There is growing evidence that our current food system faces increasing environmental and health challenges. Global food production is responsible for about one-third of all greenhouse gas emissions while threatening climate stability and ecosystem resilience(Reference Alsaffar1). At the same time, diets that are not nutritionally balanced have contributed to more than a quarter of deaths globally(2) mostly from diet-related chronic diseases requiring costly treatments(Reference Muka, Imo and Jaspers3). Many governmental bodies and health authorities now recognise the urgency required to tackle this problem. For example, the UN’s Sustainable Development Goals (SDG) include an aim to achieve food security, improve nutrition and promote sustainable agriculture (i.e. SDG 2)(4). To tackle these concerns, dietary guidelines on environmental sustainability have been published worldwide(5,Reference Martini, Tucci and Bradfield6,7,8,9,10) , and there are an increasing number of recommendations and guidelines that promote a more plant-based diet. In 2019, the EAT-Lancet commission proposed a global healthy reference diet that each country could modify to meet specific nutritional and cultural needs while focusing on environmental sustainability(Reference Willett, Rockstrom and Loken11). The EAT-Lancet reference diet recommended a predominantly plant-based diet, rich in fruits, vegetables, wholegrains, legumes, nuts and unsaturated oils, with a low-to-moderate amount of seafood and poultry, and a small quantity of red meat, milk, and dairy products. In the UK, Public Health England updated the Eatwell Guide(12) to include the promotion of more plant-based foods (e.g. milk alternatives included in the dairy products section), and the British Dietetic Association (BDA) One Blue Dot policy(13), considers environmental sustainability in the context of healthy, nutritional, diets.
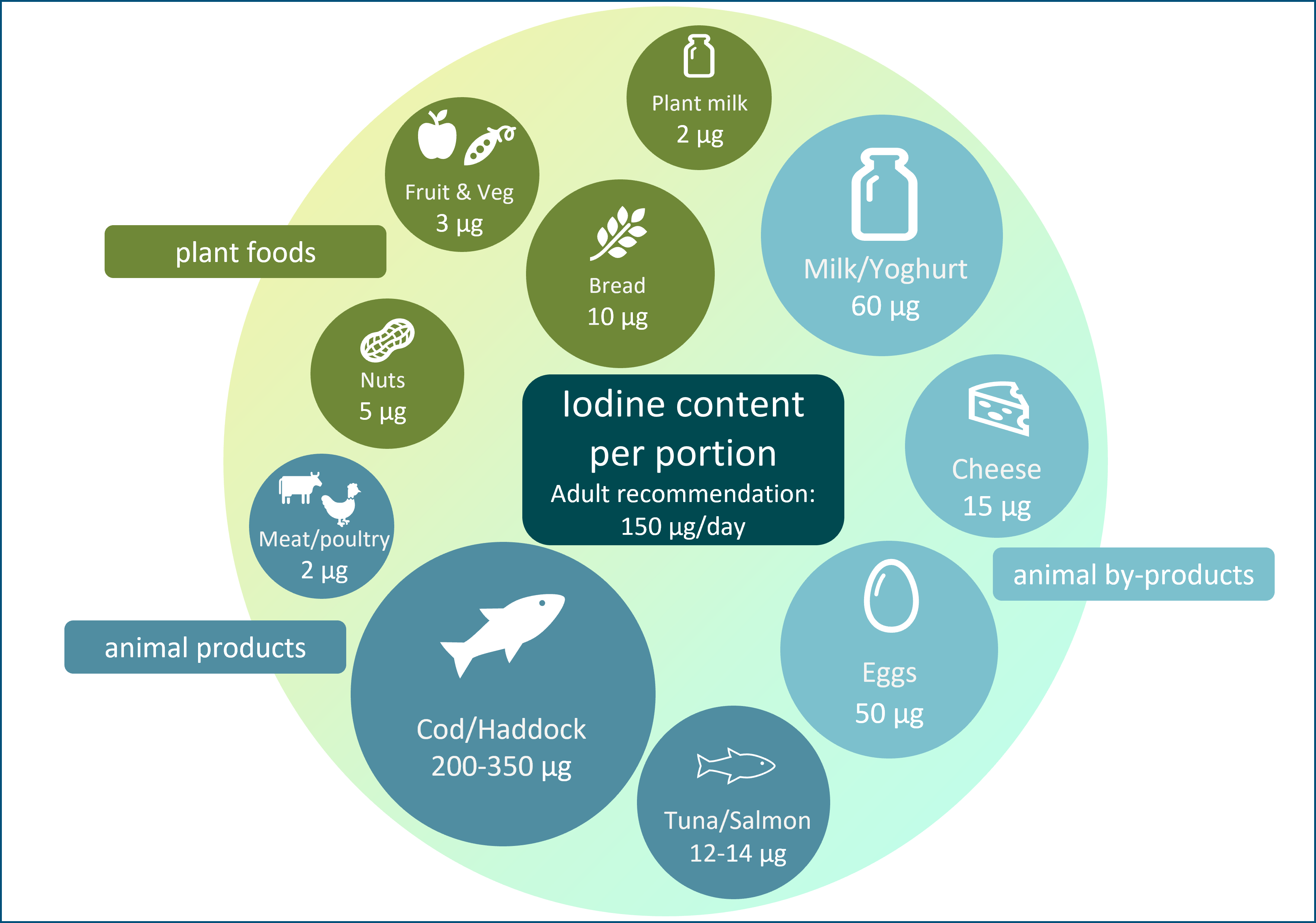
The general recommendation for predominantly plant-based diets and increased use of plant-based alternative products (e.g. milk alternatives) may have the unintended consequence of reducing iodine intake. Plant foods have a low iodine concentration (Table 1), so individuals who follow a plant-based diet may be at risk of iodine deficiency; animal sources (e.g. milk, fish and eggs; Table 1) currently provide the majority of iodine intake in many countries, including the UK(14). In some countries (UK, Ireland and Iceland)(Reference Bath, Button and Rayman15,Reference Shaw, Nugent and McNulty16,Reference Gunnarsdottir, Gustavsdottir and Thorsdottir17) , there is no iodised salt policy so moving away from animal foods will have negative impact on iodine intake to a greater degree than in countries where animal foods provide a lower proportion of total iodine intake, and where iodised salt policies exist.
