Pregnancy as a special physiological period, gradually changes in both basal and postprandial glucose metabolism occur to meet the nutritional demands of the mother and fetus(Reference Lain and Catalano1,Reference Chiefari, Arcidiacono and Foti2) . Although most women are able to maintain normoglycaemia(Reference Butte3), insulin resistance and hyperinsulinaemia deteriorate in normal pregnancy(Reference Catalano, Tyzbir and Wolfe4). Abnormal maternal glucose metabolism, such as gestational diabetes mellitus (GDM), may lead to an adverse intrauterine environment and result in increased metabolic complications(Reference Kwon, Lee and Lee5). Considerable evidence showed a continuous positive relationship between fasting plasma glucose (FPG) and postprandial glucose with caesarean section, clinical neonatal hypoglycaemia and preeclampsia(Reference Coustan, Lowe and Metzger6). Therefore, it is essential to explore effective solutions for preventing abnormal glucose metabolism during pregnancy.
Low-carbohydrate diets (LCD), as a kind of dietary pattern, is an important modifiable factor for glucose metabolism among the general population. Substantial evidence exists that LCD is linked to the development of type 2 diabetes(Reference Hite and Zamora7–Reference Namazi, Larijani and Azadbakht9). Similarly, LCD may have detrimental effects on GDM risk, with increased consumption of fat and protein sources and limited refined grains and whole grains. To assess the relative levels of LCD, Halton et al. (Reference Halton, Willett and Liu10) created a simple summary score designated the ‘LCD score’. To the best of our knowledge, only two studies have examined the role of LCD score with GDM incidence(Reference Looman, Schoenaker and Soedamah-Muthu11,Reference Bao, Bowers and Tobias12) . The Nurses’ Health Study found pre-pregnancy LCD score was associated with an increased risk of GDM(Reference Bao, Bowers and Tobias12), which was similar to the results of the Australian Longitudinal Study(Reference Looman, Schoenaker and Soedamah-Muthu11). The two previous studies investigated participants’ long-term dietary patterns before pregnancy. However, a considerable proportion of women changed their diet during pregnancy(Reference Koutelidakis13–Reference Looman, Geelen and Samlal15). Existing evidence has indicated that macronutrient components of diet in mid-pregnancy may predict the incidence of GDM(Reference Wang, Storlien and Jenkins16,Reference Bo, Menato and Lezo17) . Yet, the relationship between LCD score during pregnancy with GDM remains unknown. Secondly, previous studies were based on the Western population. The direct generalisation of their findings to other populations may be limited, especially when major food sources of macronutrients are considerably different(Reference Willett18). In the USA, the highest ranked scores were cake/pie(Reference O’Neil, Keast and Fulgoni19). In contrast, among most Asian countries, especially China, a large proportion of dietary energy is provided by carbohydrate and the main source of carbohydrate is white rice(Reference Shirani, Esmaillzadeh and Keshteli20,Reference Nanri, Mizoue and Kurotani21) . Although white rice has a high glycaemic index(Reference Sugiyama, Tang and Wakaki22), the dietary pattern including rice was associated with a significant decreased risk of GDM(Reference Hu, Oken and Aris23,Reference Zhou, Chen and Zhong24) . Therefore, we hypothesised that LCD score may be associated with impaired glucose metabolism among Chinese population. Thus, we sought to evaluate the relation between LCD score with maternal glucose concentrations and insulin resistance by using data from a population-based study in China.
Method
Study population
The current present study used the baseline data from an on-going prospective GDM cohort study (registration number: NCT03023293), which recruited pregnant women (20–28 weeks) in 2017–2018 at a hospital in Guangzhou, China. Participants aged 20–45 years were eligible for the study. Women who had a history of diabetes, CVD, haematological systemic disorder or thyroid disease, polycystic ovary syndrome, mental disorder pregnancy infection or multiple pregnancies were excluded from the study.
A total of 1022 pregnant women were enrolled. We further excluded the participants who reported unrealistic energy intakes (<2510 or >16 736 kJ/d) (n 4). Finally, 1018 women were included in the analysis. The ethics committee of the School of Public Health at Sun Yat-sen University approved the study. All participants were carefully instructed and signed informed consent at initial enrolment.
