the gestation and lactation until the offspring weaning. The mothers assigned to the groups without melatonin supplementation received an equal dose of an alcoholic-saline solution as a vehicle. In addition, the mothers’ body weight (BW) and food intake (FI) were measured daily.
At three months old, one female from each group was crossed for mating. After confirming a vaginal plug formation (day 1 of pregnancy), the mother mice were individually housed and continued their diet. At birth, offspring sex was assessed based on the anogenital distance, Reference Wolterink-Donselaar, Meerding and Fernandes26 weighed, and continued with their mother until weaning (Fig. 1).
At weaning, one male offspring was randomly taken off each litter to form the experimental groups, and they were fed with the C diet. The male offspring groups were named considering the mother’s diet and supplementation, thus: C, CMel, HF, and HFMel (n = 10/each). In addition, the offspring’s BW was measured at birth and then weekly, and FI was measured daily.
Carbohydrate metabolism
The mother’s oral glucose tolerance test (OGTT) was performed two days before mating and one day after weaning. Offspring OGTT was analyzed at 12 weeks old. First, the animals fasted for six hours and took a 2 g/kg glucose load by orogastric gavage. Then, blood was collected from the tail vein after zero, 15, 30, 60, and 120 min, and glucose was measured (glucometer Accu-Chek, Roche, SP, Brazil), allowing the “area under the curve” calculation (GraphPad Prism, v. 9.5.1 for Windows, La Jolla CA, USA).
Furthermore, the fasting insulin resistance index (FIRi) and quantitative insulin sensitivity check index (QUICKi) were performed on mothers and on offspring to measure insulin resistance and insulin sensitivity, respectively: FIRi = (fasting glucose × fasting insulin)/25) Reference Pang, Xi, Huang, Cui, Gong and Zhang27 and QUICKI = 1/[log(fasting insulin (µU/mL) + log(fasting glucose (mg/dL)]. Reference Katz, Nambi and Mather28
Sacrifice and tissue extraction
We sacrificed the mothers two days after weaning and the adult offspring at 12 weeks old. The animals fasted for six hours and were heparinized (Dalteparin Sodium, Fragmin, Pfizer, SP, Brazil, 200 mg/kg) and anesthetized (intraperitoneal Ketamine 240 mg/kg and Xylazine 30 mg/kg). Blood was collected through the cervical vessels section, and plasma was separated from the blood by centrifugation (712 xg for 15 min).
Immediately the pancreas was dissected, weighed, and fixed (n = 5, formaldehyde at 4 % w/v, phosphate buffer 0.1 M pH 7.2), then embedded in Paraplast plus (Sigma-Aldrich Co., St Louis, MO, USA) or inflated through the pancreatic duct with Hank’s solution (n = 5, supplemented with bovine serum albumin, BSA, 1.0 mg/mL). Next, the islets were isolated after collagenase digestion (type V 0.8 mg/mL, Sigma-Aldrich Co., St Louis, MO, USA), the exocrine portion was discarded, and the islets were manually collected in a Petri dish and used to analyze static insulin secretion in vitro (n = 15 islet/group) or frozen at −80°C for molecular analysis.
Plasma
In mothers, we measured adiponectin (Mouse adiponectin ELISA kit #EZMADP-60K, Millipore, Missouri, USA) and insulin (Rat/mouse Insulin ELISA Kit #EZRMI-13K, Millipore, Missouri, USA).
In offspring, we measured the C-Peptide, Glucose-dependent Insulinotropic Peptide (GIP), Glucagon, Interleukin (IL)-6, Insulin, Leptin, Peptide YY (PYY), and Tumor Necrosis Factor-alpha (TNFa) by Multiplex Biomarker Immunoassays for Luminex xMAP technology (Millipore, Billerica, MA, USA, cat. #MMHMAG-44K). Furthermore, to measure adiponectin, we used an enzyme-linked immunosorbent assay (Mouse adiponectin ELISA kit #EZMADP-60K, Millipore, Missouri, USA).
Pancreas
The pancreas prepared for light microscopy was entirely sectioned at 5 µm thickness, and sections were stained with hematoxylin and eosin or incubated with anti-insulin antibodies for immunohistochemistry analysis. The observations and digital photomicrographs were obtained in a Nikon microscope (model 80i and DS-Ri1 digital camera, Nikon Instruments, Inc., New York, USA).