Table 1. The iodine content of a range of animal and plant foods by UK recommended portion size
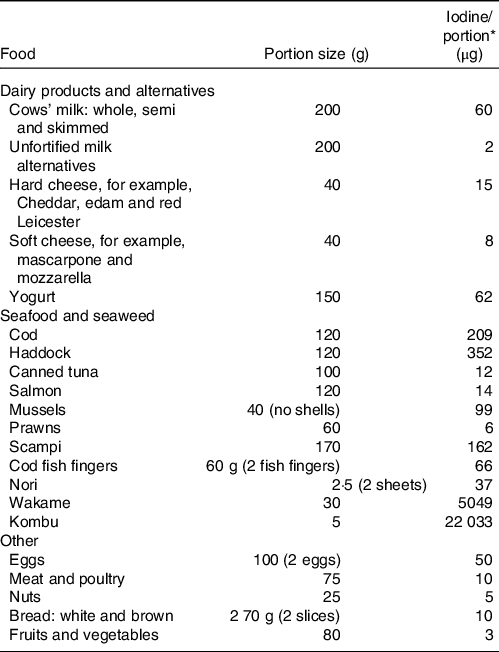
* Values are taken from UK food tables(Reference Finglas, Roe and Pinchen19).
The FAO of the UN and the WHO launched their guiding principles for sustainable and healthy diets(18). These principles included increasing intake of plant foods such as fruit and vegetables, while including moderate amounts of dairy products, eggs and fish(18). Interestingly, the FAO and WHO report specifically mentions the need to limit salt intake but to ensure that any salt is iodised(18) to ensure adequate iodine intake, and this is especially important in the context of a plant-based diet.
It is unclear whether plant-based dietary patterns, such as the EAT-Lancet reference diet, would provide a sufficient intake of iodine. Unlike other plant-based dietary recommendations, the EAT-Lancet reference diet is quantified, providing guideline daily intakes (average and range in g/d) for each food group. It is therefore possible to calculate the iodine provided, which we have done using UK data, as an example of a country without a salt iodisation programme.
Scope of this review and methodology
In this narrative review, we have summarised the current published evidence for the risk of iodine deficiency in those following a predominantly plant-based diet (not just a strict vegan diet). The literature review was conducted utilising PubMed, Google Scholar and Cochrane Library databases up until December 2022. Relevant publications were selected using a combination of keywords for plant-based diets and iodine intake (e.g. plant-based, vegan, vegetarian, iodine, intake, status and deficiency). Additional studies were identified by a manual search of bibliographic references in original papers and reviews.
In addition to the narrative review, we estimated the iodine provided by the EAT-Lancet reference diet using iodine concentration data from UK food tables(Reference Finglas, Roe and Pinchen19). This reference diet consists of food groups from which target intake levels and reference ranges are suggested (e.g. 250 g dairy foods/d with range of 0–500 g). The reference ranges are described by the EAT-Lancet authors as uncertainty ranges and considered to be compatible with optimal health in different populations(Reference Willett, Rockstrom and Loken11). We calculated the iodine concentration of each food group using the food components listed in the appendices of the EAT-Lancet report(Reference Willett, Rockstrom and Loken11) and matching this to the corresponding iodine concentration data from UK food tables. A list of the corresponding food components from UK food tables used in this analysis can be found in Supplementary Table 1.
Total daily iodine intake provision from EAT-Lancet reference diet was calculated and compared with iodine intake recommendations for iodine in adults (150 µg/d) and during pregnancy (200–250 µg/d). We used different intake recommendations for comparison, primarily using the values equivalent to the Reference/Recommended Nutrient Intake (RNI) as we calculated provision of iodine from the EAT-Lancet reference diet (in terms of dietary planning(Reference Barr20)), not the assessment of population average intakes (for which the Estimated Average Requirement would be more appropriate)(Reference Barr20,Reference Allen21) . For adults, we compared iodine provision of the diet against the recommendation of 150 µg/d (the RNI/RDA/Adequate Intake according to WHO(22), USA(23) and European(24) recommendations, respectively) and the UK value of 70 µg/d for the Lower Reference Nutrient Intake (the estimated intake required to prevent goitre)(25). For pregnancy, we present the comparison as a range to account for the variation in iodine intake recommendations around the world, from 200 µg/d in Europe (AI)(24) to 220 µg/d in the USA (RDA)(23) and 250 µg/d according to WHO (RNI)(22).
As a final part of this review, we highlight potential options for those following a plant-based diet to achieve an adequate iodine intake.
