The aim of the present study was to investigate whether high tissue concentrations of homocysteine (HCY) and its precursors S-adenosylhomocysteine (SAH) and S-adenosylmethionine (SAM) in human bone are associated with altered bone morphology. Additionally, we aimed to assess the association between cancellous bone structure and methylation capacity, which is indicated by the SAH:SAM ratio.
In rats, high bone concentrations of HCY and SAH as well as an increased SAH:SAM ratio have been shown to be associated with a reduced bone quality(Reference Herrmann, Tami and Wildemann1). A recently published in vitro study has further demonstrated that decreased SAM-dependent methylation impairs osteoblast differentiation(Reference Vaes, Lute and van der Woning2). Therefore, our hypothesis was that high bone concentrations of HCY and SAH, an increased SAH:SAM ratio as well as a low bone concentration of SAM are associated with a deteriorated cancellous bone structure also in humans.
Methods
Study design
We harvested cancellous bone samples at the femoral neck of eighty-two osteoarthritis patients undergoing hip arthroplasty. Cancellous bone structure was analysed by histomorphometry. In addition, we measured bone concentrations of total HCY, collagen-bound HCY, SAH and SAM. Blood was sampled to assess the serum concentration of HCY. For each bone metabolite, we calculated the median. In addition, we determined the median bone SAH:SAM ratio. These values were used as cut-off points. The subjects with values above the median were assigned to the high category groups, and the subjects with values less than the median were assigned to the low category groups.
Subjects
Subjects were recruited from the Orthopaedic Department of the Knappschaftskrankenhaus Püttlingen, Germany, during the time period from 1 March 2006 until 31 January 2007. The age range of the study population was 44–86 years. None of the patients had hip injury or had been immobilised. Individuals with non-normal serum concentrations of Ca and vitamin D were not included in the study. Subjects were also excluded from the study if they suffered from hyper- or hypoparathyroidism, hyper- or hypothyroidism, malignancies and inflammatory diseases, or if they received one of the following medications: oestrogen, parathormone, vitamin D, strontium ranelate, bisphosphonates, corticosteroids, anticonvulsants and cytostatic or immunosuppressive drugs. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board and Ethical Commission of the University of Saarland (Homburg/Saar, Germany). Written informed consent was obtained from all subjects.
Specimen preparation and blood sampling
Bone samples were fixed in 4 % phosphate-buffered formalin for histomorphometric evaluation or stored at − 80°C for the analyses of HCY, SAH and SAM concentrations.
Fasting blood was collected in serum tubes 1 d before surgery between 08.00 and 10.00 hours. After 20 min of clotting, samples were centrifuged at 4000 rpm for 10 min to separate serum from cellular blood components. Blood serum was stored at − 80°C until measurement of HCY.
Histomorphometry
Formalin-fixed bone samples were decalcified in 10 % EDTA solution for 5 weeks and embedded in paraffin. Using haematoxylin–eosin-stained sections of 5 μm thickness, cancellous bone structure was assessed at a magnification of 4 × (Olympus BX60 Microscope; Olympus, Tokyo, Japan; Zeiss Axio Cam and Axio Vision 3.1; Carl Zeiss, Oberkochen, Germany; ImageJ; NIH, Bethesda, MD, USA). The following histomorphometric parameters were determined and calculated in adherence to the recommendations of the American Society of Bone and Mineral Research: trabecular thickness (Tb.Th); trabecular number (Tb.N); trabecular separation (Tb.Sp); percentage of trabecular area (Tb.Ar)(Reference Parfitt, Drezner and Glorieux3). The quantitative analysis of Tb.Th, Tb.N, Tb.Sp and Tb.Ar provides important information on the quality of cancellous bone. Accordingly, a decrease in Tb.Th, Tb.N and Tb.Ar and an increase in Tb.Sp can indicate a reduced bone quality.
Bone analyses of homocysteine, S-adenosylhomocysteine and S-adenosylmethionine
Collagen-bound HCY was extracted from the bone samples using a collagenase digestion method as reported previously(Reference Herrmann, Tami and Wildemann1). Concentrations of total HCY and collagen-bound HCY in bone were measured by HPLC (Agilent 1100; Waldbronn, Germany) using a fluorescence detector and a commercial assay (Immundiagnostik, Bensheim, Germany)(Reference Herrmann, Tami and Wildemann1).