We analyzed 15 nonconsecutive random sections in each animal. First, the islet volume density (Vv [islet, pancreas]) was estimated by point-counting, and islet mass (M [islet, pancreas]) was estimated as the product of Vv [islet, pancreas], and pancreas mass. Second, the numerical density per area of the islets (QA [islet, pancreas]) was determined by taking into consideration the edge effect in the counts). Reference Gundersen29 Then, the islet cross-sectional area was determined as A [islet, pancreas] = Vv [islet, pancreas]/2*QA [islet, pancreas]. Reference Mandarim-de-Lacerda and Del-Sol30,Reference Mandarim-de-Lacerda31
Furthermore, we used image analysis in sections incubated with anti-glucagon (CSB-PA002654, Cusabio, 1:100) and anti-insulin (sc-9168, Santa Cruz Biotech, CA, USA; 1:100) to estimate the volume density of alpha and beta cells. Briefly, the sections were incubated with biotinylated secondary antibodies and streptavidin-peroxidase conjugates, washed in PBS, revealed with liquid diaminobenzidine (DAB, Histostain Plus Kit, Invitrogen, CA, USA), and counterstained with hematoxylin. Then, using the ImagePro Plus 7.1 for Windows (Media Cybernetics Corp., Rockville, MD, USA), islets were outlined, and the deconvoluted color was the DAB image measured in intensity units, then converted to the optical density. Reference Mandarim-de-Lacerda, Santos and Aguila32 Finally, alpha and beta-cell mass was estimated as the product of [Vv [alpha-cell] (or Vv [beta-cell]), and M [islet]. Reference Marinho, Aguila and Mandarim-de-Lacerda33,Reference Marinho, Martins, Cardoso, Aguila and Mandarim-de-Lacerda34
Glucose-stimulated insulin secretion in vitro
Fifteen isolated islets per group were incubated for 30 min at 37°C in a Krebs–Ringer bicarbonate buffer (KRB) growth medium containing 115 mM NaCl, 5 mM KCl, 10 mM NaHCO3, 2.56 mM CaCl2, 1 mM MgCl2, and 15 mM HEPES. This solution was supplemented with glucose 5.6 mM and BSA 0.3% (pH 7.4, Sigma-Aldrich Co., St Louis, MO, USA) and continuously gassed with 95% O2/5% CO2. Next, the culture medium was replaced with fresh buffer, and five islets per group were incubated for another hour at three different concentrations of glucose (2.8, 11.1, or 22.2 mM.). Finally, the insulin level was measured (rat/mouse Insulin ELISA Kit #EZRMI-13K, Millipore, Missouri, USA).
Quantitative real-time polymerase chain reaction (RT-qPCR)
Briefly, the isolated pancreatic islets had the total RNA extracted using Trizol (Invitrogen, CA, USA), and 1 µg of mRNA was treated with DNAse I (Invitrogen) and evaluated with Nanovue (GE Healthcare Life Sciences, Piscataway, NJ, USA). cDNA was synthesized (Oligo dT) from the mRNA of the samples, and cDNA was mixed with the gene of interest primer and SYBR Green Mix (Invitrogen). First, the expression of the TATA- box binding protein (Tbp) gene was performed and used as the reference gene for mRNA standardization. Next, negative controls were performed in wells in which the cDNA was replaced by deionized water. Then, the qPCR was evaluated with the Step One Plus real-time PCR cycler system (Applied Biosystems by Life Technologies, Waltham, Massachusetts, USA) and SYBR Green mix (Invitrogen). In addition, signal amplification was measured using the 2−ΔΔCt method to estimate the difference between target gene cycles and endogenous control. Reference Rao, Lai and Huang35 Primer sequences were designed by Primer3 software (http://bioinfo.ut.ee/primer3-0.4.0/).
Statistical analysis
Data were tested for normality (for small samples, Shapiro–Wilk test) and homogeneity of variances (Bartlett test) and then shown as mean and standard deviation. The differences between groups were tested using Student’s t-test with Welch’s correction (mothers, two groups) or two-way ANOVA followed by Tukey’s multiple comparison test (GraphPad Prism v.9.5.1 for Windows, GraphPad Software, San Diego, CA, USA). We accepted the P-value <0.05 statistical significance.
Results
Mother data
Before mating, after eight weeks of diet, the HF mother was heavier than the C mother, and melatonin supplementation did not alter BW. However, the OGTT’s area under the curve (AUC) was 20% higher in HF mothers than in C mothers and 12% lower in HFMel mothers than in HF mothers. After weaning, BW increased by +14% in HF mothers than in C mothers and decreased by −12% in HFMel mothers than in HF mothers. Food intake did not show a difference among the groups. However, the energy intake was higher in HF mothers than in C mothers (Table 1).
Table 1. Biometry and plasma analyses of mothers

AUC, area under the curve; BW, body weight; EI, energy intake; FI, food intake; FIRi, Fasting Insulin Resistance index; OGTT, oral glucose tolerance test; PW, pancreas weight; QUICKi, Quantitative Insulin Sensitivity Check Index. Groups: C, control; HF, high fat; Mel, melatonin. Mean ± SD, P < 0.05 when: †≠ C; ‡≠ HF.