Iodine deficiency as a public health concern
Iodine is essential for synthesising the thyroid hormones, triiodothyronine (T3) and tetraiodothyronine (T4), and an inadequate iodine intake can lead to various adverse effects, collectively termed the iodine deficiency disorders (IDD)(Reference Zimmermann, Jooste and Pandav26). Although goitre (enlargement of the thyroid gland) is the most visible effect of iodine deficiency, cognitive impairment has the greatest impact on individuals and populations(Reference Zimmermann27). The negative association between iodine deficiency and cognition is a result of the role that thyroid hormones have in brain and neurological development, meaning that iodine is particularly important during the first 1000 d of life, when the brain is developing(Reference Velasco, Bath and Rayman28). A study in the UK has shown that even mild-to-moderate iodine deficiency during pregnancy is associated with lower IQ and reading ability in school-aged children(Reference Bath, Steer and Golding29).
In recent years, iodine deficiency has re-emerged as a public health concern in women of reproductive age in several European countries(Reference Elorinne, Alfthan and Erlund30,Reference Henjum, Brantsæter and Kurniasari31,Reference Nyström, Brantsæter and Erlund32) , including the UK and Ireland(Reference Mullan, Hamill and Doolan33,Reference Combet, Ma and Cousins34,Reference Bath, Sleeth and McKenna35,Reference McMullan, Hamill and Doolan36) . These countries had been considered to be iodine replete for decades, but concern surrounding the re-emergence of iodine deficiency in the UK was ignited in 2011 when the first nationwide survey of iodine status for more than 50 years found mild iodine deficiency in a teenage schoolgirls(Reference Vanderpump, Lazarus and Smyth37). Since then, several regional studies in the UK and Ireland have reported mild iodine deficiency in adolescent girls and women of childbearing age(Reference Mullan, Hamill and Doolan33,Reference Combet, Ma and Cousins34,Reference Bath, Sleeth and McKenna35,Reference Nawoor, Burns and Smith38) . Results from the UK’s National Diet and Nutrition Survey (NDNS) shows iodine sufficiency in children (4–18 years) and adults (19–64 years), but mild iodine deficiency in women of childbearing age (16–49 years). The data also show a downward trend in iodine status (as measured by urinary iodine concentration) of women of childbearing age from 2013 (when first introduced) to the latest data in 2019(39).
There is concern that pregnant women in the UK are iodine-deficient – although the data are not from the nationally representative NDNS survey (as they are excluded)(Reference Roberts, Steer and Maplethorpe40), but from regional studies across the UK(Reference Bath, Steer and Golding29,Reference Kibirige, Hutchison and Owen41,Reference Pearce, Lazarus and Smyth42,Reference Knight, Shields and He43,Reference Bath, Walter and Taylor44,Reference Bath, Furmidge-Owen and Redman45,Reference Farebrother, Dalrymple and White46,Reference Snart, Keeble and Taylor47) and the thresholds for defining deficiency during pregnancy are not considered as robust as those in children(Reference Zimmermann and Andersson48). In other countries of Europe, women are often classified as deficient during pregnancy, even in countries where children are classified as iodine-sufficient(Reference Bath49), and data from dietary surveys show low iodine intake in pregnant women and those of childbearing age(Reference Bath, Verkaik-Kloosterman and Sabatier50). Indeed, the focus should be on women of childbearing age, not just in those with confirmed pregnancy. Iodine deficiency in women prior to pregnancy, where thyroidal stores of iodine are not optimised, is increasingly considered to be a risk factor for impaired maternal thyroid hormone profile(Reference Moleti, Di Bella and Giorgianni51) and may be linked to reduced cognitive outcomes of the child(Reference Robinson, Crozier and Miles52). This matters because plant-based diets are more likely to be followed by young women, and data from the UK show that women are more likely to consume alternatives to milk and dairy products than other groups(Reference Dineva, Rayman and Bath53).
Dietary sources of iodine
A plant-based diet is generally considered to be one where plant foods make up the majority of the diet, but there can be inclusion of animal products. A vegetarian, rather than a vegan, diet would be considered as plant-based, as meat and fish are not consumed, but eggs and/or dairy products might be included. However, in recent years, the term has been applied to those who follow a flexitarian style diet – where animal foods may be consumed in limited amounts (including meat and fish), but where most meals are based on plant-based foods and products.
Animal products, including milk, fish and eggs, provide over 60 % of adult iodine intake in some countries (e.g. Iceland, Norway, Spain and the UK) and contribute considerably to total iodine intake (Table 2).
Table 2. The contribution of animal products to total iodine intake in adults in the ten European countries that have available dietary data, based on a recent review(Reference Bath, Verkaik-Kloosterman and Sabatier50)

M, mandatory iodised salt policy; V, voluntary iodised salt policy; N, no iodised salt policy.
Table created using data from Bath et al. 2022(Reference Bath, Verkaik-Kloosterman and Sabatier50).
Table does not show iodised salt policy for all countries in Europe – only those with data on contribution of food groups to total intake in adults.