Dietary assessment
Pregnant women were asked to report their food intake during a face-to-face interview by using a validated eighty-one-item quantitative FFQ(Reference Zhang and Ho25). Dietary data on the frequency (per d, week or month for each food item) of intake and portion size in the past month were reported. The number of servings per frequency was exhibited in natural units (e.g. one egg), household measures (e.g. one bowl) or grams (e.g. 200 g of cooked meat). Food photographs with standard portions sizes were used for assistance during the interview. The Chinese Food Composition Table was used to calculate individual daily intake of nutrients for each food item(Reference Wgpx26). The average daily intake of nutrients was calculated by multiplying the frequency of consumption of each food by its nutrient content and summing the nutrient intake for all food items.
Calculation of the low-carbohydrate diet score
To reduce the bias of underestimating food consumption and represent dietary composition, nutrient density (percentage of total energy intake) was used to calculate LCD scores. According to the percentages of fat, protein and carbohydrate from total energy intakes, participants were divided into eleven strata. Participants in the highest strata of fat or protein intakes received ten points, the lowest strata receiving zero point. On the contrary, participants in the highest strata of carbohydrate intakes received zero points, the next strata receiving one point and so on(Reference Halton, Willett and Liu10). We calculated the overall LCD score by summing the points for three macronutrients, ranging from 0 (highest carbohydrate and lowest fat and protein intake) to 30 (lowest carbohydrate and highest fat and protein intake). We also created an animal LCD score based on the proportions of energy as carbohydrate, animal protein and animal fat, and a vegetable LCD score based on the percentages of energy as carbohydrate, vegetable protein and vegetable fat intakes (Table 1).
Table 1. Criteria for determining the low-carbohydrate diet score*
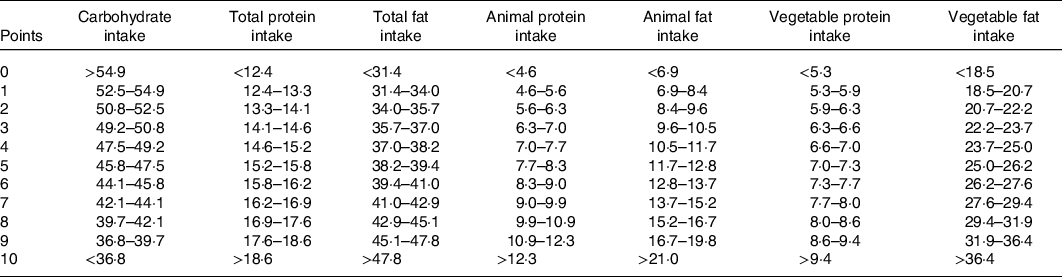
* Percentage of total energy intake is presented.
Assessment of glucose metabolism
Procedures for collection of blood samples were established for the present study. Eligible participants underwent a standard 2-h oral glucose tolerance test (OGTT). Trained clinical nurses instructed women drinking 300 ml water with 75 g of anhydrous glucose following an overnight fast of at least 10 h. Maternal plasma glucose levels during OGTT, including FPG and OGTT 1-h and 2-h glucose (postprandial glucose), were measured with clinical chemistry analyzer (ARCHITECT i2000SR; Abbott) by the glucose oxidase method.
Fasting insulin concentration was measured using ELISA (10-1113-01; Mercodia). All ELISA protocols were in accordance with the manufacturer’s instruction. The homoeostasis model assessment of insulin resistance (HOMA-IR) index was calculated as the product of fasted insulin (μU/ml) and fasted glucose (mmol/l) divided by 22·5(Reference Wallace, Levy and Matthews27). HOMA-IR is widely used to identify individuals with insulin resistance in clinical and epidemiological studies(Reference Tam, Xie and Johnson28,Reference Stern, Williams and Ferrannini29) .
GDM was diagnosed using criteria recommended by the International Association of Diabetes Pregnancy Study Group: 0-h glucose ≥ 5·10 mmol/l; 1-h glucose ≥ 10·00 mmol/l or 2-h glucose ≥ 8·50 mmol/l. If one, two or all of these criteria were met, the woman was diagnosed with GDM(30).
