Background
Older people are the fastest growing sector of the population. In France, the population over age 65 in 2015 represented 19 per cent of the total (nearly 12 million), with about 1.5 million over the age of 85 (Insee – Populations légales 2012 – 75-Paris, n.d.). In recent years, older people have accounted for the largest increase in hospital admissions (Organisation for Economic Co-operation and Development [OECD], n.d.; Office for National Statistics, 2013), with an increasing economic burden on health care systems. In France, data (Agence Technique de l’information sur l’Hospitalisation, n.d.) recorded 12.2 million persons hospitalized in an acute unit in 2016 (184 per 1,000), with 1.5 million older than 80 years of age (402 per 1,000). The French National Health Insurance (NHI) is largely controlled by the French state. NHI reimburses patients’ outlays on care-related hospitalisations, outpatient services, prescription drugs, and to a lesser extent, dental and vision care (Rodwin, Reference Rodwin2003). Unlike other countries such as the United States, the very ill patients are those who will pay the least, because the NHI then assumes the full cost of care.
Hospitalizations are frequently associated with an increased risk of functional decline (Covinsky et al., Reference Covinsky, Palmer, Fortinsky, Counsell, Stewart, Kresevic and Landefeld2003; Kortebein, Ferrando, Lombeida, Wolfe, & Evans, Reference Kortebein, Ferrando, Lombeida, Wolfe and Evans2007) both during hospitalization and following discharge. Several studies emphasise that about 30 per cent of older patients have lost the ability to perform some activities of daily living at hospital discharge in comparison with their pre-illness baseline (Covinsky et al., Reference Covinsky, Palmer, Fortinsky, Counsell, Stewart, Kresevic and Landefeld2003; McCusker, Kakuma, & Abrahamowicz, Reference McCusker, Kakuma and Abrahamowicz2002), and the oldest are at high risk of developing new functional deficits during hospitalization (Volpato et al., Reference Volpato, Onder, Cavalieri, Guerra, Sioulis and Maraldi2007), which may also precipitate cognitive decline (McCusker et al., Reference McCusker, Kakuma and Abrahamowicz2002). This loss is strongly correlated with higher use of health resources (Covinsky et al., Reference Covinsky, Palmer, Fortinsky, Counsell, Stewart, Kresevic and Landefeld2003; Hasan, Reference Hasan2001), greater caregiver burden, institutionalization, and death (Cornette et al., Reference Cornette, Swine, Malhomme, Gillet, Meert and D’Hoore2006; Teno et al., Reference Teno, Harrell, Knaus, Phillips, Wu, Connors and Lynn2000). However, functional decline in older people also occurs frequently before hospitalization, and recovery or decline in the hospital is a complex, dynamic process (Covinsky et al., Reference Covinsky, Palmer, Fortinsky, Counsell, Stewart, Kresevic and Landefeld2003; Ellis, Whitehead, Robinson, O’Neill, & Langhorne, Reference Ellis, Whitehead, Robinson, O’Neill and Langhorne2011).