Milk and dairy products
Milk and dairy products are the primary sources of iodine in many countries, contributing about 12–53 % of total daily iodine intake (Table 2)(Reference Bath, Verkaik-Kloosterman and Sabatier50). Naturally, cows’ milk has a low iodine concentration but has become a rich source of iodine through farming practices, such as providing cattle with iodine-enriched feed and using iodine-based disinfectants known as iodophors during the milking process(Reference van der Reijden, Zimmermann and Galetti54). Countries such as Australia and New Zealand have replaced iodophors with other disinfectants, which may explain the decline in milk iodine concentration in those countries(Reference Thomson55). Similarly, the considerable variability in milk iodine content within and between countries is likely a result of differences in farming practices(Reference van der Reijden, Zimmermann and Galetti54). Within some countries (including the UK), there is seasonal variation in milk iodine concentration, where summer milk has a lower concentration than winter milk; this results from greater reliance on iodine-fortified feed during the winter months rather than on pasture grazing(Reference van der Reijden, Zimmermann and Galetti54). In addition, both UK and other European studies have previously shown that organically-produced cows’ milk is lower in iodine compared with conventional cows’ milk(Reference Bath, Button and Rayman56), mainly due to restrictions on iodine-fortified cattle feed and higher goitrogen components of the cattle feed (e.g. clover-based fodders and feeds(Reference van der Reijden, Zimmermann and Galetti54)). Despite this, organic milk is still a good source of iodine, and more recent research suggests no overall difference in iodine concentration between organic and conventional milk in the UK, likely due to changing farming practices in the organic sector(Reference Qin, Faludi and Beauclercq57).
Milk-alternative drinks
While the EAT-Lancet commission does not explicitly include plant-based milk alternatives in the reference diet, the BDA One Blue Dot policy and the updated Eatwell Guide recommend that milk and dairy products be consumed interchangeably with plant-based alternatives. Notably, these recommendations do not stipulate that consumers should also ensure that these products are fortified with iodine. This is an important point because research in the UK(Reference Bath, Hill and Infante58), Norway(Reference Dahl, Aarsland and Næss59) and the USA(Reference Ma, He and Braverman60) has shown that unless fortified with iodine, plant-based milk alternatives have a very low iodine concentration – just 2·1 % of the value of UK cows’ milk(Reference Bath, Hill and Infante58). A 200-g portion of unfortified drinks would provide 0·9–4·3 µg of iodine(Reference Bath, Hill and Infante58).
In 2015, just 6 % of drinks on the UK market were fortified with iodine. We conducted an updated survey of plant-based alternatives available in the UK in 2020 and found that only 19·8 % of plant-based milk alternatives were fortified with iodine (compared with 63·0 % fortified with Ca); hence, the likelihood of consumers selecting an iodine-fortified product remains low(Reference Nicol, Thomas and Nugent61). As most of the products available on the UK market are produced abroad, these results are most likely relevant for other European countries.
Dairy products such as cheese and yogurt are also important sources of iodine in the UK (Table 1), and dairy products alone (i.e. excluding the contribution from liquid milk) contribute to 11 % of adults’ and 17 % of children’s iodine intake(Reference Roberts, Steer and Maplethorpe40). Data show that consumers are buying less liquid milk and more milk derivatives such as cheese and yogurt(62,63) . Non-milk dairy products alternatives, such as plant-based yogurt and cheese, are not usually fortified with iodine(Reference Nicol, Thomas and Nugent61) and if unfortified plant-based alternatives are used in place of dairy products, iodine intake may be reduced.
It is plausible that adherence to diets that limit milk and milk products and replace them with plant-based alternatives may result in an inadequate iodine intake. A recent study using iodine intake and status (measured as median urinary iodine concentration) from the NDNS (Years 7–9; 2014–2017) found that individuals who exclusively consumed (largely unfortified) plant-based milk alternatives had a lower iodine intake (94 v. 129 µg/d) compared with exclusive cows’ milk consumers, as well as a lower iodine status (median urinary iodine concentration: 79 v. 132 µg/l), indicating that this group was iodine-deficient according to the WHO threshold for population sufficiency (>100 µg/l)(Reference Dineva, Rayman and Bath53). The study found that plant-based milk alternatives were more likely to be consumed by young women (7·6 % of women 16–49 years v. 4·6 % of the total NDNS sample were milk-alternative consumers)(Reference Dineva, Rayman and Bath53). This is a concern as young women who consume diets low in iodine are especially vulnerable to maternal and fetal effects of iodine deficiency if they become pregnant.
Eggs
Chicken eggs are a good source of iodine, with a concentration of 50 µg/100 g, or approximately 25 µg of iodine provided by one egg (Table 1). Eggs are estimated to contribute between 2 % and 13 % of total iodine in Europe countries(Reference Bath, Verkaik-Kloosterman and Sabatier50). Though data on the variability in egg iodine content are limited, it is believed to be affected by factors such as feed content, the physical status of hens such as age and laying rate, and environment(Reference Roseland, Bahadur and Pehrsson64,Reference Pehrsson, Roseland and Patterson65) .
Fish
Fish and seafood products are naturally rich sources of iodine, although absolute levels are species-specific, creating a broad range of food options and iodine provision per portion (Table 1). Fish consumption in the UK is low, and therefore it makes a smaller contribution to population iodine status than milk and dairy products, contributing 11 % of the adult intake(66). However, on a per-portion basis, fish has the potential to contribute significantly towards iodine intake recommendations.