Covariates assessment
Anthropometric data were measured by trained clinical nurses. A standard height measuring instrument (nearest 0·1 cm) was used to measure height. Maternal weight (nearest 0·1 kg) was measured using digital body weight scales. Demographic data, including maternal age at mid-pregnancy, parity, education level, monthly household income, occupation, passive smoking, alcohol intake, physical activity and family history of diabetes, were collected using a standardised questionnaire during the interview. Education level was divided into low (senior high school or below), middle (junior college) and high (college or above). Occupation and monthly household income level were categorised into four groups. Family history of diabetes, smoking and alcohol use during pregnancy was categorised into yes or no. The intensity of physical activity was assessed using the International Physical Activity Questionnaire(Reference Smith, Reid and Matthews31). Pre-pregnancy body weight was self-reported at the interview. Pre-pregnancy BMI (kg/m2) was computed as weight (kg) divided by the square of height (m). Gestational weight gain until the glucose screening test was the deviation between self-reported pre-pregnancy weight and maternal weight measured at OGTT test.
Statistical analysis
We divided participants into quartiles according to their overall LCD score, animal LCD score and vegetable LCD score, respectively. Continuous variables are reported as means and standard deviations. Categorical variables are reported as percentages. Characteristics were compared using ANOVA or Kruskal–Wallis tests, χ 2 tests or Fisher’s exact test as appropriate. The outcome variables were GDM and the measures of glucose metabolism: FPG, OGTT 1-h glucose, OGTT 2-h glucose and HOMA-IR. Mixed linear regression analyses were conducted to evaluate the associations between LCD scores and maternal glucose levels, and generalised linear mixed models were used to analyse the relationship between LCD scores and GDM. We conducted tests of linear trend across quartiles of the LCD score by assigning the median value for each quartile and fitting this as a continuous variable in models. The model was adjusted for survey year, age, parity, gestational weeks, monthly household income, education level, family history of diabetes, pre-pregnancy BMI, gestational weight gain until the glucose screening test, physical activity, total energy intakes, passive smoking and pregnancy alcohol intake. The false discovery rate (FDR) was used for the P-value correction upon multiple comparisons, using the Benjamini–Hochberg method(Reference Yoav and Yosef32). Mediation analysis was conducted for HOMA-IR associated with both LCD score and glucose metabolism. The 95 % CI was calculated with 5000 bootstrap re-samples.
Statistical analyses were conducted using SAS Software version 9.4 (SAS Institute Inc.), while the mediating model was analysed with the PROCESS macro (www.afhayes.com) for SPSS (model 4). All P values are two-sided and statistical significance was determined at the P value < 0·05 level.
Result
Characteristics of study subjects
We diagnosed 194 (19·1 %) incident GDM in 1018 singleton pregnant women. The cumulative average LCD scores ranged from a median of 5 in the first quartile to a median of 24 in the fourth quartile (Table 2). The mean daily carbohydrate intake ranged from 254·3 g in the first quartile to 203·4 g in the fourth quartile. Women with higher overall LCD scores had higher age, educational levels, consumed more MUFA, PUFA, cholesterol and dietary fibre than those with lower scores. We observed similar results for the vegetable LCD score (online Supplementary Table S1). For animal LCD scores, participants with higher scores consumed more cholesterol and less dietary fibre (online Supplementary Table S2). No statistical differences were observed among physical activity levels, plasma insulin concentrations, HOMA-IR values and other characteristics across quartiles of overall LCD score (P > 0·05).