Readmission of older patients is frequent and poorly understood (Hasan, Reference Hasan2001). In recent years, several studies have sought to identify risk factors for hospital readmissions (Alassaad et al., Reference Alassaad, Melhus, Hammarlund-Udenaes, Bertilsson, Gillespie and Sundström2015), institutionalization, or death (Deschodt et al., Reference Deschodt, Wellens, Braes, De Vuyst, Boonen, Flamaing and Milisen2011; McCusker et al., Reference McCusker, Kakuma and Abrahamowicz2002; Mudge, O’Rourke, & Denaro, Reference Mudge, O’Rourke and Denaro2010). Older people often have coexistent medical, functional, psychological, and social needs (Ellis et al., Reference Ellis, Whitehead, Robinson, O’Neill and Langhorne2011), requiring assessment on several fronts and involving several disciplines. The goal of comprehensive geriatric assessment (Ellis et al., Reference Ellis, Whitehead, Robinson, O’Neill and Langhorne2011) is to identify each problem and to manage it appropriately. Although nutritional assessment remains a priority, nutritional scores such as Mini Nutritional Assessment (MNA) do not support accurate prediction (Dent, Chapman, Howell, Piantadosi, & Visvanathan, Reference Dent, Chapman, Howell, Piantadosi and Visvanathan2014). However, frailty syndromes are a valid predictor of outcomes after acute hospitalization (Dent et al., Reference Dent, Chapman, Howell, Piantadosi and Visvanathan2014; Warnier et al., Reference Warnier, van Rossum, van Velthuijsen, Mulder, Schols and Kempen2016). Several screening tools are in use to predict readmission or functional decline (Cornette et al., Reference Cornette, Swine, Malhomme, Gillet, Meert and D’Hoore2006; Covinsky et al., Reference Covinsky, Palmer, Fortinsky, Counsell, Stewart, Kresevic and Landefeld2003; McCusker et al., Reference McCusker, Kakuma and Abrahamowicz2002). Variables include patient demographic factors, medical co-morbidity data, laboratory data, social determinants of health, patient functional status, and prior use of health care services. For example, frequently used scores – Identification of Seniors At Risk (ISAR) (Dendukuri, McCusker, & Belzile, Reference Dendukuri, McCusker and Belzile2004), Variable Indicative of Placement Risk (VIP), and the Flemish version of the Triage Risk Screening Tool (fTRST) (Braes et al., Reference Braes, Flamaing, Sterckx, Lipkens, Sabbe, Rooij and Milisen2009; Deschodt et al., Reference Deschodt, Wellens, Braes, De Vuyst, Boonen, Flamaing and Milisen2011) – are about equally effective in predicting functional decline and use of health services (Braes et al., Reference Braes, Flamaing, Sterckx, Lipkens, Sabbe, Rooij and Milisen2009) but with a high level of false positives. These scores either fail to provide accurate risk prediction for hospitalized older patients or are too complicated for routine use.
It would be useful, nonetheless, to understand the processes and criteria that underlie decisions of discharge from hospital. To our knowledge, few studies (Ellis et al., Reference Ellis, Whitehead, Robinson, O’Neill and Langhorne2011; Mudge et al., Reference Mudge, O’Rourke and Denaro2010) have analysed them.
Our main objective was to understand clinical decision-making concerning the discharge of older hospital patients. We compared discharge decisions (i.e., discharge home or offer of a nursing home stay) by the hospital team with those of an independent expert panel, and looked at the proportion of patients still at home 3 months after being discharged from hospital. Our second objective was to analyse the main parameters associated with the hospital team’s decisions and the panel’s decisions.
Method
Population
This case note review was carried out at the Bretonneau Hospital (Assistance Publique, Paris) during the period between January and June 2016. Bretonneau Hospital was created in 2000 as a resource centre for older patients with dementia or psychiatric conditions in the region (the 9th, 17th, and 18th Paris arrondissements). All hospitalized patients previously living at home were considered in this study. Only patients with a Mini-Mental State Examination (MMSE) score of less than 15 were excluded.
Bretonneau Hospital has had a specific database since 2006 (regulated for data protection purposes by the Commission Nationale de l’Informatique et des Libertés, authorization n°1858079). Socio-demographic and medical data are systematically collected.
We retained the following items for our study:
(1) Socio-demographic characteristics including age, gender, marital status, household composition, formal and informal caregivers, and years of formal education. We used 7 categories for educational level: 1 for no school, 2 for 10 years of education, 3 for 14 years of education, 4 for 17 years of education, 5 for 18 years of education, 6 for 20 years of education, and 7 for more than 20 years of education (Amieva et al., Reference Amieva, Jacqmin-Gadda, Orgogozo, Le Carret, Helmer, Letenneur and Dartigues2005).
(2) Overall cognitive function was assessed with the MMSE as a screening test in accordance with the French National Alzheimer’s Database (Pradier et al., Reference Pradier, Sakarovitch, Le Duff, Layese, Metelkina, Anthony and Robert2014); functional status was assessed using the Instrumental Activities of Daily Living (IADL) score (Law, Barnett, Yau, & Gray, Reference Law, Barnett, Yau and Gray2011). If there was already a diagnosis of dementia, we noted it.