The current UK recommendation for fish and seafood includes eating two 140 g portions per week (280 g fish per week or 40 g/d), one of which should be from oily fish(12). According to NDNS data(66), UK adults are currently not meeting this recommendation, with a median daily intake of total fish at 13 g/d (median 0 g/d of oily fish(66), reflecting the high proportion of non-consumers). By contrast, Nordic dietary patterns emphasise fish intake, and seafood is consumed more regularly in those countries. The adult mean daily fish intake is higher in Norway (67 g/d(Reference Totland, Melnæs and Lundberg Hallén67)) and contributes more to iodine intake. Indeed a recent study found that only women aged 18–35 years in Norway had a fish intake below the recommended two portions per week(Reference Janowska-Miasik, Waśkiewicz and Witkowska68). Consequently, implementing plant-based dietary recommendations in countries with a high fish intake, such as Norway, may decrease iodine intake.
There is a potential conflict between dietary recommendations for fish intake and sustainability considerations, as an increase in global fish intake may put additional strain on global fish stocks. The EAT-Lancet commission combats this by recommending consumers choose farmed seafood rather than wild-caught to conserve wild fish populations. However, iodine-rich fish such as cod and haddock are not widely farmed(Reference Cai, Zhou and Yan69), and therefore a diet prioritising farmed seafood will provide less iodine than one without this restriction, even if the recommended portions are consumed. Additionally, aquaculture production has a range of environmental concerns of its own. Increased aquaculture production will result in significant resource constraints due to limited freshwater resources(Reference Ahmed, Thompson and Glaser70). There is also widespread irreversible habitat destruction due to aquaculture which is likely to affect biodiversity negatively(Reference Diana71).
To guide consumers in making an environmentally responsible choice, the Marine Conservation Society has developed their Good Fish Guide(72), which rates seafood based on its sustainability and where and how it is caught. Approximately 80 % of all seafood sold in the UK is from five species (cod, haddock, salmon, tuna and prawns)(72). Of these five fish species, Atlantic cod, Atlantic salmon, tuna and prawns all received a mixed rating, and consumers are recommended to look for more sustainable alternatives. However, haddock and Pacific cod are currently rated as good options, both of which are rich sources of iodine and so should be encouraged in those striving to meet the dual goals of iodine sufficiency and diet sustainability.
Seaweed
Seaweed is often cited as a rich plant-based source of iodine; however, the iodine content is highly variable and unreliable as a source of iodine and could even result in excessive iodine intake(73,Reference Konno, Makita and Yuri74,Reference Nishiyama, Mikeda and Okada75,Reference Katagiri, Asakura and Sasaki76,Reference Tsubota-Utsugi, Imai and Nakade77,Reference Jeon, Kim and Kwon78) . For example, kelp has a high iodine concentration (Table 1); therefore, even a 5-g portion could provide 22 033 µg of iodine, over 14 000 % of the adult recommended intake(22), well in excess of the safe upper limit of 600 µg/d(Reference Zimmermann79). Indeed, in a sub-national study in Denmark, researchers found that regular seaweed consumers had a high or excessive iodine intake (>900 µg/d)(Reference Kristensen, Madsen and Hansen80). Kelp supplements should not be used as a source of iodine, and regular brown seaweed intake is not recommended(Reference Zimmermann and Delange81). However, not all seaweed species have very high iodine content, and species with an appropriate iodine concentration might be able to reduce the risk of iodine insufficiency in those following a plant-based diet. Seaweed is gaining popularity as a functional ingredient in plant-based products to enhance taste or food matrix or to enrich the product with bioactive compounds(Reference Bouga and Combet82,Reference Kyriakopoulou, Keppler and van der Goot83,Reference Ainsa, Honrado and Marquina84,Reference Garciarena, Poulsen and Lindholm Bøgh85) . However, additional research is required to understand the contribution of seaweed to iodine intake and whether this is a safe source of iodine, given the potential for toxicity. In addition, the bioavailability of iodine from seaweed is not well understood(Reference Combet, Ma and Cousins86).
Iodised salt as a source of iodine
To prevent and control iodine deficiency disorders, the WHO recommends the iodisation of all food-grade salt(22). Consequently, many countries such as Austria, Denmark, Italy and Turkey have enacted mandatory fortification of salt (Table 2)(Reference Bath, Verkaik-Kloosterman and Sabatier50). However, only 40 % of European countries have a mandatory salt iodisation policy(Reference Bath, Verkaik-Kloosterman and Sabatier50), with countries including the UK and Ireland having no such requirement. Therefore, there is a lack of iodine fortification in the UK and Ireland, and the availability of iodised salt is low(Reference Bath, Button and Rayman15,Reference Shaw, Nugent and McNulty16) . As a result, iodine status in these countries depends on individual food choices, with milk and dairy products often the primary dietary source. For example, milk and dairy products in the UK provide 51 % and 33 % of children’s and adult’s iodine intake, respectively(14). Other countries are similarly heavily reliant on appropriate food choices in the absence of iodine fortification policies, for example, in Norway, where, although officially noted as having a voluntary iodised salt policy, the permitted iodine content of salt is low, and there is limited use an availability of iodised salt in the country(Reference Nyström, Brantsæter and Erlund32).
Other countries, such as the Netherlands, Denmark and Belgium, have fortified bread with iodine through the use of iodised salt in bread making(Reference Jakobsen, Nielsen and Paulsen87,88,Reference Moreno-Reyes, Van Oyen and Vandevijvere89) . This provides a non-animal source of iodine in those countries. Indeed, 49 % and 53 % of adult iodine intake in Belgium and the Netherlands are from bread and cereals due to the iodine fortification of bread(Reference Bath, Verkaik-Kloosterman and Sabatier50). By contrast, the contribution of bread and cereals is low in countries where bread is not fortified with iodine; for example, it provides just 6 % and 12 % of total iodine intake in Norway and the UK, respectively(Reference Bath, Verkaik-Kloosterman and Sabatier50).
