Intra-uterine growth retardation (IUGR) is usually ascribed to ‘uteroplacental insufficiency’, a poorly defined clinical term suggesting compromised maternal–fetal relations, which limits availability of substrates to the fetus and retards growth during gestation( Reference Gmenwald 1 – Reference Bell and Ehrhardt 4 ). IUGR affects approximately 5 % of human neonates and is considered a major health problem worldwide( 5 ). Epidemiological studies suggested that IUGR was associated with an increased risk of the metabolic syndrome in adulthood, a clustering of cardiovascular risk factors such as diabetes, hypertension, dyslipidaemia and obesity( Reference Barker, Bull and Osmond 6 , Reference Hales, Barker and Clark 7 ). Mitochondrial changes might serve as a link during this process. Previous studies have reported that IUGR impaired mitochondrial biogenesis and oxidative phosphorylation (OXPHOS) in the liver of rats, and this derangement might predispose the IUGR rats to the development of type 2 diabetes mellitus( Reference Peterside, Selak and Simmons 8 , Reference Park, Kim and Kim 9 ). Thus, early nutritional intervention to improve mitochondrial function and energy metabolism for the IUGR offspring may be an effective approach to decreasing the risk of metabolic syndrome in later life.
Medium-chain TAG (MCT) are six- to twelve-carbon fatty acid esters of glycerol, and they have several physiological advantages because of their unique gastrointestinal absorption and transport processes( Reference Zentek, Buchheit-Renko and Ferrara 10 ). Commercial MCT products predominantly comprise C8 : 0 and C10 : 0, and they are obtained through lipid fractionation from edible fats, such as coconut, palm oils and Cuphea seed oils( Reference Zentek, Buchheit-Renko and Ferrara 10 ). Unlike long-chain TAG (LCT), which are transported as chylomicrons from the intestinal tract in the lymphatics to the blood, MCT are efficiently absorbed and carried directly to the liver via the portal vein for energy production( Reference Wojtczak and Schönfeld 11 ). Consequently, MCT represent immediately available sources of energy that have been used in the formulas of premature infants. Treatment with MCT may be an effective approach to alleviating energy deficiency caused by IUGR.
Dietary use of MCT feeding to improve growth performance of the young pigs is gaining increasing momentum in the swine industry( Reference Dove 12 – Reference Dierick, Decuypere and Molly 14 ). We previously reported that replacement of dietary LCT with MCT had a positive role in alleviating growth lag of the IUGR piglets( Reference Zhang, Chen and Li 15 ). In addition, we found the beneficial effects that MCT had in improving hepatic oxidative status of the IUGR piglets, as evidenced by increased metabolic efficiency of glutathione redox cycle and pentose phosphate pathway (PPP), which might be associated with the decreased expenditure of glucose as a source of energy( Reference Zhang, Chen and Li 15 ). Therefore, we hypothesised that there is a possible link between oxidative status and energy metabolism in the liver, a process related to substrate availability and glucose flux. The present study was conducted to investigate the effects of IUGR and MCT on hepatic energy metabolism and mitochondrial function of weanling piglets. Moreover, it has been widely accepted that the pig can act as an animal model for IUGR studies in humans because of its biological similarity to humans( Reference Merrifield, Lewis and Claus 16 ). The study may give us some guidance to guarantee appropriate development of the IUGR offspring during the early period post weaning.
Methods
Materials
The MCT used in the present study were isolated from coconuts. The test oils, including soyabean oil (SO) and MCT (consisting of 55·8 % caprylin and 43·8 % decanoin), were obtained from Yihai Oils & Grains Industries Co. Ltd (for detail information see the online Supplementary Material S1).
Before fatty acid analysis, 50 mg of SO or MCT sample was placed into a 50-ml glass flask and diluted in 3 ml of 0·5 m-sodium hydroxides methanol solution. The sample was then refluxed at 55°C for 20 min, followed by supplementing with 3 ml of 13 % boron trifluoride methanol solution and continuing to reflux for 20 min. Finally, 1 ml of n-hexane was added to the mixture. The reaction was terminated by adding saturated sodium chloride solution and cooled down to room temperature. The n-hexane layer was carefully collected, and the sample was dried over anhydrous sodium sulphate, frozen and stored in sealed vials at 4°C before GC analysis.
The fatty acid compositions were determined by a GC (Shimadzu GC-17A; Shimadzu) with a capillary column (SP2340; Supelco) and a flame ionising detector. The temperature at the injector port and detector was set at 260 and 300°C, respectively. The GC oven temperature was programmed from 70°C (2 min) to 200°C at a rate of 15°C/min. Then, it was programmed to 230°C at a rate of 1·5°C/min and held for 20 min. The carrier gas was He, with a flow rate of 2 ml/min. The injector was used in split mode with a ratio of 1:30. The fatty acids were identified by comparing their retention times with those of synthetic standards (47885-U; Sigma-Aldrich).