(3) Geriatric assessment included the risk of falls, variations in weight (which is a strong predictor of adverse outcome; Soto et al., Reference Soto, Secher, Gillette-Guyonnet, Abellan van Kan, Andrieu, Nourhashemi and Vellas2012), asthenia, number of co-morbid conditions, number of treatments, and Framingham score (Amieva et al., Reference Amieva, Jacqmin-Gadda, Orgogozo, Le Carret, Helmer, Letenneur and Dartigues2005). All patients underwent an assessment of their daily living needs using the French “Iso Resource Group” (Groupe Iso Ressources or GIR) scale (Somme & de Stampa, Reference Somme and de Stampa2011), which is an overall assessment of social needs in six categories. GIR category 6 is for those who are not dependent on others, and category 1 is for the most dependent.
(4) Psychiatric assessment included the French version of the Neuro-Psychiatric Inventory (NPI; Koskas, Henry-Feugeas, Feugeas, Ou, & Drunat, Reference Koskas, Henry-Feugeas, Feugeas, Ou and Drunat2017), psychiatric illness, and personality disorders.
Blind Assessment
A panel of three experts (a neurologist, a psychiatrist, and a geriatrician) reached consistent decisions on patient hospital discharge (i.e., discharge home or offer of nursing home stay) after studying the patient’s data during the last days of the hospitalization for each patient included in the study. These practitioners met once a week to give a consensus opinion for hospital discharge. For this study, we considered that the expert panel constituted the “gold standard” of decision-making.
The expert review panel was systemically required, and so both reviews occurred with all patients being discharged. If a patient’s MMSE was less than 15, the expert panel did not pursue their assessment.
The study was blinded in that neither the hospital team nor the expert panel saw the other team’s decision before making their own. However, this process did not affect the final decision, which always aligned with the recommendation of the hospital staff.
Main Outcomes
We compared the hospital team decision with the expert panel decision for each patient to determine how closely these two processes corresponded. We analysed the association between the hospital discharge decision (i.e., discharge home or offer of nursing home stay) with the social and medical parameters listed in Tables 1 and 2.
Table 1: Patients’ main characteristics (n = 102)
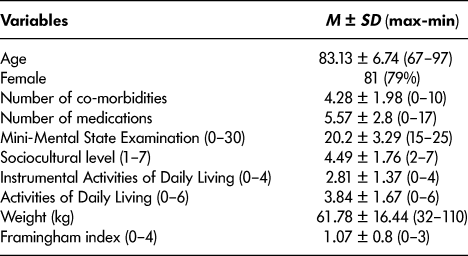
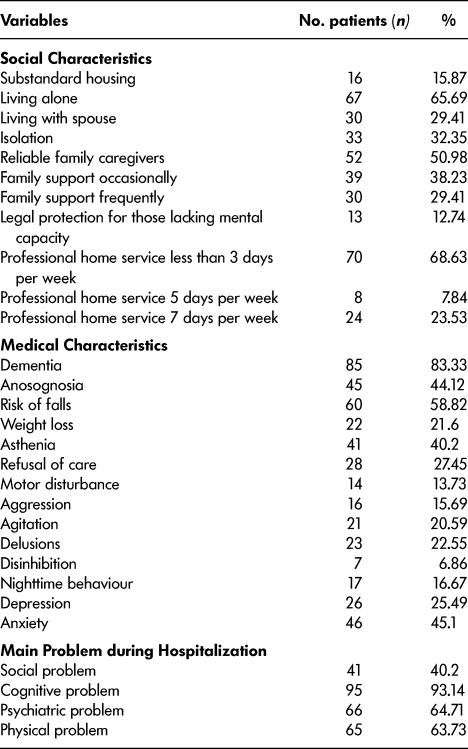
Table 2: Patients’ social and medical characteristics (n = 102)
For patients discharged home, a phone interview was carried out 3 months after discharge with either the patient or a designated contact (caregiver or general practitioner) to find out if the patient was still at home. It should be noted that, as the final decision was made by the hospital team, we could not compare the number of patients still at home at 3 months with the panel decision.