The HF mothers showed hyperinsulinemia (+130%), insulin resistance (FIRi +236 %), and diminished insulin sensitivity (QUICKi −21%) compared to the C mothers, which were restored in the HFMel vs. HF. In addition, lower plasmatic adiponectin levels (−34%) were observed in the HF mothers than in the C mothers, but higher levels of adiponectin (+58%) and an improvement of glucose metabolism was seen in the HFMel mothers in comparison to the HF mothers (Table 1).
Offspring data
At birth, the HF offspring was heavier by +16%, and the CMel by +8% than the C offspring (Fig. 2B). The initial difference observed in CMel vs. C was not maintained in the following weeks, but a heavier HF offspring existed until week 12 compared to the C offspring. However, BW decreased in the HFMel offspring from the fourth week compared to the HF offspring. At twelve weeks, the HF offspring was heavier by +9% than the C offspring, and HFMel lost weight by −7% than HF (Fig. 2A). In addition, FI and EI were increased in the HF offspring compared to the C offspring, but reduced in HFMel than in HF.

Figure 2. Offspring body weight evolution and oral glucose tolerance test. A. Body weight evolution; B. Birthday body weight. C. Oral glucose tolerance test curves; D. Area under the curve. Data are mean ± SD, n = 10/group, Groups: C (control), CMel (control melatonin), HF (high fat), and HFMel (high-fat melatonin) P < 0.05 when †≠ C e ‡≠ HF.
There was an “area under the curve” increase (OGTT +15%, Fig. 1B-C), hyperinsulinemia (+30%), insulin resistance (FIRi +46%), and reduced insulin sensitivity (QUICKi −8%) in HF than in C (Table 2). However, HFMel vs. HF showed lessened OGTT (−8%, Fig. 2C-D), plasma insulin (−40%), insulin resistance (FIRi −50%), and increased insulin sensitivity (QUICKi +18%) (Table 2).
Table 2. Biometry and plasma analyses of offspring

BW, body weight; EI, energy intake; FI, food intake; FIRi, Fasting Insulin Resistance index; GIP, gastric inhibitory polypeptide; IL, Interleukin; PW, pancreas weight; PYY, intestinal polypeptide; QUICKi, Quantitative Insulin Sensitivity Check Index; TNFa, tumoral necrosis factor-alpha. Groups: C, control; HF, high fat; Mel, melatonin; Mean ± SD, P < 0.05 when: †≠ C; ‡≠ HF; #≠CMel.
Plasma
The HF offspring, compared to the C one, showed lower adiponectin (−25%), GIP (−60%), and PYY (−45%), and higher IL6 (+88%), TNFalpha (+18%), C-Peptide (+45%), and leptin (+27%). Also, HFMel vs. HF showed higher adiponectin (+18%) and diminished IL6 and TNF alpha (−30%). In addition, the groups did not differ in glucagon (Table 2).
Pancreatic islets
Typical rodent Islet alpha and beta-cell distributions were observed in C and CMel and showed in the first two rows of photomicrographs of Fig. 3. However, islets were hypertrophied in the HF group, but restored in the HFMel group (Fig. 3A). Consequently, alpha and beta cells were hypertrophied in the HF group than in the C group but reduced in the HFMel vs. HF (Fig. 3B-C).

Figure 3. Pancreatic islets in adult offspring. The two rows show islets immunolabeled by anti-glucagon (alpha cells) and anti-insulin (beta cells) (same magnification in all images). A. Islet cross-sectional area, B. alpha-cell mass, C. beta-cell mass. Data are mean ± SD, n = 5/group, *P < 0.05, **P < 0.01. Groups: C (control), CMel (control melatonin), HF (high fat), HFMel (high-fat melatonin).
Glucose-stimulated insulin secretion in vitro
Islet insulin secretion was higher in the HF offspring than in the C one (+530 % at 2.8 mM) but lower in the HFMel offspring than in the HF one (−85 %) (Fig. 4A) as well as at 11.1 and 22.2 mM of glucose (higher secretion in HF than in C, and lower secretion in HFMel than in HF) (Fig. 4B-C). In addition, at 22.2 mM, insulin secretion was lower in CMel than in C (Fig. 4C).

Figure 4. Insulin secretion in isolated islets in adult offspring. Data are mean ± SD, n = 5/group, ***P < 0.001. Groups: Groups: C (control), CMel (control melatonin), HF (high fat), HFMel (high-fat melatonin).
Pro-inflammatory cytokines
Il6, Il1b, and Tnfa were augmented, but Sirt1 was diminished in the HF offspring than in the C offspring. These cytokines were mitigated, and Sirt1 was increased in the HFMel offspring compared to the HF one. Also, CMel offspring showed diminished Il1b and augmented Sirt1 expressions compared to the HFMel offspring (Fig. 5A-D).