Vegan diets and iodine intake
It is estimated that between 2 and 3 % of the UK population are following a vegan diet(90,91) , with 620 000 participating in ‘Veganuary’ (the pledge to follow a vegan diet in January) in 2022, a 268 % increase from 2018(92,93) . Vegan diets, where there is complete removal of animal products from the diet, provide an example of the effect of strict plant-based diets on iodine intake. Although the scientific evidence in this regard is relatively scarce, and the sample sizes of most studies are insufficient to draw a definitive conclusion, available evidence shows that those following a vegan diet are at high risk of iodine deficiency(Reference Eveleigh, Coneyworth and Avery94,Reference Groufh-Jacobsen, Hess and Aakre95,Reference Eveleigh, Coneyworth and Zhou96,Reference Brantsæter, Knutsen and Johansen97) .
Three reviews have investigated the iodine status of those consuming vegan and vegetarian diets(Reference Eveleigh, Coneyworth and Avery94,Reference Fields, Dourson and Borak98,Reference Preedy, Burrow and Watson99) . The most recent review published in 2020(Reference Eveleigh, Coneyworth and Avery94) identified fifteen relevant studies that examined iodine intake or status by different dietary groups such as vegetarian, vegan, omnivore and lacto-vegetarian; the data show that adults following a vegan diet (not taking an iodine-containing supplement) have an increased risk of low iodine status, iodine deficiency and inadequate iodine intake than those following an omnivorous diet(Reference Eveleigh, Coneyworth and Avery94). It should be noted that several of the studies included in the analysis did not measure the iodine intake from salt, and any iodine from dietary supplements was often excluded from the dietary intake. Notably, the highest iodine intake was recorded in vegans who habitually consume seaweed, with intakes well above the RNI. In these circumstances, sudden and sustained excess of iodine can inhibit hormonal biosynthesis (Wolff–Chaikoff effect)(Reference Wolff and Chaikoff100,Reference Miyai, Tokushige and Kondo101) . This inhibition is typically transient and of short duration if iodine intake returns to the recommended range. However, prolonged excess intake of seaweed, such as kelp, a brown seaweed often called kombu, has been linked to cases of thyroid dysfunction, including both hyper- and hypothyroidism(Reference Crawford, Cowell and Emder102).
There are case reports of goitre (i.e. a symptom of severe iodine deficiency) in those following a vegan diet, resulting in a severely low iodine intake, including cases in a UK neonate (as a result of maternal vegan diet in pregnancy)(Reference Shaikh, Anderson and Hall103), toddler in the USA(Reference Yeliosof and Silverman104), children in the UK(Reference Brandt, Ajzensztein and Sakka105) and UK women of childbearing age(Reference Park, Watson and Bevan106). In addition, there are individual case reports that have highlighted the risk of severe iodine deficiency in those following a restrictive diet as a result of medical conditions, such as cows’ milk allergy(Reference Gordon, Hall and Amin107,Reference Caprio, Umano and Luongo108) or other reasons(Reference Moore, Sasidharan Pillai and Austin109). Finally, a randomised controlled trial in Sweden found that adults following a palaeolithic diet for 2 years (that restricted salt and dairy product intake) had a higher risk of iodine deficiency (as measured by urinary iodine concentration) than those on a diet following Nordic Nutrition Recommendations(Reference Manousou, Stal and Larsson110). These studies all underline the susceptibility to iodine deficiency in those following a diet restricting the consumption of iodine-rich foods.
Iodine provided by predominantly plant-based diets
While the effect of a strict vegan has been explored in relation to iodine intake, and risk of iodine deficiency, less is known about a more moderate following of a plant-based diet. We therefore calculated the iodine provision of the EAT-Lancet reference diet, which recommends a moderate intake of dairy products, and limited meat, fish, and egg intake, with plenty of fruit and vegetables. The specific quantities of iodine-rich foods recommended by the EAT-Lancet reference diet (Table 3) are daily intake of 250 g dairy products (i.e. both milk and dairy products, range 0–500 g), 28 g fish (196 g/week) and 13 g egg (approximately 1·5 eggs/week)(Reference Willett, Rockstrom and Loken11).
Table 3. The EAT-Lancet reference diet, with recommended daily food intake (including possible ranges) for an adult consuming 2500 kcal/d with estimated iodine content of each food group per 100 g/d, and contribution to WHO recommendation for iodine intake(22)

* If using unfortified milk alternative (at 1 µg/100 g), iodine provision from dairy category would be 2·5 µg/d (range 0–5µg/d) and the total daily iodine intake would be 54 µg/d (range 8·3–147 µg/d) or 36 % (5·5–98 %) of adult intake recommendations.
† If using fortified milk alternative (at 22·5 µg/100 g), iodine provision from dairy category would be 56·25 µg/d (range 0·00–5) and the total daily iodine intake would be 108 µg/d (range 8·25–255 µg/d) or 72 % (5·5–170 %) of adult intake recommendations.