Ethics
This is a retrospective study without clinical or therapeutic intervention that did not require the approval of the ethics committee. Nevertheless, all patients and families were notified of the protocol and signed an agreement form for their participation.
Data Analysis
Quantitative variables were indicated as mean ± standard deviation. Qualitative variables were indicated as number and percentage of the total.
We compared the team and panel decisions using chi-square, and determined a kappa coefficient.
For quantitative and qualitative variables, we carried out regression analysis with three models using the expert panel decision, the hospital team decision, and “patients still at home at 3 months” as dependent variables. We retained p < .05 for all analysis except for the last regression analysis using 17 comparative variables. Significant levels of p were .003 corrected for multiple comparisons using the Bonferroni correction.
Results
Population
We included 102 patients in our study. It was a community-dwelling geriatric population (mean age 83.13 years ± 6.74). About 65 per cent were living alone before their hospitalization, and only half had a reliable caregiver. Although 83 per cent had mild or moderate dementia (MMSE 20.2 ± 3.29; ADL 3.84 ± 1.67), about 44 per cent had anosognosia, and about 13 per cent were subject to the French regime of legal protection for those lacking mental capacity. Social problems were present in 40 per cent of cases and substandard housing in 16 per cent. All characteristics are summarized in Tables 1 and 2.
Correlation of Hospital Team and Expert Panel Decisions
The expert panel and the hospital team made the same decision for 76 patients (74.5% of the cases): 24 times opting for admission to a nursing home and 53 times for a return home. There is a statistically significant difference between their decisions (p < .001). The agreement is moderate with a kappa coefficient of 0.468 [0.95 CI: 0.29–0.65] (Table 3).
Table 3: Decision agreement between hospital team and expert-panel hospital team

Note. The agreement is moderate with a kappa coefficient of 0.468 [0.95 CI: 0.29–0.65] and p = 4.24.10-6 statistically significant difference between their decisions.
Of 102 patients, the hospital team decided to discharge 63 patients home. The phone interview carried out 3 months after discharge found 45 patients still at home, 13 persons in nursing homes, and 5 deceased patients. As mentioned above, blind expert decision did not influence final hospital team decision.
Regression Analysis
For quantitative variables in the two models – namely, “panel’s decisions” and “hospital team’s decisions” – only MMSE (p = .017) reached a significant threshold for expert decision (Table 4a).
Table 4a: Linear regression (a) with expert panel decision as dependent variable (n = 102); (b) with hospital team decision as dependent variable (n = 102); (c) with “at home at 3 months” as dependent variable (n = 63)

Note. ADL = Katz Activities of Daily Living; IADL = Lawton Instrumental Activities of Daily Living; MMSE = Mini-Mental State Examination; Pr = probability.
* p < .05.
For qualitative variables, the panel’s decisions were associated with isolation (p = .018), reliable caregivers (p = .004), and social problems (p = .001). The “hospital team’s decisions” were associated with legal protection for those lacking mental capacity (p = .045) and behavioural symptoms perceived as aggressive (p = .001). We would emphasise the significant association for both decision processes with refusal of care (respectively p = .025 and .016) and social problems (respectively p = .001 and < .001) (Table 4b).