SAM and SAH were measured in the bone samples using a modified liquid chromatography–tandem MS method as described previously(Reference Herrmann, Tami and Wildemann1, Reference Gellekink, van Oppenraaij-Emmerzaal and van Rooij4). Inter-assay CV were 3·8 % for HCY, 8·0 % for SAH and 4·8 % for SAM.
Serum analysis of homocysteine
HCY was analysed with an enzymatic fluorescence polarisation immunoassay on an AxSYM automated analyser (Abbott, Wiesbaden, Germany). Intra- and inter-assay CV were 4·5 and 4·6 %, respectively.
Statistics
All data are given as means and standard deviations. The comparison between the experimental groups was performed by Student's t test for those outcome variables that were normally distributed and by the Mann–Whitney U test for those outcome variables that were not normally distributed. Comparison of different categories between the groups was performed by the χ2 test or Fisher's exact test. Spearman's rank correlation coefficient was used to determine the strength of association between serum and bone concentrations of HCY. A P value < 0·05 was considered to indicate significant differences. All statistical analyses were performed using the SPSS software package, version 17.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Bone analyses of homocysteine, S-adenosylhomocysteine and S-adenosylmethionine
Median bone concentrations of HCY, SAH and SAM were 0·0546, 0·0159 and 0·0836 μmol/l, respectively. The interquartile ranges of bone metabolite concentrations were 0·0405–0·0732 μmol/l for HCY, 0·0113–0·0243 μmol/l for SAH and 0·0450–0·1326 μmol/l for SAM. Based on the medians, individuals were grouped into high concentrations of HCY (HCY+ group, 0·0547–0·0937 μmol/l), SAH (SAH+ group, 0·0161–0·1712 μmol/l) and SAM (SAM+ group, 0·0837–0·5743 μmol/l) or low concentrations of HCY (HCY− group, 0·0031–0·0545 μmol/l), SAH (SAH− group, 0·0054–0·0157 μmol/l) and SAM (SAM− group, 0·0039–0·0835 μmol/l). The median SAH:SAM ratio was 0·206 with a range of 0·030–0·205 in the high-methylation capacity group (SAH:SAM− group) and 0·207–3·262 in the low-methylation capacity group (SAH:SAM+ group). It was found that 48 % of the total HCY was bound to collagen.
Anthropometric characteristics
Individuals of the SAH+ group showed a significantly lower BMI compared with individuals of the SAH− group. In the SAM+ group, the number of females was significantly higher than that in the SAM− group. No significant differences in the anthropometric characteristics were found between individuals of the HCY+ and HCY− groups as well as between the individuals of the SAH:SAM+ and SAH:SAM− groups (Table 1).
Table 1 Anthropometric characteristics and histomorphometric data of the total study population and of individuals with high or low bone concentrations of homocysteine (HCY), S-adenosylhomocysteine (SAH) and S-adenosylmethionine (SAM) as well as a low or high methylation capacity*
(Mean values, standard deviations and percentages)
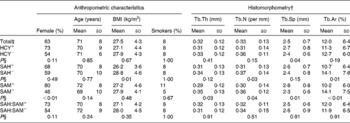
Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Ar, trabecular area.
* Comparisons between the experimental groups were performed by the χ2 test (male and female), the Mann–Whitney U test (age, Tb.Th and Tb.Ar), Student's t test (BMI, Tb.N and Tb.Sp) or Fisher's exact test (smokers).
† Histomorphometric parameters include Tb.Th, Tb.N, Tb.Sp and Tb.Ar.
‡ The medians of the total study population (total) were defined as cut-off values indicating high (+) or low ( − ) bone concentrations of HCY, SAH and SAM as well as a low or high methylation capacity (SAH:SAM+ v. SAH:SAM− ). Total, n 82. HCY+, HCY− , SAH+, SAH− , SAM+, SAM− , SAH:SAM+ and SAH:SAM− groups, n 41 (each).
§ A P value < 0·05 was considered to indicate significant differences.