Following the diet would result in an estimated daily iodine intake of 128 µg/d (Table 3), which is 85 % of the recommended intake for adults (at 150 µg/d(22,23,24) ); as the provision of iodine is below the RNI, the distribution of intake in a population would mean that is likely that a high proportion of individuals would have low intake (i.e. intake below the Estimated Average Requirement or Lower Reference Nutrient Intake). When considering the range of intake suggested for each food category, the iodine provision could be as low as 8 µg/d (6 % of adult RNI) or as high as 295 µg/d (196 % of daily adult RNI). However, the higher end of the range includes 100 g of fish and 500 g of dairy products per d which does not line up with the overall message of the EAT-Lancet commission to have a low-to-moderate intake of animal-based products. The calculated provision of iodine in the EAT-Lancet reference diet, at 128 µg/d, is below the RNI for pregnancy – the diet would provide just 51–64 % of the pregnancy recommended intake of iodine (200–250 µg/d)(22,23,24) and therefore would place individuals at risk of iodine deficiency during pregnancy. The calculation of iodine provision is based on food sources only, and we recognise that in countries with iodised salt programmes, additional iodine may be provided through iodised table salt use, although this would be unlikely in the UK(Reference Bath, Button and Rayman15) or other countries such as Norway.
It is clear from Table 3 that the majority of iodine in the EAT-Lancet reference diet is from the dairy foods category (250 g of cows’ milk would provide 76 μg/d, or 51 % of the WHO recommended iodine intake for adults). When considering the range of potential dairy product intake (0–500 g/d), the higher end of dairy product intake would provide a sufficient iodine for an adult (500 g of UK milk would provide an estimated 150 μg/d). However, the overall message diet, and plant-based diets, in general, is to reduce the consumption of foods of animal origin. Indeed, the commission suggests that increasing milk consumption from 250 g to 500 g would increase greenhouse gas emissions and total environmental effects regardless of changes to production practices. Consequently, the commission recommends that the optimal intake of dairy products should be at the lower end of the reference range which will lower the overall iodine content of the reference diet. To meet this recommendation, some may turn to plant-based milk alternatives and if cows’ milk is replaced with a fortified plant-based alternative (fortified at 22·5 µg/100 ml, the most commonly used level of iodine fortification(Reference Nicol, Thomas and Nugent61)), the reference diet would provide a total of 108 µg iodine/d, 72 % of the adult RNI and 43–54 % of iodine intake recommendations in pregnancy(22,23,24) . However, it is far more likely that consumers would replace cows’ milk with an unfortified milk alternative, as the majority of the products on the market do not add iodine to replace that found in cows’ milk(Reference Nicol, Thomas and Nugent61). We estimate that 250 g of a plant-based milk alternative not fortified with iodine (estimated iodine concentration = 1 µg/100 g) would provide just 2·5 µg iodine/d (Table 3). Therefore, consumers replaced cows’ milk with a unfortified plant-based milk alternative the diet would provide a total of just 54 µg iodine/d, 35 % of the adult RNI and 22–27 % of the RNI in pregnancy. The provision of just 54 µg iodine/d is below the Lower Reference Nutrient Intake for adults (of 70 µg/d) which was set as the intake required to prevent goitre(25). Therefore, without adequate advice on supplementation, individuals following the EAT-Lancet reference diet using unfortified milk alternatives would be at high risk of deficiency, and if this was during pregnancy this would be of public health concern in terms of risk to fetal neurodevelopment(Reference Bath111).
The egg category provides 6 μg of iodine per d (4 % of adult RNI; Table 3). The average UK consumer consumes 2·9 eggs per week(Reference Guo, Hobbs and Cockcroft112), not including the eggs added to prepared food products; therefore, adhering to the diet would necessitate a reduction in egg consumption and, therefore, in iodine provision from this food source in the UK.
The fish category provides an estimated 25 µg iodine/d (Table 3), based on the iodine content of the fish examples provided in the EAT-Lancet report (one portion (98 g) of sockeye salmon and one portion (98 g) of Atlantic cod per week)). However, if the consumer chose alternative fish sources, the iodine provision from fish would be much lower – 196 g of salmon or canned tuna per week would provide just 4 and 2 μg/d, respectively. This highlights that the iodine provision is highly dependent on the species of fish consumed, as well as the overall quantity. The commission recommend that oily fish is chosen in preference as it provides greater quantities of n-3 fatty acids; however, white fish has a higher iodine concentration than oily fish (Table 1). While some plant-based diets may exclude fish consumption altogether, the amount of fish recommended by the EAT-Lancet commission is higher than usual intake in the UK (13 v. 28 g/d). However, encouraging people to meet the recommendation of 196 g/week may be challenging in the UK with consumer trends indicating that fish consumption is already declining(14).
There are several caveats regarding the calculation of iodine in the EAT-Lancet reference diet. Firstly, there is the variation in milk iodine concentration, both by farming type (organic or conventional) and by season. This makes it difficult to determine the exact iodine provision by the EAT-Lancet reference diet. In the summer months, a 250-g portion of UK milk would provide 50 μg of iodine compared with 103 μg in winter, thereby changing the total iodine provided from 102 to 155 μg/d. Secondly, we calculated the contribution of milk and dairy products to iodine intake using values for liquid milk, which may underestimate the amount of iodine provided by the reference diet, as other dairy products such as certain types of cheese may have a higher iodine concentration (on a per gram basis) than milk. For example, while Cheddar cheese (30 μg/100 g) provides a similar amount of iodine to cows’ milk, halloumi (60 μg/100 g) and red Leicester cheese (46 μg/100 g) would contribute more to overall intake. However, the majority of dairy product intake will likely come from milk(62), and therefore this is unlikely to affect the overall iodine content of the EAT-Lancet reference diet. The iodine content of the EAT-Lancet reference diet was calculated using UK data with the assumption of no iodised table salt, as the UK does not have a universal salt iodisation programme and the availability of iodised salt is low(Reference Bath, Button and Rayman113). Finally, the iodine provision may be lower than we calculate as the quantities of food recommended by the commission are based on calorie requirements (2500 kcal/d) of a 70-kg male or a 60-kg female (aged 30 years) whose physical activity level is moderate to high. If quantifies are adjusted to meet 2000 kcal/d instead, it is likely that the recommendations for animal-based products would be lower, and consequently, so would the iodine provided.