Table 2. Characteristics of the participants according to the low-carbohydrate diet (LCD) score
(Mean values and standard deviations; medians and interquartile ranges; numbers and percentages)
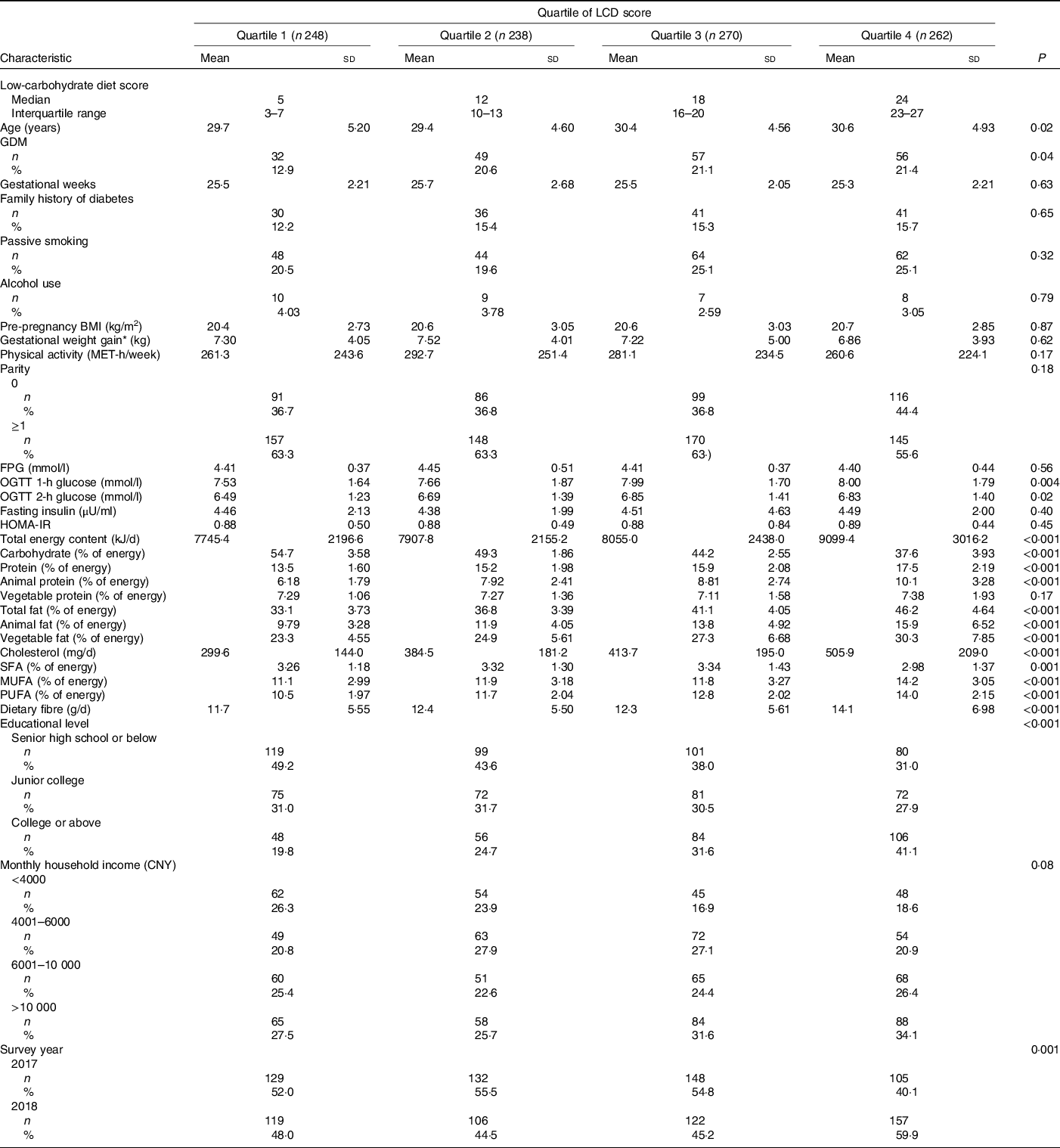
GDM, gestational diabetes mellitus; MET, metabolic equivalent; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test; HOMA-IR, homoeostasis model assessment of insulin resistance; CNY, China yuan (1 China yuan = 0·14 US dollars).
* Gestational weight gain until the glucose screening test.
Association between low-carbohydrate diets scores and glucose metabolism
Table 3 presented the relationship of three LCD scores with glucose levels and insulin resistance. After controlling for potential confounding variables (model 2), overall and animal LCD scores were positively associated with OGTT 1-h glucose (β: 0·024, 0·023; se 0·008, 0·008, P FDR = 0·02, 0·02, respectively). In contrast, no significant relationships were observed between three LCD scores with FPG, OGTT 2-h glucose and HOMA-IR.
Table 3. Multivariable linear regression of low-carbohydrate diet (LCD) scores during pregnancy with glucose metabolism
(β-Coefficients and standard errors)
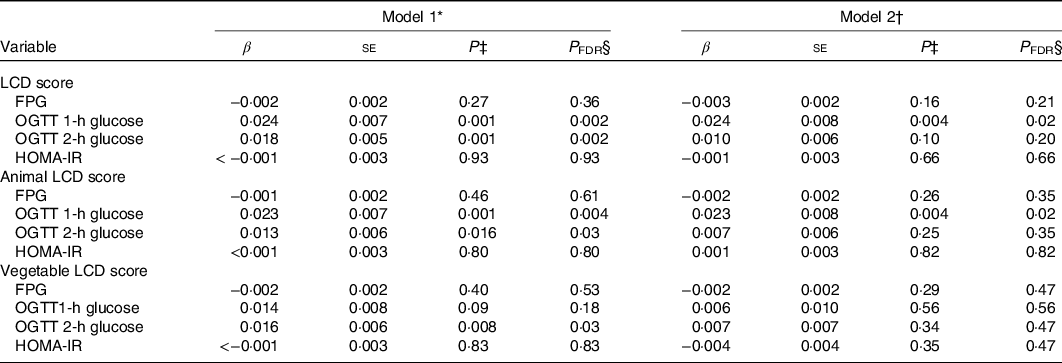
FPG, fasting plasma glucose; OGTT, oral glucose tolerance test; HOMA-IR, homoeostasis model assessment of insulin resistance.
