Introduction
Marine macroalgae and invertebrates form rich benthic communities along the northern portion of the western Antarctic Peninsula (WAP). In shallow waters, macroalgae dominate on hard substrates, often covering > 80% of the seafloor and with standing biomass levels comparable to temperate kelp forests (Wiencke & Amsler Reference Wiencke, Amsler, Wiencke and Bischof2012, Quartino et al. Reference Quartino, Saravia, Campana, Deregibus, Matula, Boraso, Momo, Gómez and Huovinen2020). Large, perennial brown algae predominate in terms of both cover and biomass, but smaller red macroalgae are common as understory algae and can also be locally dominant in terms of cover (Wiencke et al. Reference Wiencke, Amsler, Clayton, De Broyer, Koubbi, Griffiths, Raymond, d'Udekem d'Acoz and Van de Putte2014, Gómez & Huovinen Reference Gómez, Huovinen, Gómez and Huovinen2020).
The abundant macroalgae support rich assemblages of small invertebrates including amphipods, which are the most abundant macroalgal-associated invertebrates (Huang et al. Reference Huang, Amsler, McClintock, Amsler and Baker2007, Aumack et al. Reference Aumack, Amsler, McClintock and Baker2011a). Although the vast majority of WAP macroalgal biomass is chemically defended from consumption by most amphipods and other grazers (von Salm et al. Reference von Salm, Schoenrock, McClintock, Amsler, Baker, Puglisi-Weening and Becerro2019, Amsler et al. Reference Amsler, McClintock, Baker, Gómez and Huovinen2020), amphipods and macroalgae have a mutualistic relationship in which the amphipods benefit their macroalgal hosts by removing small epiphytic and emergent endophytic algae. In turn, the amphipods benefit by gaining a refuge from fish predation when associated with chemically defended macroalgae (Amsler et al. Reference Amsler, McClintock and Baker2014, Reference Amsler, McClintock, Baker, Gómez and Huovinen2020, Heiser et al. Reference Heiser, Amsler, McClintock, Shilling and Baker2020).
Gastropods are also widespread and abundant along the WAP and elsewhere in Antarctica (Engl Reference Engl2012, Schiaparelli & Linse Reference Schiaparelli, Linse, Broyer, Koubbi, Griffiths, Raymond, d'Udekem d'Acoz, Van de Putte and A.P.2014) including in association with WAP macroalgae (Richardson Reference Richardson1977, Picken Reference Picken1979, Reference Picken1980a, Iken Reference Iken1999, Amsler et al. Reference Amsler, Huang, Engl, McClintock and Amsler2015, Rosenfeld et al. Reference Rosenfeld, Aldea, Ojeda, Marambio, Hüne, Troncoso and Mansilla2017, Elias-Piera et al. Reference Elias-Piera, Rossi, Petti, Campos, Valério-Berardo and Corbisier2020). We have hypothesized that macroalgal-associated gastropods may play a similar role to amphipods in benefitting their larger macroalgal hosts by removing epiphytic microalgae and filamentous macroalgae, and we provided support for this hypothesis in a mesocosm study (Amsler et al. Reference Amsler, Amsler, Curtis, McClintock and Baker2019). Although gastropods presumably benefit from an associational refuge from fish predation as amphipods do, particularly on more structurally complex, finely branched macroalgal species, we were unable to support a hypothesis that the macroalgae provide an associational refuge to gastropods from sea stars (Amsler et al. Reference Amsler, Amsler, Curtis, McClintock and Baker2019), which are common predators of small molluscs on the WAP (McClintock Reference McClintock1994, Mahon et al. Reference Mahon, Amsler, McClintock and Baker2002, Schram et al. Reference Schram, Amsler and McClintock2019).
We also hypothesize that the importance of gastropod grazers to their macroalgal hosts may be greater on large, blade-forming macroalgae than on more finely branched macroalgae. The most common, large blade-forming macroalga along the WAP is the huge brown alga Himantothallus grandifolius (A. Gepp & E.S. Gepp) Zinova, which often dominates the benthos from 10 to 15 m depth down to depths of 30–40 m or greater (Wiencke & Amsler Reference Wiencke, Amsler, Wiencke and Bischof2012, Wiencke et al. Reference Wiencke, Amsler, Clayton, De Broyer, Koubbi, Griffiths, Raymond, d'Udekem d'Acoz and Van de Putte2014). Individual H. grandifolius have one or up to a few blades that can exceed 17 m in length and be nearly 1 m wide (Dieckmann et al. Reference Dieckmann, Reichardt, Zielinski, Siegfried, Condy and Laws1985) and that lie decumbent, sometimes covering nearly 100% of the seafloor (authors’ personal observations). Because of their large size, we have not been able to sample quantitatively their associated amphipod assemblages, but based on our casual observations during scuba dives, gastropods are much more abundant on these blades than amphipods, at least on the exposed upper surfaces and during the daytime. Indeed, one might expect that an amphipod on the upper surface of one of these huge blades would be no more protected from fish predation than were it associated with bare rock.
The other common, large blade-forming macroalga along the WAP is the red alga Sarcopeltis antarctica Hommersand, Hughey, Leister, & P.W. Gabrielson, which until recently (Hughey et al. Reference Hughey, Leister, Gabrielson and Hommersand2020) has been referred to as Gigartina skottsbergii Setchell & N.L. Gardner. S. antarctica forms lobed, peltate thalli up to 2 m in diameter (Hughey et al. Reference Hughey, Leister, Gabrielson and Hommersand2020). Relatively small individuals (mean total surface area ~0.6 m2) of S. antarctica were included in an earlier study of amphipod assemblages associated with eight common macroalgal species (Huang et al. Reference Huang, Amsler, McClintock, Amsler and Baker2007), and we later enumerated gastropods from the same samples (Amsler et al. Reference Amsler, Huang, Engl, McClintock and Amsler2015). Although, as noted below, there were limitations to the gastropod analyses and all eight macroalgal species supported more amphipods than gastropods, S. antarctica had the highest ratio of gastropods to amphipods of these eight species.