Figure 5. Pro-inflammatory markers in the pancreatic islet of adult offspring. A. Il6, interleukin6; B. Il1b, interleukin1 beta; C. Tnfa, tumor necrosis factor-alpha; D. Sirt1, Sirtuin 1. Data are mean ± SD, n = 5/group, *P < 0.05, **P < 0.01, ***P < 0.001. Groups: Groups: C (control), CMel (control melatonin), HF (high fat), HFMel (high-fat melatonin).
Oxidative stress and ER stress
Sod, Catalase, and Gpx were reduced, and Chop, Gadd45, and activating transcription factor 4 (Atf4) were improved in HF than in C. However, HFMel showed higher Sod, Catalase, and Gpx and lower Chop, Gadd45, and Atf4 than HF. In addition, Chop and Atf4 genes were downregulated in the CMel vs. HFMel (Fig. 6A-F).
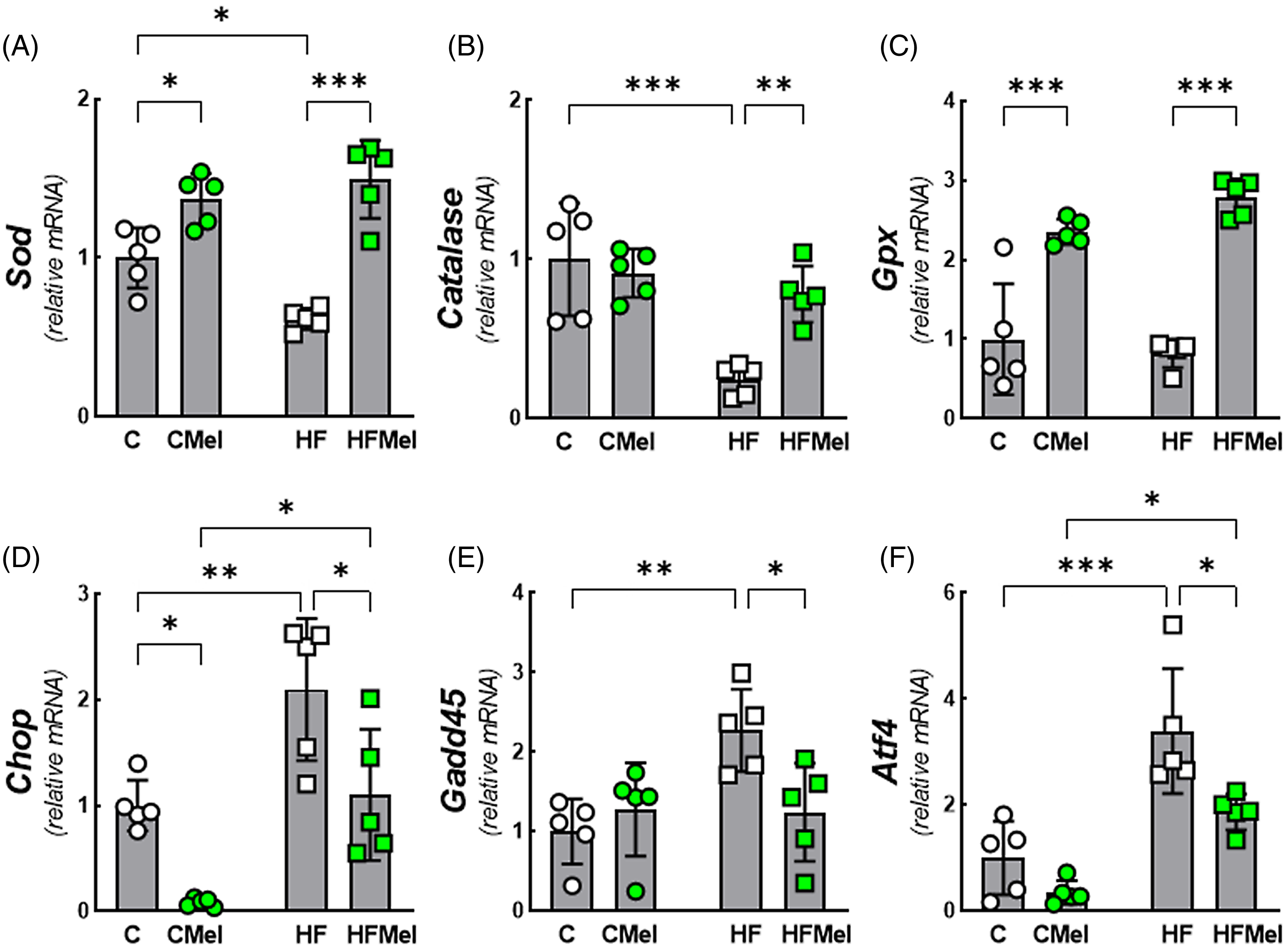
Figure 6. Obese mother melatonin supplementation restored biomarkers of oxidative and endoplasmatic reticulum stress in pancreatic islets of adult offspring. A. Sod, superoxide dismutase; B. Catalase; C. Gpx, glutathione peroxidase. D. Chop, DNA-damage-inducible transcript 3; E. Gadd45, growth arrest and DNA-damage-inducible 45; F. Atf4, activating transcription factor 4. Data are mean ± SD, n = 5/group, *P < 0.05, **P < 0.01, ***P < 0.001. Groups: C (control), CMel (control melatonin), HF (high fat), and HFMel (high-fat melatonin).