Table 4b: Regression analysis (a) with expert panel decision as dependent variable (n = 102); (b) with hospital team decision as dependent variable (n = 102); (c) with “at home at 3 months” as dependent variable (n = 63)
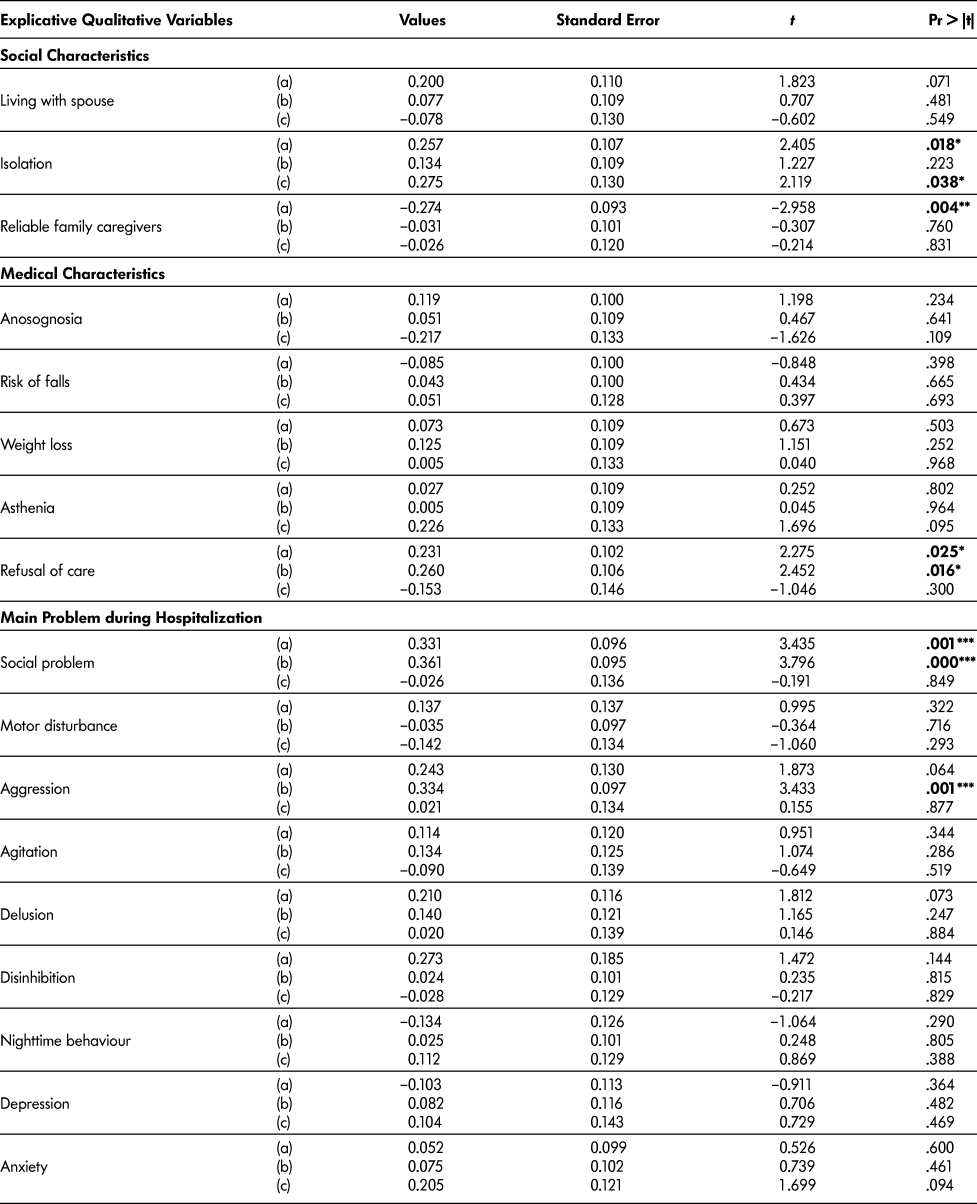
Note. * p < .05. ** p < .005. *** p corrected for multiple comparisons using the Bonferroni’s correction p < 0.003.
We explored the model using “patient still at home at 3 months” as the dependent variable (n = 63 patients; patients not returning home were excluded) with the same parameters. We obtained information for 100 per cent of patients (either from the patient or from the designated contact). Of these 63 patients, 13 (32.5%) were no longer at home when the 3-month telephone survey was carried out. We found that MMSE remained the only variable significantly associated with “at home at 3 months” in the quantitative regression analysis. Only “living alone” (p = .037) and “isolation” (p = .038) reached a significant threshold in qualitative analysis (Tables 4a and 4b).