The main goal of the present study was to document the gastropod assemblages associated with H. grandifolius and S. antarctica across multiple sites to facilitate future studies of their roles in the ecology of their specific macroalgal hosts and in the overall ecology of these macroalgal-dominated WAP communities. As is discussed below, gastropod densities can vary with depth, and consequently we compared two depth zones across four collection sites spatially separated by at least 1 km. For comparison, as logistical constraints allowed, we also collected gastropods from smaller numbers of individuals of three other common macroalgal species (Desmarestia anceps Montagne, Desmarestia antarctica R.L. Moe & P.C. Silva and Plocamium sp.) from two sites each. We did so even though we knew from our previous work (Huang et al. Reference Huang, Amsler, McClintock, Amsler and Baker2007, Amsler et al. Reference Amsler, Huang, Engl, McClintock and Amsler2015) that gastropod densities on macroalgae are more variable than amphipod densities and thus larger sample sizes than we were able to obtain for these other species would probably be necessary for statistical rigour. Consequently, the results from the additional species are primarily presented in the Supplemental Material.
Materials and methods
Collection sites and methods
Collections of macroalgae and their associated gastropod assemblages were made between late February and mid-May 2017 at six sites within 3.5 km of Palmer Station on the WAP (Fig. S1). The sites for H. grandifolius and S. antarctica were 1) ‘East Litchfield’, the unofficial name of a small islet off the north-east corner of Litchfield Island (64°46.112'S, 64°05.031′W; purple circle in Fig. S1), 2) ‘Southeast Bonaparte’, an unofficial name for the extreme south-east portion of Bonaparte Point which is not covered by glacier (64°46.747′S, 64°02.514′W; orange square in Fig. S1), 3) the north-eastern side of Stepping Stones Islands (64°47.027′S, 63°59.464′W; brownish-green diamond in Fig. S1), 4) the north-east corner of Christine Island (64°47.486′S, 64°00.997′W; blue triangle in Fig. S1) and 5) ‘Hermit Cove’, which is the unofficial name for a semi-protected slight embayment on the south-east end of Hermit Island (64°47.917′S, 64°00.439′W; inverted red triangle in Fig. S1).
Collections of H. grandifolius were made by scuba divers at 9 ± 1 and 18 ± 1 m depths (holdfast attachment depths) at ‘East Litchfield’, ‘Southeast Bonaparte’, Stepping Stones Islands and Christine Island, with five individuals collected at each site and depth except for six individuals collected at 18 m depth at Christine Island. The shallow depth of 9 m was chosen as this is near the shallowest depth at which H. grandifolius can be commonly found at many sites. The deep depth of 18 m was chosen as this is twice the shallow depth. S. antarctica was collected at 9 ± 1 and 18 ± 0.3 m depths at ‘Southeast Bonaparte’, Stepping Stones Islands and ‘Hermit Cove’ and at 9 m depth only at ‘East Litchfield’ (where it did not occur at 18 m depth), with five individuals collected at each site and depth.
To quantitatively collect the gastropod assemblages, macroalgae were cut just above the holdfast (H. grandifolius) or carefully pried from the substrate with a flat-bladed knife (S. antarctica) and then gently raised above the substrate as needed and teased into fine mesh (~0.25 mm) bags made from shear curtain cloth (one alga per bag). The bags had flexible wire rings at their mouths that helped keep them open while the macroalgae were being enveloped and that could subsequently be twisted closed and secured with small clips. The wire-supported openings ranged from ~0.6 to 1.0 m in diameter and the bags were ~1.2 to 2.0 m in length. Upon surfacing at the end of collection dives, the mesh bags were transferred to buckets of fresh seawater immediately and transported to Palmer Station, where they were held in flowing seawater aquaria for up to several hours before being processed.
To remove gastropods from the algae, the mesh bags were inverted into buckets and thoroughly rinsed with seawater followed by visual inspection and by hand removal of any gastropods remaining in the bags. Gastropods remaining on the algae after transfer into the buckets were brushed off. The gastropods were concentrated using a mesh screen (0.2 mm) and then preserved in 10% buffered formalin seawater for later identification (per Engl Reference Engl2012) and enumeration. The macroalgal thalli were weighed wet after removing as much seawater as possible by centrifugal force. Squares of thalli 10–25 cm on a side were cut out of five to six individuals each of H. grandifolius and S. antarctica and weighed to determine the surface area to weight conversion factors. Overall assemblage data were expressed as the total abundance of individuals per m2 thallus, the total number of species and the Shannon diversity index (H' = -Σipiloge(pi); pi is the proportion of the total count). The collections commonly included very small, juvenile gastropods that could not be identified to the genus or species level, and they occasionally contained larger gastropods that were damaged in processing and could not be identified. These were included in the analysis of total individuals but were not included for any of the other parameters. The collections also included juvenile members of three species in the genus Prosipho that could be distinguished as distinct species but not assigned to a specific species. These were included in all analyses.
Statistical analyses
Comparisons of total abundances of gastropods per m2, numbers of gastropod species and Shannon diversity index values of gastropod species between H. grandifolius or S. antarctica and collection depths utilized SPSS v.25 (IBM Corp.). The numbers of individual gastropods on individual algae were rank transformed and gastropod species number data were transformed by √(x + 0.5) as recommended by Zar (Reference Zar1998) in order to meet equality of error variances as determined by Levene's tests and normality as determined by both the Kolmogorov-Smirnov and Shapiro-Wilk tests. These were then compared using both one-way and three-way univariate general linear models tests. For the one-way tests, comparisons were made between data compiled from all sites at each depth for each species, and post hoc analyses between individual species and depths were conducted using a Tukey multiple comparison test. For the three-way analyses, species and depth were treated as fixed factors and site was treated as a random factor. Shannon diversity index data could not be transformed to satisfy the assumptions of parametric statistics so they could only be analysed using the one-way equivalent non-parametric Kruskal-Wallis H test. Post hoc pairwise comparisons of diversity between species and depths utilized pairwise Mann-Whitney U tests corrected for type I error using the sequential Dunn-Sidak method (Sokal & Rohlf Reference Sokal and Rohlf1995). Comparisons between the numbers of Nacella concinna (Strebel) at the two depths utilized Mann-Whitney U tests as the data were not normally distributed.