* Model 1: unadjusted.
† Model 2: adjusted for survey year, age, parity, gestational weeks, monthly household income and education level, family history of diabetes, pre-pregnancy BMI, gestational weight gain until the glucose screening test, physical activity, total energy intakes, passive smoking and pregnancy alcohol intake.
‡ P value thresholds for generalised linear mixed regression.
§ Correct P value thresholds for false discovery rate.
Association between low-carbohydrate diets score and gestational diabetes mellitus
Multivariate-adjusted OR for GDM across quartiles of three LCD scores are presented in Table 4. The crude OR of GDM for comparisons of highest with lowest quartile were 1·84 (95 % CI 1·14, 2·95) for the overall LCD score (P for trend = 0·02), 1·56 (95 % CI 1·00, 2·45) for animal LCD score (P for trend = 0·02) and 1·07 (95 % CI 0·69, 1·65) for vegetable LCD score (P for trend = 0·62). However, the associations were attenuated after adjustment for potential confounding factors.
Table 4. Risk of gestational diabetes mellitus according to quartile (Q) of pregnancy low-carbohydrate diet (LCD) scores
(Odds ratios and 95% confidence intervals)
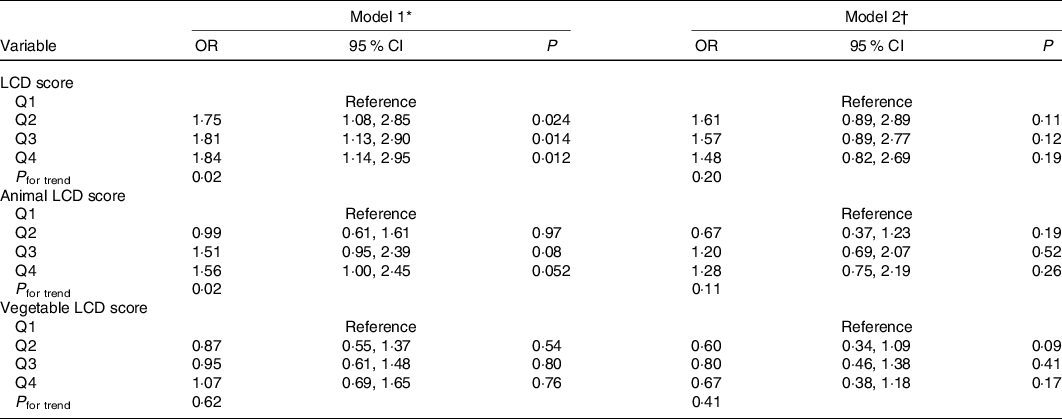
* Model 1: unadjusted.
† Model 2: adjusted for survey year, age, parity, gestational weeks, monthly household income and education level, family history of diabetes, pre-pregnancy BMI, gestational weight gain until the glucose screening test, physical activity, total energy intakes, passive smoking and pregnancy alcohol intake.
Mediating effect of homoeostasis model assessment of insulin resistance
The result presented no significantly mediating effect of HOMA-IR on the relationship between LCD scores and maternal glucose metabolism (online Supplementary Table S3).
Discussion
We observed that the overall and animal LCD scores were positively associated with OGTT 1-h glucose in pregnant women, and there were no significant associations between the three LCD scores with FPG, OGTT 2-h glucose, HOMA-IR and GDM after adjustment for covariates. No significantly mediating effects of HOMA-IR between LCD scores with glucose metabolism were found.