Transcriptional factors and beta-cell identity markers
There were decreased expressions of Pdx1 (−36%, Fig. 7A), Mafa (−60%, Fig. 7B), Neurod1 (−65%, Fig. 7C), Pax6 (−31%, Fig. 7F), and Pparg (−75%, Fig. 7H) in HF than in C. However, the expressions of these genes were regulated in HFMel than in HF, except Pax6. In addition, although it has not been decreased in the HF group, aristaless-related homeobox (Arx) (+70%, Fig. 7D) and Ppara (+400%, Fig. 7G) were enhanced in HFMel vs. HF. Conversely, Pax4 was augmented in HF than in C (+260%) but diminished in HFMel vs. HF (−25%, Fig. 7E). Furthermore, Neurod1 and Pax6 were enhanced in CMel vs. HFMel (Fig. 7C and 7F).

Figure 7. Melatonin supplementation of obese mothers improves transcription factors and beta-cell identity markers in adult offspring. A. Pdx 1, pancreatic duodenal homeobox 1; Mafa, v-maf musculoaponeurotic fibrosarcoma oncogene family; C. Neurod1, neurogenic differentiation 1; D. Arx, transcription factor aristaless-related homeobox gene; E. Pax4, paired box 4; F. Pax6, paired box 6; G. Ppara, peroxisome proliferator-activated receptor alpha; H. Pparg, peroxisome proliferator-activated receptor gamma. Data are mean ± SD, n = 5/group, *P < 0.05, **P < 0.01, ***P < 0.001. Groups: C (control), CMel (control melatonin), HF (high fat), HFMel (high-fat melatonin).
Discussion
It is known that obesity in the mother might implicate the offspring’s adverse pancreatic islet cell remodeling and altered metabolism. Reference Mandarim-de-Lacerda36-Reference Ornellas, Karise, Aguila, Mandarim-de-Lacerda, Faintuch and Faintuch38 These alterations are associated with changes in islet pro-inflammatory regulators, oxidative stress, ER stress, and beta-cell integrity in adult offspring, consequently deteriorating insulin production and glycemic control. Reference Cerf39,Reference Cerf40 However, these alterations were mitigated in the offspring when the obese mother received melatonin supplements during pregnancy and lactation, which agreed with our initial hypothesis that melatonin treatment improves insulin sensitivity and glucose tolerance in mice. Reference Sartori, Dessen and Mathieu41 Fig. 8 summarizes our findings.