Non-parametric, multivariate analyses using PRIMER-e (Quest Research Ltd) were performed to compare gastropod assemblages on individual algae with specific statistical methodologies following the recommendations of Clarke et al. (Reference Clarke, Gorley, Somerfield and Warwick2014). Because gastropod abundances varied by several orders of magnitude across samples, the data were fourth-root transformed to down-weight the influence of the most abundant species. To compare the similarity between samples, Bray-Curtis coefficients were calculated and represented via non-metric multidimensional scaling (nMDS) ordination plots. Statistical differences between groupings were determined by CLUSTER analysis using similarity profile (SIMPROF) tests (P = 0.05) and by one-way and (fully nested) two-way analysis of similarities (ANOSIM) tests. To determine which species made the greatest contribution to some of the observed patterns, we used species contributions to sample (dis)similarities (SIMPER) tests.
Results
A total of 30 shelled gastropod species were identified on H. grandifolius and S. antarctica (Table I), with five additional species present on D. anceps and/or Plocamium sp. (Table S1). One individual of the nudibranch Doris kerguelenensis (Bergh) was collected on a H. grandifolius but not included in the analyses. Conversion factors to surface area were determined as 0.16 m2 surface area per 100 wet g alga for H. grandifolius and 0.325 m2 surface area per 100 wet g alga for S. antarctica. Calculated surface areas of H. grandifolius ranged from 0.9 to 13.1 m2 (mean 2.9 m2) and for S. antarctica they ranged from 0.4 to 4.6 m2 (mean 1.8 m2).
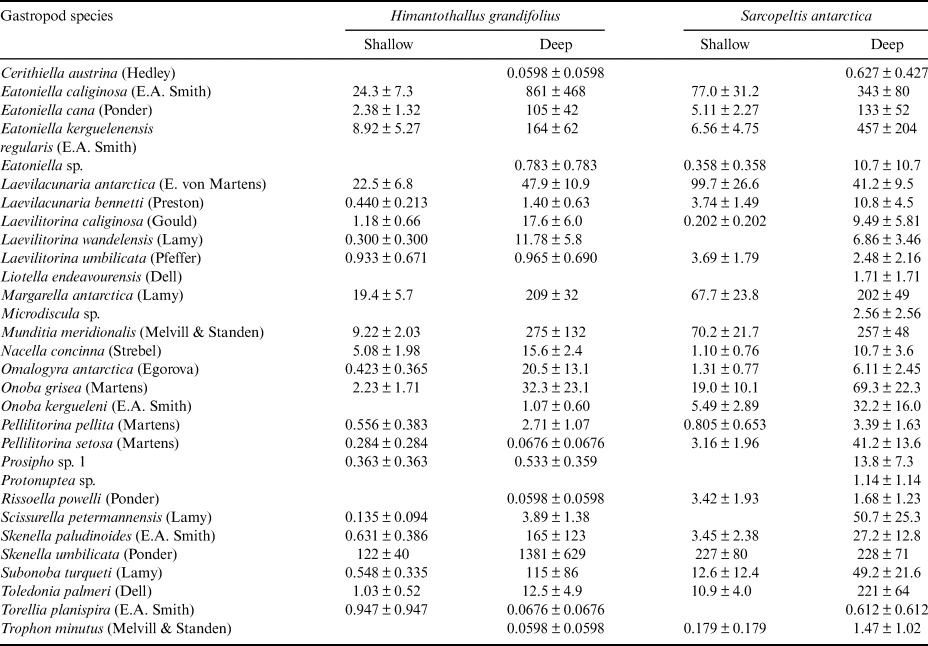
Table I. Numbers of individual gastropods by species per 100 cm2 surface area of Himantothallus grandifolius and Sarcopeltis antarctica (mean ± SE).
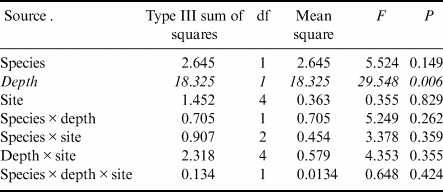
Gastropod numbers per unit surface area differed significantly across depths and species in the one-way analysis (F 3,72 = 22.928, P < 0.0005; Fig. 1a). Both H. grandifolius and S. antarctica supported significantly higher total numbers of gastropods at 18 m depth compared to at 9 m depth, with H. grandifolius supporting significantly fewer gastropods than S. antarctica at 9 m depth (Fig. 1a). The same result for depth differences was apparent in the three-way analysis (Table II), but the difference between species did not reach the level of statistical significance (P = 0.074). Both macroalgal species also supported significantly more gastropod species at 18 m depth than 9 m depth, as revealed both by the one-way (F 3,72 = 46.414, P < 0.0005; Fig. 1b) and three-way (Table III) analyses, but there were no significant differences between species at either depth (Fig. 1b & Table III). In the three-way analyses, neither collection site nor any interaction between species, depths or sites was significant (Tables II & III). Non-parametric analysis of the Shannon diversity index revealed significant differences overall (H3 = 26.357, P < 0.0005), with the only significant differences between species and depths being higher H’ in the 18 m S. antarctica (Fig. 1c).