Discussion
Our main finding was the discrepancy between the hospital team and expert panel decisions for patient hospital discharge. Our analyses showed that different parameters were being used in the decision processes of the hospital team and the panel, but it should be noted that “social problems” and “refusal of care” were common parameters. The association between patients who were still able to remain at home 3 months after discharge and various social parameters, particularly “isolation” and “living alone”, is also noteworthy. Although our study focused on a French psychogeriatric unit, we emphasised the similarities between geriatric acute units and psychogeriatric units with a high frequency of persons living with dementia and frailty (Peyneau, Koskas, Romdhani, Houenou-Quenum, & Drunat, Reference Peyneau, Koskas, Romdhani, Houenou-Quenum and Drunat2016) and also with psychogeriatric units in other countries (Clerencia-Sierra et al., Reference Clerencia-Sierra, Calderón-Larrañaga, Martínez-Velilla, Vergara-Mitxeltorena, Aldaz-Herce, Poblador-Plou and Prados-Torres2015).
We were surprised by the discrepancy between the hospital team and expert panel decisions. Hospital discharge (Bowles, Foust, & Naylor, Reference Bowles, Foust and Naylor2003) requires careful, comprehensive assessment of patients’ needs (Bowles, Naylor, & Foust, Reference Bowles, Naylor and Foust2002). Hospital teams assessing medical and social needs make empirical decisions based on the practitioners’ skills and knowledge. However, there is a wide variation in hospital policies, and/or discharge procedures and planning (Bowles et al., Reference Bowles, Foust and Naylor2003; Mamon et al., Reference Mamon, Steinwachs, Fahey, Bone, Oktay and Klein1992). We explain the discrepancy in terms of specific human and environmental factors that provide the empirical knowledge base that discharge decision-makers use in everyday hospital care.
Moreover, interdisciplinary input and especially nurse opinion in planning the patient’s home care is a key factor missing from the panel decisions. We found that the MMSE score was statistically associated with the expert panel decisions but not with the hospital team decisions. For example, the Hospital Admission Risk Profile (HARP 3) puts the emphasis on a number of independent predictors of functional decline: increasing age, lower admission scores on MMSE and on the Instrumental Activities of Daily Living scale (IADL; Sager et al., Reference Sager, Rudberg, Jalaluddin, Franke, Inouye, Landefeld and Winograd1996), and the Score Hospitalier d’Evaluation du Risque de Perte d’Autonomie scale (SHERPA; Cornette et al., Reference Cornette, Swine, Malhomme, Gillet, Meert and D’Hoore2006), which includes five factors: (a) age, (b) impairment in premorbid instrumental ADLs, (c) falls in the year before hospitalization, (d) cognitive impairment, and (e) poor self-rated health. Sensitivity and specificity are low (67.9% and 70.8% respectively). MMSE is a well-known screening tool but with low sensitivity (Roalf et al., Reference Roalf, Moberg, Xie, Wolk, Moelter and Arnold2013) and is potentially misleading. Similarly, the IADL and Katz ADL scales are not sufficiently sensitive (Koskas et al., Reference Koskas, Henry-Feugeas, Feugeas, Poissonnet, Pons-Peyneau, Wolmark and Drunat2014) and were not associated with decision-making by the hospital team or the expert panel. Everyday observation of the patient by the team is certainly more pertinent.
We tried to identify the patterns of information gathering used by health professionals in making discharge decisions. Social problems (isolation, lack of reliable caregivers, and refusal of care) were associated with both the hospital team and panel hospital discharge decisions in our study. It is generally agreed that these factors are important when reaching appropriate discharge decisions (Bowles et al., Reference Bowles, Foust and Naylor2003; Naylor et al., Reference Naylor, Brooten, Jones, Lavizzo-Mourey, Mezey and Pauly1994). Several indices that focus on hospital discharge (Cornette et al., Reference Cornette, Swine, Malhomme, Gillet, Meert and D’Hoore2006; Dendukuri et al., Reference Dendukuri, McCusker and Belzile2004; Deschodt et al., Reference Deschodt, Wellens, Braes, De Vuyst, Boonen, Flamaing and Milisen2011; Donze et al., 2013; Prescott, Soeken, & Griggs, Reference Prescott, Soeken and Griggs1995; Teno et al., Reference Teno, Harrell, Knaus, Phillips, Wu, Connors and Lynn2000) seem less pertinent for social assessment. We think that hospital discharge is better characterized as a continuous process instead of an isolated event (Bowles et al., Reference Bowles, Foust and Naylor2003; Mamon et al., Reference Mamon, Steinwachs, Fahey, Bone, Oktay and Klein1992; Naylor et al., Reference Naylor, Brooten, Jones, Lavizzo-Mourey, Mezey and Pauly1994; Nyweide et al., Reference Nyweide, Anthony, Bynum, Strawderman, Weeks, Casalino and Fisher2013). Continuity of hospital and ambulatory care reduced the rate of hospitalization (Amjad, Carmichael, Austin, Chang, & Bynum, Reference Amjad, Carmichael, Austin, Chang and Bynum2016; Nyweide et al., Reference Nyweide, Anthony, Bynum, Strawderman, Weeks, Casalino and Fisher2013;). Although a hospital team usually assesses the patient’s clinical characteristics when making these decisions, they also have to coordinate follow-up services and home care. Anticipating future needs will help them make appropriate decisions (Amjad et al., Reference Amjad, Carmichael, Austin, Chang and Bynum2016). In France, a geriatric network (Somme, Reference Somme2014) has been in place for several years. However, this integrated care structure is faced with problems due in particular to the difficulty of coordinating the various facilities and their cost (Pimouguet, Lavaud, Dartigues, & Helmer, Reference Pimouguet, Lavaud, Dartigues and Helmer2010).