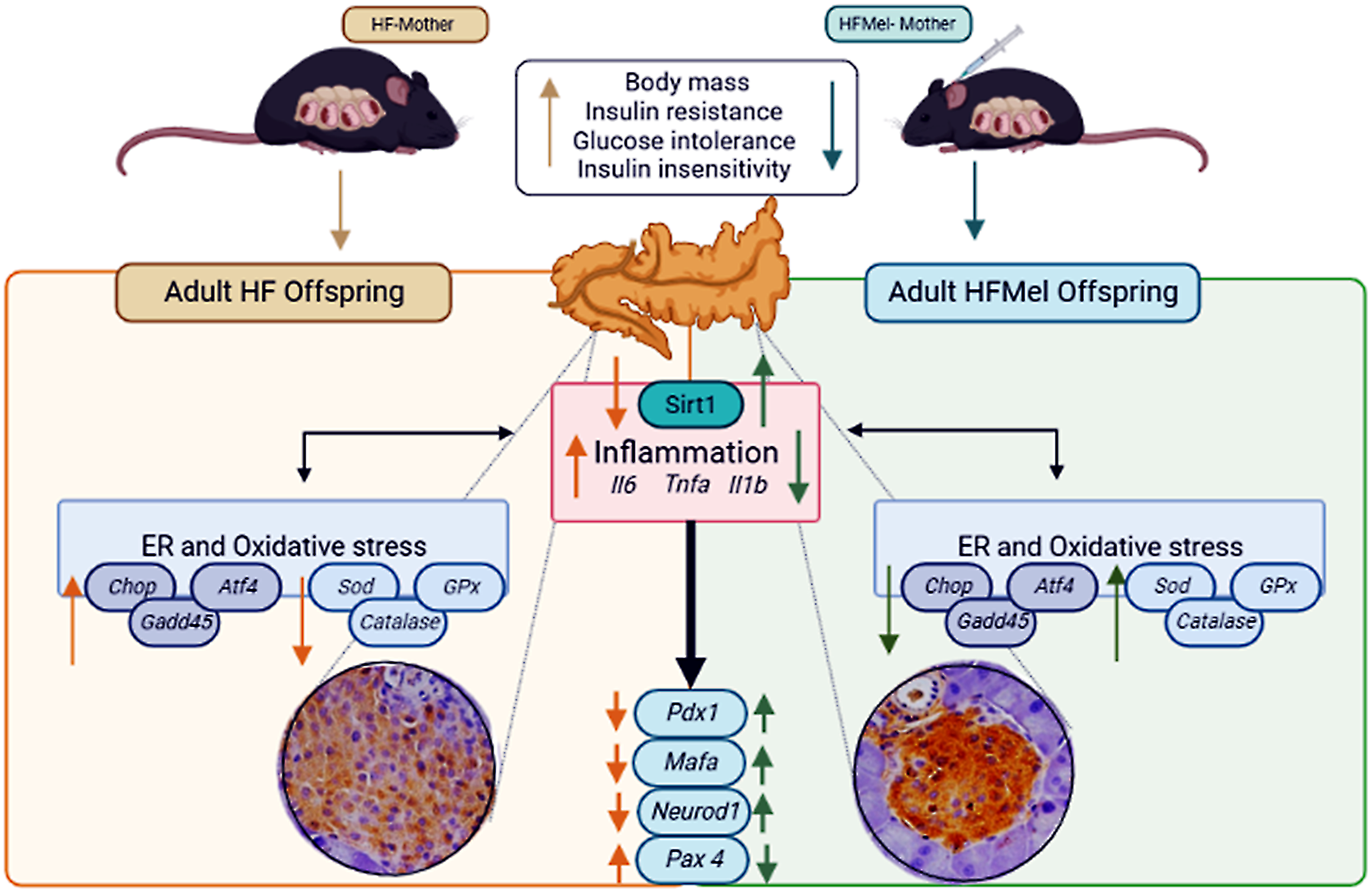
Figure 8. Obese mothers supplemented with melatonin have beneficial effects on their adult offspring (reduced body weight and insulin resistance). In addition, pro-inflammatory cytokines and endoplasmic reticulum stress markers were mitigated, and enzymes related to oxidative stress increased. The hypertrophied islets due to obese mothers were mitigated by melatonin supplementation to mothers. Arrow-up indicates an increase, and arrow-down indicates a decrease. Atf4, activating transcription factor 4; Catalase; Chop, DNA-damage inducible transcript; Gadd45, growth arrest and DNA-damage-inducible 45; Gpx, glutathione peroxidase.; Il1b, interleukin1 beta; Il6, interleukin6; Mafa, v-maf musculoaponeurotic fibrosarcoma oncogene family; Neurod1, neurogenic differentiation 1; Pax4, paired box 4; Pdx 1, pancreatic duodenal homeobox 1; Sirt1, Sirtuin 1; Sod, superoxide dismutase; Tnfa, tumor necrosis factor-alpha.
Fetal programming affects male and female offspring differently. Reference Ornellas, Mello, Mandarim-de-Lacerda and Aguila42-Reference Thompson, Song and Polster44 Therefore, sexual dimorphism merits a more detailed study to investigate the link with maternal melatonin supplementation. Usually, studying male offspring is suitable when we are not interested in sexual dimorphism because males suffer less influence from the variety of sexual hormones. On the contrary, mature female cycle periodically (they go through the four phases of the estrous cycle at each period), which would be another variable to consider in the study, including the moment of sacrifice. Hence, to avoid the inherent aspect of females and focus on the study’s central theme, only males were sampled.