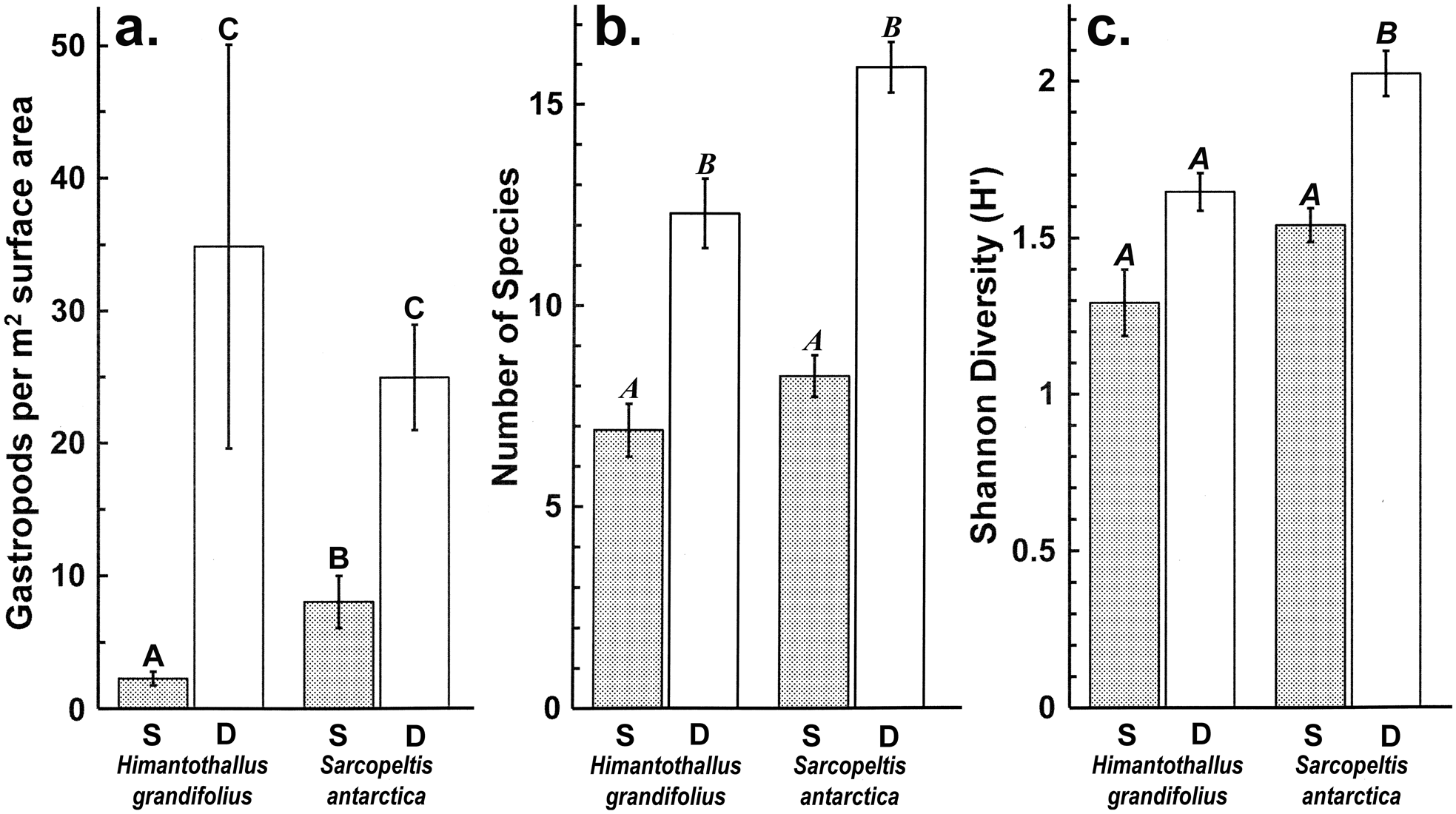
Fig. 1. Gastropods on Himantothallus grandifolius and Sarcopeltis antarctica (means ± SE). a. Number of individual gastropods per m2 macroalgal thallus. b. Number of individual gastropod species per individual macroalgae. c. Shannon diversity index (H') for gastropod assemblages on macroalgae. Bars with the same letters above are not significantly different from each other (P = 0.05). D = deep samples (18 m depth); S = shallow samples (9 m depth).
Table II. Three-way general linear models table for numbers of individual gastropods (rank transformed) on shallow and deep Himantothallus grandifolius and Sarcopeltis antarctica across sample sites. Significant differences shown in italics.
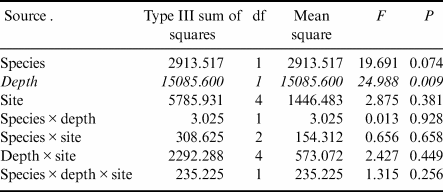
Table III. Three-way general linear models table for numbers of individual gastropod species (square root transformed) on shallow and deep Himantothallus grandifolius and Sarcopeltis antarctica across sample sites. Significant differences shown in italics.
Because the limpet N. concinna is the most commonly studied Antarctic gastropod, its abundance was analysed individually. N. concinna was slightly more abundant on H. grandifolius than S. antarctica, but it was not one of the more abundant gastropod species on either species. It occurred more commonly on deeper individuals (28 of 36 total) than shallow individuals (10 of 40 total). N. concinna was significantly (U = 307.500, P < 0.0005) higher in density on the deeper individuals (0.135 ± 0.021 gastropods m-2; mean ± SE) than the shallow individuals (0.031 ± 0.011). However, when comparing densities of N. concinna only on individual macroalgae on which it occurred (which greatly reduced the sample size, particularly for the shallow collections), this difference was no longer significant (U = 107.500, P = 0.281), even though the trend for higher density on the deeper (0.174 ± 0.022) vs shallower (0.124 ± 0.028) individuals remained.