The literature is sparse on the association between LCD score with glucose metabolism in pregnant women; thus, it is difficult to directly compare our results with other studies. The Nurses’ Health Study (NHS) and the Australian Longitudinal Study have observed that the LCD score before pregnancy was significantly associated with increased risk of GDM during extended follow-up(Reference Looman, Schoenaker and Soedamah-Muthu11,Reference Bao, Bowers and Tobias12) . Similar to LCD score, a multivariate nutrient density model is another method to examine the balance of carbohydrate, fat and protein intake(Reference Willett, Howe and Kushi33). The effect estimate from the model can be interpreted as the effect of increasing intake of one macronutrient at the expense of the other macronutrient while keeping energy content constant. Previously, several prospective studies have examined the association between substitution of macronutrients and abnormal maternal glucose metabolism using this method(Reference Pang, Colega and Cai34,Reference Saldana, Siega-Riz and Adair35) . Replacement of protein with carbohydrate was associated with an increased risk of GDM in a Multiethnic Asian cohort among mid-pregnancy women(Reference Pang, Colega and Cai34). Substituting fat for carbohydrate resulted in a significant increase in risk of both impaired glucose tolerance and GDM in American women during mid-pregnancy(Reference Saldana, Siega-Riz and Adair35). Our findings of the relationship between LCD score with OGTT 1-h glucose also support the hypothesis that LCD is associated with abnormal maternal glucose metabolism. To interpret the association between LCD score and postprandial glucose, each of the macronutrients should be considered because an individual with a higher LCD score tends to have a relatively lower intake of carbohydrate and higher intake of fat and protein to compensate energy requirements. A previous study has shown a negative association between carbohydrate intake during mid-pregnancy with abnormal glucose metabolism(Reference Ley, Hanley and Retnakaran36). We observed a positive significant association between animal LCD, not vegetable LCD scores, and OGTT 1-h glucose. The divergent results indicated that associations may partly be ascribed to the detrimental effects of animal fat and animal protein. Epidemiological studies observed that a high intake of animal fat and protein was associated with an increased risk of abnormal maternal glucose metabolism(Reference Zhou, Chen and Zhong24,Reference Bowers, Tobias and Yeung37,Reference Bao, Bowers and Tobias38) .
Although the biological mechanisms which may account for the relationship between LCD score and postprandial glucose were not well understood, there are several potential interpretations for this finding. For dietary protein, compared with a vegetable protein-rich meal, an animal protein-rich meal resulted in higher plasma concentrations of branched-chain amino acids(Reference Brandsch, Shukla and Hirche39), which have been reported to be positively linked to the development of insulin resistance and incident diabetes in recent metabolomics studies(Reference Newgard, An and Bain40–Reference Wang, Larson and Vasan42). Additionally, experimental data demonstrated high-fat diets result in impaired glucose tolerance. Changes in the fatty acid composition of the membrane induced by dietary fat, especially animal fat modification, has been related to impaired insulin binding and/or GLUT(Reference Lichtenstein and Schwab43). However, we found no significant association between LCD score with OGTT 2-h glucose. It is possible that OGTT 1-h glucose, compared with OGTT 2-h glucose, was more linked to insulin resistance(Reference Abdul-Ghani, Abdul-Ghani and Ali44,Reference Abdul-Ghani, Williams and DeFronzo45) . Moreover, our study found a non-significant relationship between LCD score and FPG. Ley et al. (Reference de Seymour, Chia and Colega46) reported no association between three macronutrient intakes during mid-pregnancy and FPG(Reference Ley, Hanley and Retnakaran36). A multi-ethnic Asian cohort study also found a low-carbohydrate–high-protein and fat diet-based seafood, noodle and soup were not associated with FPG. It is possible that FPG, compared with postprandial glucose, may be less likely influenced by external factors, such as carbohydrate intakes and gastrointestinal absorption function(47).