Maternal obesity induces inflammation in offspring, Reference Kretschmer, Schulze-Edinghausen and Turnwald45,Reference Enstad, Cheema and Thomas46 leading to impaired oxidative stress, Reference Igosheva, Abramov and Poston47,Reference Napso, Lean and Lu48 and ER stress. Reference Park, Jang and Bouret49 Melatonin might restore regular physiological function during pregnancy by alleviating oxidative damage in the placenta, favoring nutrient transfer, and improving the placenta vascular dynamics at the uterine-placental interface. Reference Saat, Risvanli and Dogan50-Reference Chuffa, Lupi, Cucielo, Silveira, Reiter and Seiva52 Additionally, maternal melatonin freely crosses the placenta and can influence offspring development, programming several functions related to neural and brain development, energy, and glucose metabolism. Reference Gomes, Motta-Teixeira and Gallo53
Melatonin plays an anti-oxidative effect and protects against obesity-related insulin resistance Reference Xu, Li and Li54 and prevents the generation of reactive oxygen species, regulating the redox state in the beta cell. Reference Zephy and Ahmad55 Also, melatonin is a potent antioxidant molecule and has been reported to increase the antioxidant enzyme expression and activity, Reference Mayo, Sainz, Antoli, Herrera, Martin and Rodriguez56 and melatonin reduces SODK68 acetylation in oocytes in culture. Reference Han, Wang and Li57 In agreement, our findings demonstrated enhanced Sod, catalase, and Gpx, decreasing pro-inflammatory cytokines in offspring, emphasizing the interplay between inflammation and oxidative stress. Reference Dandekar, Mendez and Zhang58
Melatonin regulates GLUT4 expression and triggering via its G-protein-coupled membrane receptors, the phosphorylation of the insulin receptor, and intracellular substrates mobilizing the insulin-signaling pathway. Reference Cipolla-Neto, Amaral, Afeche, Tan and Reiter59 In the current study, melatonin supplementation was linked with weight loss in obese mothers and their offspring, possibly because melatonin determined an adequate energy balance mainly by regulating energy flow to and from the stores. Reference Cipolla-Neto, Amaral, Afeche, Tan and Reiter59 Furthermore, maternal melatonin supplementation diminished glucose intolerance and insulin resistance in mothers and their offspring, improving Pparg expression, an insulin sensitizer.
The upregulation of the oxidative stress enzymes may explain the relief of ER stress observed in the offspring, possibly associated with the increased expression of Sirt1, which alleviates oxidative stress and ER stress Reference Xu, Liu and Zhao14 in HFMel offspring. In addition, melatonin strongly inhibits oxidative stress and partially inhibits ER stress in pancreatic beta cell in vitro. Reference Park, Shim and Na60
Maternal obesity impairs offspring’s beta-cell function in rodents Reference Cerf, Williams and Nkomo61 and humans. Reference Black, Watanabe and Trigo62 Here, maternal obesity negatively affects the genetic regulation of beta-cell differentiation, maturation, and glucose metabolism in the offspring, such as pancreatic duodenal homeobox 1 (Pdx1), v-maf musculoaponeurotic fibrosarcoma oncogene family (Mafa); neurogenic differentiation 1 (Neurod1); paired box 4 (Pax4) and paired box 6 (Pax6). Moreover, some transcription factors are required at specific stages of the islet cell formation stimulating a network of transcription factors regulating cell differentiation genes in the embryonic stages. Reference Murtaugh63
Pdx1 is a crucial transcription factor in different stages of pancreatic development, and the process of differentiation of beta cells starts early in the embryonic period. Reference Offield, Jetton and Labosky64 The expression of Pdx1 assures beta-cell function but decreases during insulin resistance and type 2 diabetes. Reference Yang, Dayeh and Volkov65,Reference Lin and Vuguin66
Mafa is found exclusively in developing and adult insulin cells, linked to insulin cell production. Reference Matsuoka, Artner, Henderson, Means, Sander and Stein67 Moreover, induction of Mafa expression is essential for regenerative approaches to regenerate functional and mature beta cells from pluripotent stem cells. Reference Rezania, Bruin and Arora68
An insulin resistance environment includes hyperglycemia, hyperinsulinemia, increased FIRi, and diminished QUICKi. Insulin seems not to perform its role in reducing blood glucose in our HF animals, even with the pancreatic islet hypersecreting insulin at different glucose concentrations. Besides, Pdx1 deficiency increases beta-cell susceptibility to ER stress, as Pdx1 regulates a wide range of genes involved in diverse ER functions, including the proper formation of disulfide bonds and protein folding, and the unfolded protein response. Therefore, the reduced expression of the Pdx1 gene in HF offspring may favor the beta-cell failure to compensate for insulin resistance related to impaired critical ER functions. Reference Sachdeva, Claiborn and Khoo69 However, it might indicate an early beta-cell failure in this group, programmed by maternal obesity.
Neurod1 is detected in the developing embryonic pancreas Reference Naya, Huang and Qiu70 and, in elderly life, plays a predominant role in the maintenance of functional beta cells. Reference Gasa, Mrejen and Lynn71 Pax4 is transiently expressed in all endocrine progenitors during pancreatic development and downregulated shortly after birth. Reference Lin and Vuguin66 Pax4 appears essential for the appropriate initiation of beta-cell differentiation. Reference Collombat, Hecksher-Sorensen and Broccoli72,Reference Sosa-Pineda73 Remarkably, upregulated gene expressions of Pdx1, Mafa, and Neurod1 were observed in the offspring of obese mothers supplemented with melatonin.