Multivariate analyses of H. grandifolius revealed a distinction between depths that is apparent in a two-dimensional nMDS plot of Bray-Curtis similarities (Fig. 2). A CLUSTER analysis with a SIMPER test revealed five significant SIMPROF groups (Fig. 2). The two largest groups (14 and 16 individuals) included individuals from multiple sites, but all but one individual in each group were from shallow or deep depths, respectively. One group of seven individuals was mixed between depths and the other two groups of only two individuals each were both from the same depth. A global ANOSIM test indicated significant differences between sites and depths (R = 0.448, P = 0.0001). Pairwise comparisons within sites (Table S2) indicated significant differences between depths at all but the ‘Southeast Bonaparte’ site (R = -0.04, P = 0.516). A two-way ANOSIM test across depth and site indicated the strongest effect of depth (R = 0.519, P = 0.0001) and a weaker but significant effect of site (R = 0.283, P = 0.0001). SIMPER analysis of contributions to similarity and dissimilarity between depths indicated that the shallow samples had an overall similarity of 53.07%, the deep samples had an overall similarity of 60.60% and the depths had an average dissimilarity of 54.06%. The species making the greatest contributions to these similarities and dissimilarities are listed in Table S3.
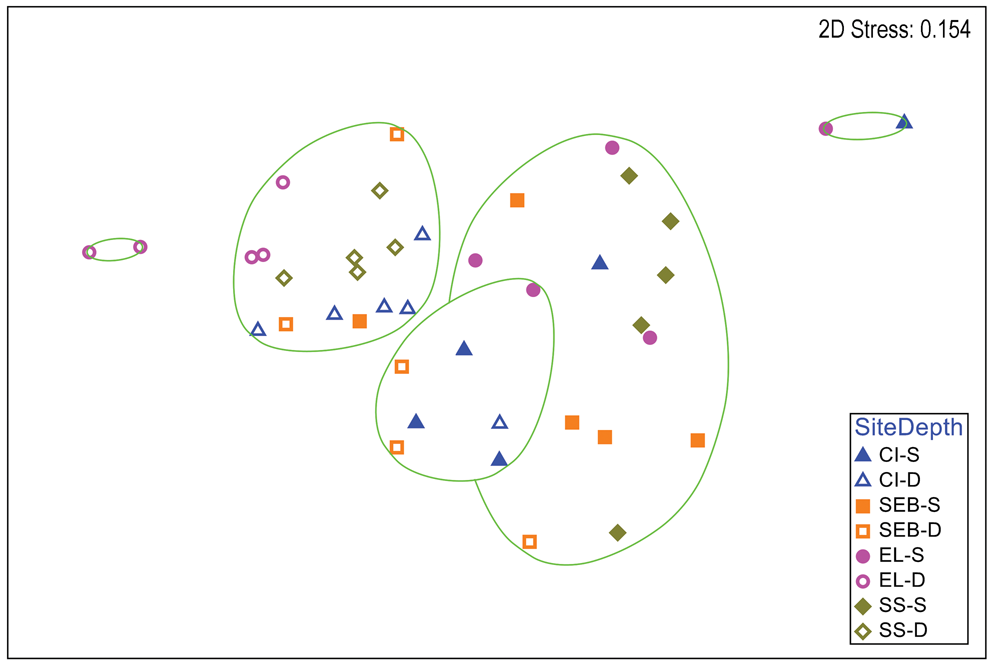
Fig. 2. Two-dimensional non-metric multidimensional scaling ordination of gastropod assemblages on Himantothallus grandifolius by collection site and depth. Bray-Curtis similarities calculated from fourth-root-transformed data. Site symbol shapes and colours match Fig. S1. Green lines indicate significantly different groups from CLUSTER analysis and SIMPER tests (P = 0.05). CI = Christine Island; D = deep samples (18 m); EL = 'East Litchfield'; S = shallow samples (9 m); SEB = 'Southeast Bonaparte'; SS = Stepping Stones Islands.
A clear distinction between depths is also apparent in a two-dimensional nMDS plot of Bray-Curtis similarities of S. antarctica (Fig. 3), but some similarities within depths at each site are also apparent, particularly for the deep collections. A CLUSTER analysis with a SIMPER test revealed six significant SIMPROF groups (Fig. 3). With the exception of a single individual collected at 18 m depth at ‘Southeast Bonaparte’, all individuals grouped with others from the same collection depth. All of the deep Stepping Stones individuals grouped together and alone, all of the deep ‘Hermit Cove’ individuals grouped together with one individual from deep ‘Southeast Bonaparte’ and the three remaining deep ‘Southeast Bonaparte’ individuals grouped together and alone. Although most shallow individuals formed a single large group, three shallow ‘East Litchfield’ individuals grouped together and alone. A global ANOSIM test indicated significant differences between sites and depths (R = 0.606, P = 0.0001). Pairwise comparisons within the sites that had two depth collections indicated significant differences between depths at all three sites (Table S2). A two-way ANOSIM test across depth and site indicated the strongest effect of depth (R = 0.772, P = 0.0001) and a somewhat weaker but significant effect of site (R = 0.445, P = 0.0001). SIMPER analysis of contributions to similarity and dissimilarity between depths indicated that the shallow samples had an overall similarity of 55.78%, the deep samples had an overall similarity of 65.03% and the depths had an average dissimilarity of 52.34%. The species making the greatest contributions to these similarities and dissimilarities are listed in Table S4.