Our study features telephone follow-up of the patient or a referral at 3 months. Other studies have been carried out with shorter follow-up (Bowles et al., Reference Bowles, Naylor and Foust2002; Naylor et al., Reference Naylor, Brooten, Jones, Lavizzo-Mourey, Mezey and Pauly1994). It seems likely that patients who are readmitted to hospital within two weeks of discharge had often been discharged before their treatment had had its full effect, to some extent because shorter hospital stays are an objective of public services, with the goal of reducing insurance costs.
Our study found that a high percentage of patients (32.5%) were still able to remain at home 3 months after discharge from hospitalization. The literature (Hasan, Reference Hasan2001) reveals a wide range of possible reasons for hospital readmission. Poor patient compliance and living alone (Williams & Fitton, Reference Williams and Fitton1988) have previously been described, as in our study. These problems are a major obstacle to the establishment of home care and follow-up at home. Studies have shown that aftercare services are often difficult to implement in these cases. Although the French national Alzheimer’s Plan (Rapport d’évaluation du -2012) created several specialized bodies and facilities to reinforce home care, poor patient compliance or lack of a reliable caregiver remain major obstacles. Our study highlighted that a patient’s needs could not be met without a well-organized post-discharge referral.
Some criteria (anosognosia, dementia, and frailty criteria such as asthenia and weight loss) were not statistically associated either with the hospital team or expert panel decisions, or with patients remaining at home after 3 months. We explain these findings by the effectiveness of the hospital team in assessing and solving medical problems. Paradoxically in our study, behavioural criteria were not a factor in decisions made by the expert panel or by the hospital teams, although they are generally associated with a greater risk of institutionalization (Afram et al., Reference Afram, Stephan, Verbeek, Bleijlevens, Suhonen and Sutcliffe2014; Luppa, Luck, Brähler, König, & Riedel-Heller, Reference Luppa, Luck, Brähler, König and Riedel-Heller2008). We think that the specialization of the psychogeriatric unit made it possible to ensure better support for behavioural symptoms. “Aggression” was the only behavioral factor associated with the team’s decision in favour of a nursing home.
This study has several weaknesses. Our work was limited to one hospital, and the sample was small. The findings may differ in hospitals in other locations, discharge planning models, patients, or staff. Our findings may not be broadly generalizable over time or place since discharge policies vary in different settings and can change over time. The lack of feedback from the expert panel to the clinical staff is a limitation of our study.
We held meetings throughout our work with the various teams, but this was done informally. Also, we cannot explain in our work the factors of agreement and disagreement of teams with the results found. However, our findings are based on two different models of decision, evaluated blindly in our protocol, and the study focused on hospitalized older adults.
Although our study suggests the need for improved methods to support clinical decision-making, we note that the clinical staff generally acquires considerable information from direct observations and non-quantifiable assessments. Additionally, clinical staff also need to negotiate with patients and families or institutions, which would have influenced decision-making.