Pax6 is detected at the end of the embryonic period as a critical transcriptional regulator of adult beta-cell identity and function. Reference Lorberbaum, Docherty and Sussel74 Therefore, diminished Pax6 expression might be associated with beta-cell failure in diabetes. Reference Swisa, Avrahami and Eden75 Here, we determined that maternal obesity reduced Pax6 expression, which was not altered by maternal melatonin supplementation.
Arx expression begins during mouse pancreatic development and persists into mature alpha cells. Reference Collombat, Mansouri and Hecksher-Sorensen76 Furthermore, maternal melatonin supplementation increased Arx in offspring. Although Arx is required for early specification and maintenance of alpha-cell mass, Reference Bramswig and Kaestner77 is not directly involved in glucagon expression. Reference Gosmain, Cheyssac, Heddad Masson, Dibner and Philippe78 Therefore, the effect of melatonin on glucagon secretion is controversial. Reference Bähr, Mühlbauer, Schucht and Peschke79,Reference Peschke, Schucht and Mühlbauer80 Here, the impact of melatonin on the offspring was indirect (administered to the mothers), which might explain the absence of changes in glucagon secretion in programming offspring.
The Pax4 and Arx balance is crucial for cell fate determination of islet alpha and beta cells. Reference Collombat, Hecksher-Sorensen and Broccoli72 In the current study, we determined a decrease in the Pax4 gene related to an immature beta cell in the offspring of obese mothers supplemented with melatonin.
Our findings demonstrated that obese mothers supplemented with melatonin favored cell remodeling in the adult offspring’s pancreatic islet and preserved glucose-stimulated insulin secretion in vitro. These findings agree with a report in pinealectomized pregnant rats and impaired glucose metabolism, insulin secretion dysregulation, and failure in the glucose-stimulated insulin secretion. Reference Gomes, Vilas-Boas and Leite81
Beta-cell dedifferentiation is characterized by lessened gene expressions related to mature beta-cell function and enhanced endocrine precursor cells, an adaptive response to avoid apoptosis. Reference Wang and Zhang82 Our obese mother’s offspring showed alterations indicating more susceptibility to beta-cell failure with advancing age. In addition, maternal melatonin supplementation seems to favor adult offspring beta-cell function.
In conclusion, obese mothers supplemented with melatonin benefit their offspring’s islet cell remodeling and function. In addition, improving pro-inflammatory markers, oxidative stress, and ER stress resulted in better glucose and insulin level control. Consequently, pancreatic islets and functioning beta cells were preserved in the offspring of obese mothers supplemented with melatonin.
Supplementary materials
The supplementary material for this article can be found at https://doi.org/10.1017/S2040174423000168.
Acknowledgments
The authors thank Aline Penna and Andrea Bertoldo for their technical assistance.
Authors’ contribution
All authors discussed the results, commented on the manuscript, and approved the article’s final version. BAN and MA performed the measurements, processed the experimental data, performed the analysis, and drafted the manuscript; FO and MBA aided in interpreting the results and worked on the manuscript; MBA and CAM-L conceived the research, planned and supervised the work, supported the research, designed the figures, and edited the manuscript’s final version.
Financial support
The study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil) (Grant Nos 305993/2021-6 CAM-L and 302215/2022-0 MBA) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (E-26/200.936/2021 to CAM-L, E-26/200.796/2021 and E-26/211384/2021 to MBA). In addition, BAN and MA received a bursary from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Finance code 001). These foundations had no interference in the execution and submission of the manuscript.
Competing interests
None.
Ethical standard
The animals came from the University of the State of Rio de Janeiro vivarium, cared for in the laboratory during the experimentation until sacrifice. Animal care followed the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Pu. No. 85-23, revised in 1996 and National Academies Press, 8th Edition, Washington, DC, 2011) and ARRIVE guidelines. 22 The institutional committee approved the study (CEUA protocol n° 003/2021).
Significance of study and contribution to science
Maternal melatonin supplementation might act as an epigenetic regulator of fetal development, helping prevent disease in adult progeny like that induced by maternal obesity. However, maternal melatonin supplementation’s long-term metabolic effects are not well-known. Therefore, we hypothesized that maternal melatonin supplementation during gestation and lactation in a known model of diet-induced obesity (DIO) in mice would mitigate the development of altered glucose metabolism and insulin resistance, inflammation, ER stress, oxidative stress, and islet remodeling and beta-cell dysfunction in adult male offspring. In the current study, we observed the benefits of melatonin supplementation to obese mothers on their offspring’s islet cell remodeling and function. In addition, there were benefits in pro-inflammatory markers, oxidative stress, and ER stress resulting in glucose and insulin improvement. Consequently, pancreatic islets and functioning beta cells were preserved in the offspring of obese mothers supplemented with melatonin.