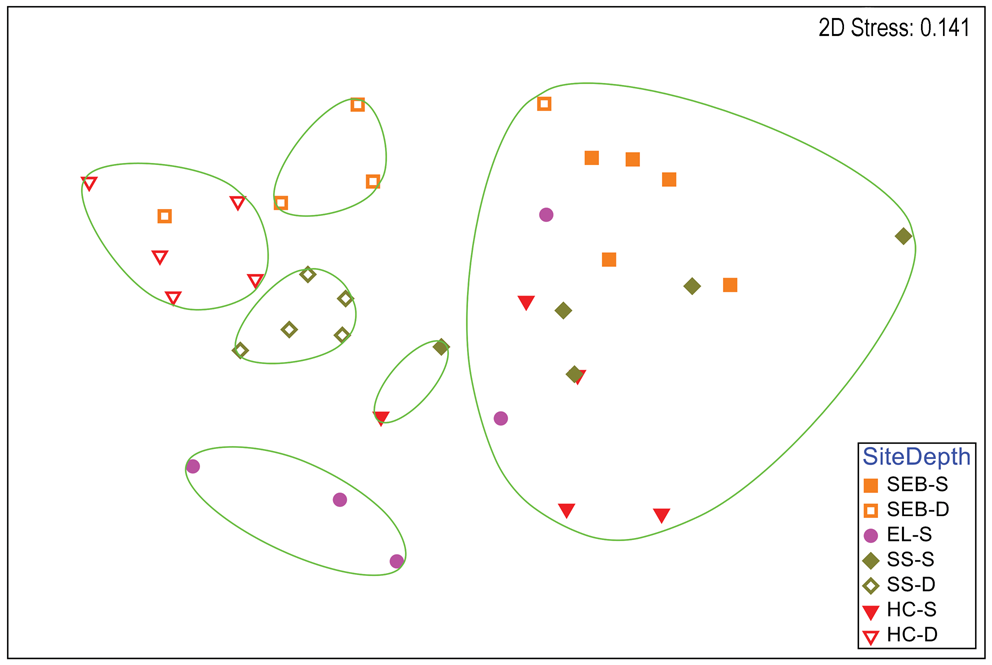
Fig. 3. Two-dimensional non-metric multidimensional scaling ordination of gastropod assemblages on Sarcopeltis antarctica by collection site and depth. Bray-Curtis similarities calculated from fourth-root-transformed data. Site symbol shapes and colours match Fig. S1. Green lines indicate significantly different groups from CLUSTER analysis and SIMPER tests (P = 0.05). D = deep samples (18 m); EL = 'East Litchfield'; HC = 'Hermit Cove'; S = shallow samples (9 m); SEB = 'Southeast Bonaparte'; SS = Stepping Stones Islands.
When comparing gastropod assemblages on H. grandifolius and S. antarctica, the only obvious pattern in a two-dimensional nMDS plot of Bray-Curtis similarities (Fig. 4) was with depth, with shallow individuals to the left side and deep individuals to the right side on the plot. However, a CLUSTER analysis with a SIMPER test revealed 14 significantly different SIMPROF groups with a pattern too complex to be overlain on Fig. 4 but that are displayed on the CLUSTER dendrogram in Fig. S2. By depth, in 10 of the 14 groups all individuals were either all shallow or all deep, with 2 additional groups of 9 or 10 individuals with a single individual not matching the collection depth of the others. By species, in 8 of the 14 groups all individuals were of a single species, with 2 additional groups of 9 or 10 individuals with a single individual not being the same species as the others (Fig. S2). Pairwise ANOSIM comparisons for the five depth-site combinations where both species were collected indicated no significant differences between gastropod assemblages on the two species at the three common shallow sites but significant differences between them at the two common deep sites (Table IV).
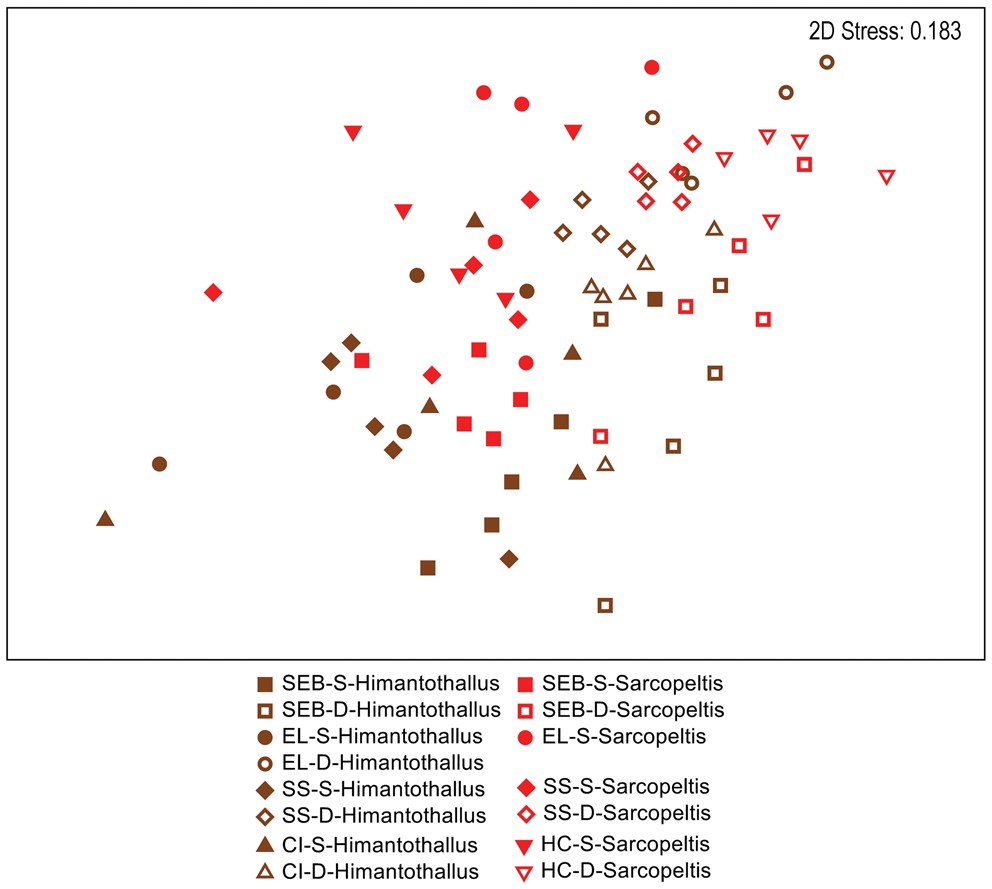
Fig. 4. Two-dimensional non-metric multidimensional scaling ordination of gastropod assemblages on Himantothallus grandifolius (brown symbols) and Sarcopeltis antarctica (red symbols) individuals. Bray-Curtis similarities calculated from fourth-root-transformed data. CI = Christine Island; D = deep samples (18 m); EL = 'East Litchfield'; HC = 'Hermit Cove'; S = shallow samples (9 m); SEB = 'Southeast Bonaparte'; SS = Stepping Stones Islands.