Serum analysis of homocysteine
Mean serum concentrations of HCY were 11·7 (sd 3·1) μmol/l in the low and 14·1 (sd 6·7) μmol/l in the high-HCY bone concentration groups, 11·9 (sd 3·1) μmol/l in the low- and 13·8 (sd 6·7) μmol/l in the high-SAH bone concentration groups, as well as 13·2 (sd 6·8) μmol/l in the low- and 12·5 (sd 3·2) μmol/l in the high-SAM bone concentration groups. Correlation analysis showed a significant correlation between the serum and bone concentrations of HCY (r 0·26, P = 0·03).
Histomorphometry
Individuals with high HCY bone concentrations demonstrated a significantly higher Tb.Sp compared with individuals with low-HCY bone concentrations. In the SAH+ group, Tb.N and Tb.Ar were significantly lower than in the SAH− group. In SAM+ individuals, Tb.Th, Tb.N and Tb.Ar were significantly lower, and Tb.Sp was significantly higher compared with individuals of the SAM− group. In contrast, there were no significant differences in bone morphology between individuals of the SAH:SAM+ and SAH:SAM− groups (Table 1).
Discussion
To the best of our knowledge, the present study reports for the first time on tissue concentrations of HCY and its precursors in human bone. Our first hypothesis was that high tissue concentrations of HCY and SAH are related to a deteriorated cancellous bone structure in humans. The results of the present study confirm this hypothesis, showing a positive correlation between high tissue concentrations of HCY and SAH in bone and a deteriorated cancellous bone structure. Our second hypothesis was that low tissue concentrations of SAM as well as a high SAH:SAM ratio in bone are associated with an impaired cancellous bone structure in humans. In contrast to our first hypothesis, we had to reject this second hypothesis, because we did not find a significant association between the SAH:SAM ratio and cancellous bone structure, and because a low SAM concentration was not associated with an impaired but an improved cancellous bone structure. This result is surprising and seemingly contradictory to the results of previous in vitro and animal studies but may be explained by the role of B vitamins in HCY metabolism. The B vitamins folate, B6 and B12 are important cofactors of the HCY-degrading remethylation and trans-sulfuration pathways(Reference Herrmann, Herrmann and Obeid5). Accordingly, deficiency of these B vitamins is the main cause of increased serum and probably also bone concentrations of HCY(Reference Herrmann, Herrmann and Obeid5, Reference Herrmann, Peter Schmidt and Umanskaya6). Furthermore, a reduced metabolism of HCY caused by B vitamin deficiency leads to an accumulation of its precursors SAH and SAM(Reference Herrmann, Herrmann and Obeid5). Therefore, we suggest that high bone SAM concentrations are a consequence of high concentrations of SAH and HCY. This relationship might explain why not only high concentrations of HCY and SAH but also of SAM are related to a deteriorated cancellous bone structure.
We could demonstrate that about 50 % of HCY is bound to collagen in human bone. It has been shown in vitro that HCY is capable of binding to aldehyde groups of collagen, and thereby affecting the formation of stable collagen cross-links(Reference Herrmann, Peter Schmidt and Umanskaya6). Of interest, collagen I is the dominant protein of the organic bone matrix. In accordance, a disturbance of collagen cross-linking through HCY might be one explanation for the alteration of bone morphology. Apart from a disturbed collagen cross-linking, a HCY-induced stimulation of osteoclasts has also been discussed as one possible pathomechanism explaining the adverse effects of HCY on bone(Reference Herrmann, Peter Schmidt and Umanskaya6).