Previous studies found pre-pregnancy LCD score was positively associated with GDM(Reference Looman, Schoenaker and Soedamah-Muthu11,Reference Bao, Bowers and Tobias12) . In the NHS, women with a high overall or animal LCD score had a higher risk of GDM(Reference Bao, Bowers and Tobias12). Our study also found the overall and animal LCD scores were significantly positively associated with GDM in the crude model. Although the relationship was attenuated after adjusting potential confounders, the dissimilarities between our results with those of NHS do not necessarily imply incompatibility. As diet was measured as many as 4 years prior to GDM incidence, the NHS aimed at long-term GDM risk, while we specifically assessed dietary quality during pregnancy. Previous studies observed pregnant women changed their dietary habits during pregnancy(Reference Radesky, Oken and Rifas-Shiman48). It is possible that the inconsistent findings might be explained by differences in the effect of diet on GDM risk according to trimester. Moreover, the difference was likely due to dietary variation between the studies. Compared with women in the NHS(Reference Halton, Willett and Liu10), women in our study had less variation of carbohydrate intake (29·3–56·0 % v. 38·6–56·4 % of energy), protein (14·1–24·0 % v. 12·3–19·1 % of energy) and fat (26·0–46·9 % v. 30·2–45·5 % of energy). Less variation could possibly lead to the absence of a correlation between LCD scores with GDM in adjusted models.
Our study found no association between LCD scores with HOMA-IR. Similar to our result, a Canadian study found that macronutrients were not associated with insulin resistance during the second trimester of pregnancy(Reference Ley, Hanley and Retnakaran36). Several possible mechanisms have been suggested. Short-term high-fat/low-carbohydrate dietary intake did not induce whole-body insulin resistance but caused a shift in glucose metabolism from oxidation to glycogen storage(Reference Chokkalingam, Jewell and Norton49). Animal experiments also showed the ketogenic LCD-fed mice developed systemic glucose intolerance with their livers exhibiting hepatic endoplasmic reticulum stress, steatosis, cellular injury and macrophage accumulation but preserved no impaired insulin-induced hepatic Akt phosphorylation and whole-body insulin responsiveness(Reference Garbow, Doherty and Schugar50). Taken together, LCD can affect maternal glucose metabolism via multiple potential mechanism pathway without improving insulin resistance. It may be the reason why we found the positive association between LCD score with OGTT 1-h glucose and no mediate effect of HOMA-IR between them.
This is the first study to investigate the relationship between LCD scores during pregnancy with glucose metabolism. We used comprehensive assessments of diet during the second trimester of pregnancy and detailed assessments of the glucose metabolism, including GDM status, fasting and postprandial glucose, and insulin resistance. These findings provide evidence to support the recommendation of a balanced diet for pregnant women in Chinese population. Pregnant women who follow a low-carbohydrate dietary pattern should avoid consuming excess animal sources of protein and fat to minimise their risk of higher glucose concentrations.
Several potential limitations should be acknowledged. Firstly, this is a cross-sectional study that may limit the cause–effect relationship. However, women were informed of their GDM diagnosis after the dietary assessments, which limited the possibility of reverse causation. Secondly, most participants of our study were Han Chinese (96·76 %); thus, cautions may be needed when generalising our results to other ethnic populations. However, the relative homogeneity of our participants advantageously reduced unmeasured confounding. Finally, recall bias of maternal dietary intakes with FFQ data is inevitable. Nonetheless, we used a validated semi-quantitative FFQ(Reference Zhang and Ho25) and food photographs with standard portions sizes for assistance, which could minimise the bias.
In conclusion, a dietary pattern relatively low in carbohydrate and high in animal protein and fat is positively associated with OGTT 1-h glucose. No significant relationship was found between LCD score with maternal FPG and insulin resistance. Further longitudinal studies are needed to confirm our results and explore the potential mechanisms.
Acknowledgements
We thank all the pregnant women who willingly took part in our study. We also appreciate the doctors and nurses for the anthropometric measurements, and postgraduate students for their contribution to the data collection.
This work was supported by the Key-Area Research and Development Program of Guangdong Province (2019B03035001) and the National Natural Science Foundation of China (81602862 and 81571452) and the Sanming Project of Medicine in Shenzhen (SZSM201803061).
The authors’ contributions are as follows: conceptualisation, L. C. and J. J.; methodology, L. C., Y. J. C., Y. M. C. and J. J.; software, N/A; validation, L. C., Q. C., Y. J. C., W. J. W. and N. T.; formal analysis, Q. C., W. J. W. and N. T.; investigation, Q. C., W. J. W., N. T. and D. Y. W.; resources, L. C., J. J. and D. Y. W.; data curation, Y. J. C., W. J. W., N. T. and Q. C.; writing – original draft preparation, Q. C., N. T. and W. J. W.; writing – review and editing, L. C., Y. J. C. and J. J.; visualisation, Q. C.; supervision, L. C. and J. J.; project administration, L. C. and J. J.; funding acquisition, L. C. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004092