Table IV. Pairwise analysis of similarities tests for differences between gastropod assemblages between Himantothallus grandifolius and Sarcopeltis antarctica at sites where each was collected from the same depth. Significant differences shown in italics.

Discussion
There were significant and often large differences between the gastropod assemblages on deep vs shallow H. grandifolius and S. antarctica in every univariate parameter examined other than the Shannon diversity index of gastropods on H. grandifolius. Although the small sample size for the other macroalgal species precluded robust analyses, the same overall trends were apparent (Figs S3–S5). Significant differences between gastropod assemblages in shallow vs deep H. grandifolius and S. antarctica were also observed when comparing each species individually at all sites except for H. grandifolius at ‘Southeast Bonaparte'. In two-dimensional nMDS ordinations of Bray-Curtis similarities (Figs 2 & 3), within each species individuals from the same depth and site often cluster near each other, particularly for the deep-depth S. antarctica. When comparing the gastropod assemblages at the five site-depth pairs where both macroalgal species were collected (Table IV), at the two deep sites the gastropod assemblages on the two macroalgal species were significantly different. However, there were no significant differences between gastropod assemblages on the two macroalgal species at the three shallow sites. This suggests that host species can be but is not always more important than the site in influencing the associated gastropod assemblage. H. grandifolius can be much larger than S. antarctica and attaches to the substrate with a single holdfast. S. antarctica attaches with multiple peg-like haptera and females produce numerous pedicellate papillae with carposporophytes that extend above the thallus and may provide some protection for very small gastropods. Perhaps these morphological differences are related to the differences in gastropod assemblages when they occur. Why the differences should partition by depth is unknown, but perhaps the lack of assemblage differentiation at shallower depths could be related to having fewer numbers of individuals, fewer species and sometimes slightly lower diversity.
Differences in Antarctic gastropod assemblages across both wide and narrow depth ranges have previously been reported (Brandt et al. Reference Brandt, Linse and Schüller2009). Within the shallower depths studied here, we are aware of no published studies in the general WAP region that examine the depth distribution of complete gastropod assemblages, but several studies have reported differences in numbers of N. concinna with depth. North of the WAP, Picken (Reference Picken1980a) sampled from 2–3 to 11–12 m depths at Signy Island (60°43′S, 45°36′W) and reported peak abundance at 4–5 m depths. Brêthes et al. (Reference Brêthes, Ferreyra and Vega1994) reported that N. concinna was more abundant at 5 m depth compared to at 10 m depth during most months of the year at Hope Bay, Trinity Peninsula (63°18′S, 56°55′W). Barnes & Brockington (Reference Barnes and Brockington2003) and Bowden (Reference Bowden2005) reported on the depth distributions of N. concinna at Adelaide Island (67°35′, 68°07′W). Barnes & Brockington (Reference Barnes and Brockington2003) reported that overall mollusc density (including bivalves, gastropods and chitons) peaked at 6 m depth and declined below that and that this was mostly driven by N. concinna. Bowden (Reference Bowden2005) sampled at 8 and 20 m depths and reported that N. concinna was more abundant at 8 m depth. In contrast, in the present study N. concinna was much more common and in higher overall densities on the deeper macroalgae, following the overall pattern observed for the macroalgal-associated amphipod assemblages. Part of this difference could be due to the fact that the present study looked only at macroalgal-associated gastropods and the other, previous studies were looking at the overall distributions of N. concinna on any substrate. The N. concinna we observed on the macroalgae were much smaller than most that we observe on rocky substrata in our study area and, indeed, the larger limpets on rocks are much more commonly observed in shallower waters, even more so at depths shallower than our 9 m ‘shallow’ depth collections (authors’ personal observations). This probably explains the apparent difference between our and previous reports of the depth distribution of this species.
Rosenfeld et al. (Reference Rosenfeld, Aldea, Ojeda, Marambio, Hüne, Troncoso and Mansilla2017) reported on the mollusc fauna in beds of S. antarctica collected from random quadrats between 0 and 7 m depths at King George Island (62°12′S, 58°54′W) and Livingston Island (62°39′S, 60°35′W). The samples included molluscs not only on the algae but also on the rocky substrate within the quadrats. The authors did not report on depth distributions within their sample range and reported only six species of shelled gastropods. Four of these species were also present on S. antarctica in the present study, and the other two were in genera (Laevilitorina and Trophon) that had congeners present in our study. We report a total of 30 species of shelled gastropods on S. antarctica (Table I). This marked difference could possibly be due to differences between the collection sites or the different depth ranges sampled, but of the 30 gastropod species we found, 22 were present in the 9 m depth samples (all were present at 18 m depth; data not shown).