According to a review by Herrmann et al. (Reference Herrmann, Peter Schmidt and Umanskaya6) on the role of hyperhomocysteinaemia in osteoporosis, the majority of large epidemiological trials found a significant positive relationship between serum HCY concentrations and fracture risk. In contrast, the association between hyperhomocysteinaemia and decreased bone mineral density is controversially discussed. About half of all studies, including a total of almost 20 000 individuals, found a significant inverse relationship between serum HCY and bone mineral density(Reference Herrmann, Peter Schmidt and Umanskaya6). Existing data of analyses of bone turnover markers suggest a shift in bone metabolism towards bone resorption(Reference Dhonukshe-Rutten, Pluijm and de Groot7). So far, two large intervention studies have investigated whether a HCY-lowering treatment with B vitamins is capable of decreasing the incidence of osteoporotic fractures(Reference Herrmann, Peter Schmidt and Umanskaya6, Reference Sawka, Ray and Yi8). In the first study, which included 615 individuals, fracture risk was found to be significantly reduced in patients receiving B vitamin supplementation, while the second study, which included more than 5000 patients, could not demonstrate a fracture-preventing effect of B vitamin supplementation. In summary, the entirety of data derived from epidemiological and interventional studies analysing the association between hyperhomocysteinaemia and fracture risk, bone mineral density as well as serological bone turnover markers, is conflicting. The data of the present study indicate a negative bone turnover in patients with high tissue concentrations of HCY in bone, while the deteriorated cancellous bone structure might be additionally an indication of an increased fracture risk. Thus, the results of the present study confirm the data of those epidemiological studies, which have reported an association between hyperhomocysteinaemia and a negative bone turnover as well as an increased risk of osteoporotic fractures.
Anthropometric data of the present study indicate a significantly lower BMI of individuals with high bone concentrations of SAH compared with individuals with low bone concentrations of SAH. These results indicate a relationship but do not prove causality between bone SAH and BMI. In mice, it has been shown that the application of a methyl-deficient diet prevents weight gain and increases SAH concentrations in a variety of different tissues(Reference Caudill, Wang and Melnyk9). However, these data do not allow conclusions on whether changes in body weight were dependent on tissue concentrations of SAH or other effects of methyl deficiency. In humans, it has been shown that osteoporosis is related – beside other factors – also to decreased body weight(Reference Lane, Russell and Khan10). This relation is reflected by the results of the present study demonstrating that high bone concentrations of SAH are associated with both lower BMI and deteriorated cancellous bone structure. Furthermore, it has to be assumed that total bone mass is decreased in osteoporotic patients, which in turn might also affect total body weight and thus BMI(Reference Lane, Russell and Khan10). Accordingly, it might be possible that the decrease in cancellous bone mass in individuals with high bone concentrations of SAH results in a reduced BMI.
A significant difference in sex distribution has been found between the SAM+ and SAM− groups, showing a significantly higher number of women in the SAM+ group than in the SAM− group. For rats, it has been reported that the activity of the methionine-activating enzyme methionine adenosyltransferase is higher in the liver of female than in that of male animals(Reference Natori11). To the best of our knowledge, there is no information on a sex-dependent activity of methionine adenosyltransferase in humans.
Some limitations of the present study have to be considered. As subjects who were recruited for the present study underwent hip arthroplasty due to osteoarthritis, it has to be addressed that bone of the proximal femur might differ between hip osteoarthritis patients and non-hip osteoarthritis patients(Reference Dequeker, Aerssens and Luyten12). A second limitation is that the mean age of the study population was relatively high (71 (sd 8) years), which might explain the relatively high mean serum concentration of HCY(Reference Herrmann, Kraenzlin and Pape13).
We conclude that high tissue concentrations of HCY and SAH in bone are related to reduced bone quality, while the relationship between SAM and altered bone properties is most probably only affected in situations of high HCY and SAH concentrations. We further conclude that altered methylation capacity is not associated with deteriorated bone morphology.
Acknowledgements
We thank Janine Becker for excellent technical assistance. All authors have no conflicts of interest. The present study was financed by the Institute for Clinical and Experimental Surgery, University of Saarland. The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors' contributions were as follows: J. H. H., M. H., W. H., T. S., T. P. and M. D. M. designed the study; J. H. H., M. H., C. S., P. G., T. H., M. K., K. K., T. S., T. P. and M. D. M. conducted the study; W. H., T. S., T. P. and M. D. M. provided essential reagents or provided essential materials; J. H. H., M. H., C. S., W. H. and M. D. M. analysed the data or performed the statistical analysis; J. H. H., M. H. and M. D. M. wrote the manuscript; J. H. H., M. H., T. P. and M. D. M. had the primary responsibility for the final content. All authors read and approved the final manuscript.