The narratives and perceptions of plant-based diets concerning iodine intake
Iodine is often overlooked when it comes to dietary recommendations. The EAT-Lancet commission does not mention iodine in its report and does not consider it an essential micronutrient when creating the reference diet. The dairy product recommendation is primarily based on Ca intake, and the fish recommendation is based on n-3 fatty acids. However, it is not just the EAT-Lancet reference diet that overlooks iodine. Many plant-based dietary recommendations focus on Ca when discussing dairy products and plant-based alternatives. UK-based recommendations such as the Eatwell Guide and the BDA One Blue Dot policy both recommend that Ca-fortified plant-based milk alternatives can be consumed interchangeably with cows’ milk without mentioning iodine. As plant-based diets continue to gain popularity, the lack of attention given to iodine will become an even greater issue.
There is inaccurate information found online (e.g. popular websites and social media) about plant-based sources of iodine. While The Vegan Society (UK) has accurate information on iodine on their website(114), other information online (including across social media) often incorrectly cites plant-based food sources as being rich in iodine – including particular fruits (e.g. prunes, strawberries or cranberries), potato skins, and navy or kidney beans(Reference Berkheiser115,Reference James116) . The iodine content of plant crops is affected by the content of iodine in the soil, but, in general, plant-based foods such as vegetables and fruits are relatively poor sources of iodine(Reference Ershow, Skeaff and Merkel117). Based on UK food table data, strawberries contain just 1 µg/100 g, dried prunes (100 g or 10 prunes) would contribute only 2 µg(Reference Finglas, Roe and Pinchen19), potatoes contain approximately 1 µg/100 g and a 400-g can of kidney beans would provide approximately 12 µg of iodine(Reference Finglas, Roe and Pinchen19). It is important to counter these myths around plant sources of iodine, where possible, and provide suitable and safe sources of iodine to those following a plant-based diet.
The fact that the EAT-Lancet reference diet provides 51–64 % of the iodine intake recommended in pregnancy, or just 22–27 % if an unfortified milk alternative is chosen, is of concern and should be highlighted to consumers. It is particularly notable because the EAT-Lancet reference diet includes a recommendation for pregnant women to supplement their diet with vitamin B12, as it is acknowledged that the diet does not provide enough B12 for pregnancy. The EAT-Lancet commission does not mention iodine supplementation in pregnancy, but our analysis highlights that the diet, without adequate iodine supplementation, would not provide sufficient iodine, especially if plant-based alternatives to cows’ milk were consumed. We acknowledge that there is a degree of debate around the recommended iodine intake in pregnancy(Reference Bath, Verkaik-Kloosterman and Sabatier50), and whether needs can be met if thyroid stores are optimised prior to pregnancy(118); however, the EAT-Lancet reference diet may not provide enough iodine for women of childbearing age so that thyroidal iodine stores can be maintained, particularly if unfortified milk alternatives are used in place of cows’ milk.
Options for those following a plant-based diet to optimise iodine intake
It is important to note that plant-based diets and iodine sufficiency do not have to be mutually exclusive. For those following a plant-based diet, there are ways to optimise iodine intake. For example, if avoiding milk, individuals should opt for iodine-fortified plant-based alternatives or increase their iodine intake from other food sources. A suitable iodine-containing supplement would be a more consistent and reliable way of ensuring an adequate iodine intake if this cannot be achieved through the diet. If a supplement is considered, this should not be a seaweed supplement; instead, iodine should be in the form of potassium iodide or potassium iodate with a dose that does not exceed 150 µg/d.
Conclusion
Although dietary recommendations, such as the EAT-Lancet reference diet, provide a valuable roadmap for healthy, sustainable diets, this review shows that they may not provide enough iodine to meet adult or pregnancy intake recommendations. With an overarching message to reduce animal product intake, consumers and health professionals must be aware that plant-based diets may exacerbate dietary and nutritional inadequacies such that of iodine, unless there is clear guidance for the consumer on use of fortified foods or appropriate supplementation. As evidenced in this review, those transitioning to a plant-based diet in the UK, a country without widespread use of iodised salt, may be at risk of iodine deficiency.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523001873
Acknowledgements
K.N. was supported by the Biotechnology and Biological Sciences Research Council FoodBioSystems Doctoral Training Programme (Grant number: BB/T008776/1, Reference number: 2459093). The funder had no role in this article’s design, analysis or writing.
S. C. B. has received an honorarium from Oatly UK and Dairy UK for delivering webinars/online videos for Healthcare Professionals. Other authors have no conflicts of interest to delcare.
K. N. and S. C. B. contributed to the research’s conception, design and interpretation. K. N. and S. C. B. contributed to the drafting of the manuscript. All authors have edited and agreed to the published version of the manuscript.