Rosenfeld et al. (Reference Rosenfeld, Aldea, Ojeda, Marambio, Hüne, Troncoso and Mansilla2017) also reviewed all reports on intertidal and shallow-water gastropod abundances in the WAP region from 1994 to 2017, and Chelchowski et al. (Reference Chelchowski, Balazy, Grzelak, Grzelak, Kędra, Legezynska and Kuklinski2022) recently reported gastropod abundances in the intertidal zone at another site on King George Island. With the exception of our prior work from Palmer Station (Amsler et al. Reference Amsler, Huang, Engl, McClintock and Amsler2015), which reported 20 species, no other published study from shallow waters in the general WAP region has reported more than nine gastropod species, including in some cases nudibranchs, which are not included here. However, in an unpublished dissertation section Picken (Reference Picken1980b) reported 31 species of gastropods associated with a variety of macroalgal species and the substrate between 2 and 12 m depths at Signy Island. Of these 31 species, at least 11 were also found in the present study, with an additional 12 at least having congeners in the present study. Another (mostly) unpublished dissertation from Signy Island based on an intensive, year-round study of fauna associated with D. anceps reported 29 macroalgal-associated gastropod species (Richardson Reference Richardson1977), with the most abundant gastropod species being similar to our D. anceps collections (Table S1). Although Elias-Piera et al. (Reference Elias-Piera, Rossi, Petti, Campos, Valério-Berardo and Corbisier2020) did not report numbers of species, they reported that gastropods at King George Island were common on subtidal macroalgae, sometimes being the dominant macroalgal-associated taxonomic group on blade-forming species. Most of the WAP area studies that have reported relatively few gastropod species have focused on the intertidal zone or were in areas (e.g. Adelaide Island; Bowden Reference Bowden2005, Waller et al. Reference Waller, Barnes and Convey2006) with much lower macroalgal percentage cover. A study from King George Island that did include subtidal macroalgae (Valdivia et al. Reference Valdivia, Díaz, Garrido and Gómez2015) had a size cut-off of 5 mm, which would have excluded many of the macroalgal-associated gastropod species we report here, and the authors noted that their methodology could have under-sampled substrate-associated gastropods. It seems probable that our observations of numerous gastropod species associated with subtidal macroalgae are representative of many if not most areas along the WAP region.
We have previously shown that gastropods were able to benefit H. grandifolius in a mesocosm experiment by reducing the densities of epiphytic diatoms and filamentous algae that can otherwise overgrow H. grandifolius and compete with it for light and nutrients (Amsler et al. Reference Amsler, Amsler, Curtis, McClintock and Baker2019). We concluded, however, that amphipods, which have previously been shown to benefit their macroalgal hosts in this manner (Amsler et al. Reference Amsler, McClintock and Baker2014, Reference Amsler, McClintock, Baker, Gómez and Huovinen2020), are probably more important in nature because the impacts of the gastropods on the epiphytes were not as pronounced as in an earlier, analogous mesocosm experiment with amphipods (Aumack et al. Reference Aumack, Amsler, McClintock and Baker2011b). The gastropod densities in the mesocosms from our 2019 report were based on the densities of gastropods on H. grandifolius sampled for the present study (Amsler et al. Reference Amsler, Amsler, Curtis, McClintock and Baker2019). Because of the > 15-fold difference in these densities between shallow and deep H. grandifolius (Fig. 1a), this means that the densities used in the experiment were approximately eight-fold higher than would be found in nature at 9 m depth, but nearly half as dense as would be found in nature at 18 m depth. Thus, the relative contribution of gastropods to keeping H. grandifolius clean of epiphytes is probably greater at deeper depths. Although we do not know whether gastropod densities remain high or perhaps even become higher at depths > 18 m, 9 m is at the shallower end of the typical depth range of H. grandifolius, and the species typically extends much deeper than our 18 m ‘deep’ depth (Wiencke & Amsler Reference Wiencke, Amsler, Wiencke and Bischof2012). Therefore, it is probable that our mesocosm study underestimated the overall importance of gastropods to their H. grandifolius hosts. We have not noticed shallower H. grandifolius, S. antarctica or other macroalgal species in nature having higher epiphyte loads than deeper individuals, but we have not specifically looked for this, and as amphipods clearly also play an important role, any differences due to differences in gastropod densities across depths could be subtle or compensated for by amphipods.
While outside the scope of the present study, at the ecosystem level the present study begs the question as to what environmental or biotic factor or factors are regulating such stark differences in gastropod density at the two depths examined. As the depths examined spanned a relatively narrow depth range, differences in environmental factors seem improbable. Future studies, however, may shed light on whether depth-dependent differences in levels of food availability or predation intensity by visual predators such as fish or chemosensory predators such as sea stars may be important.
Acknowledgements
The authors gratefully thank Dr Robert Angus for advice on statistical analyses. We are also grateful to S. Heiser, A. Shilling and S. Thomas for assistance in the field, as well as to the Antarctic Support Contract staff of Palmer Station for outstanding logistical support of this research. The final version of the manuscript was greatly improved based on the comments and suggestions of two anonymous reviewers.
Financial support
This research was funded by National Science Foundation awards PLR-1341333 (CDA, JBM) and PLR-1341339 (BJB) from the Antarctic Organisms and Ecosystems Program. JBM acknowledges the support of an Endowed University Professorship in Polar and Marine Biology provided by the University of Alabama at Birmingham.
Author contributions
CDA, LRM, JBM and BJB designed the study. LRM collected the gastropods. MOA identified the gastropods based on voucher specimens identified by WE. LRM, RAE and MOA enumerated the gastropods and MOA compiled these data. CDA analysed the data, prepared the figures and wrote the first draft of the manuscript. All authors contributed to editing the final manuscript.
Details of data deposit
Data are available at the United States Antarctic Program Data Center: https://www.usap-dc.org/view/project/p0010016.
Supplemental material
Seven supplemental figures, two supplemental tables, a supplemental materials and methods section and a supplemental results section will be found at https://doi.org/10.1017/S0954102022000153.