Animals and treatments
The experimental protocols were permitted by the Institutional Animal Care and Use Committee of Nanjing Agricultural University, and followed the current law of animal protection( 17 ). During the preparation, forty healthy pregnant sows (Landrace×Yorkshire) with similar expected dates of confinement (<4 d) and parity (second or third, 2·75 (sd 0·44)) were selected. The sows were fed a commercial diet during their pregnancies. At birth, the sows that had similar litter sizes (i.e. about eleven to twelve piglets) and that met the selection criteria for IUGR were selected; the birth weight (BW) and sex of each newborn pig (Duroc×(Landrace×Yorkshire)) were carefully recorded. A piglet was defined as IUGR when its BW was two standard deviations below the mean BW of the total population( Reference Zhang, Chen and Li 15 , Reference Wang, Huo and Shi 18 ). In each litter, one male IUGR piglet with a BW of 0·95 (sd 0·04) kg and one normal same-sex littermate with a BW of 1·58 (sd 0·04) kg were chosen. At weaning (21 (sd 1·06) d of age), sows were excluded from the study if their selected piglets died, and then twenty-four IUGR and twenty-four normal-birth-weight (NBW) piglets remained (IUGR: 5·26 (sd 0·15) kg and NBW: 6·98 (sd 0·19) kg, P<0·001). Both the IUGR and the NBW groups were transferred to the weaning unit, and each group included two sub-groups. Each sub-group was fed a diet of LCT or MCT for 28 d (Table 1). Considering the uncontrollable factors, such as individual differences in growth and development, we decided to assign three piglets to each replicate (with one pen per replicate). Thus, all piglets were distributed into four sub-groups (i.e. NBW-SO, NBW-MCT, IUGR-SO or IUGR-MCT), each conducted in four pens, with three piglets per pen. Piglets were given free access to food and water until the day of sampling.
Table 1 Composition and nutrient level of the diets (as-fed basis)
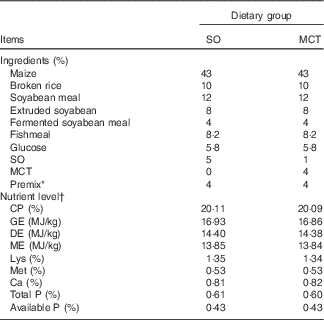
SO, soyabean oil; MCT, medium-chain TAG; CP, crude protein; GE, gross energy; DE, digestible energy; ME, metabolisable energy.
* The premix provided per mg/kg diet: retinyl acetate, 4·79; cholecalciferol, 0·075; all-rac-α-tocopherol acetate, 100; menadione, 3; thiamin, 3; riboflavin, 8; nicotinamide, 5; cobalamin, 0·04; biotin, 0·3; pantothenic acid, 20; niacin, 45; folic acid, 2; choline chloride, 450; Fe (as FeSO4.H2O), 180; Cu (as CuSO4.5H2O), 230; Zn (as ZnO), 65; Mn (as MnSO4.H2O), 50; I (as KIO3), 0·5; Se (as Na2SeO3), 0·2.
† All nutrient contents, except DE and ME, were analysed values.
Sample collection
After treatment for 4 weeks, four piglets from each treatment (one piglet per pen) with nearly equal body weight were selected. Heparinised blood samples were drawn by jugular venepuncture and then centrifuged at 2000 g for 10 min at 4°C, and plasma was stored at –80°C for further analyses. All piglets were killed by exsanguination after electrical stunning, following which the liver samples were rapidly collected. A portion of the liver was rapidly treated for mitochondria isolation, whereas the remaining parts were stored in liquid N2 until analysis.
Plasma hormone assay
The concentrations of 3,3',5-triiodo-l-thyronine and 3,3',5,5'-tetraiodo-l-thyronine (T4) in the plasma were determined using a commercially available 125I-RIA kit (Beijing Research Institute of Biotechnology). The contents of plasma adiponectin and total ghrelin were measured by ELISA using a commercially available procine-specific kit (Cusabio) according to the manufacturer’s guidelines. The detection limits were 1·875 μg/ml for adiponectin and 62·5 pg/ml for total ghrelin, respectively.
Mitochondria isolation and oxygraphic measurement
Hepatic mitochondria were prepared by the method previously described( Reference Tang, Gao and Wang 19 ), and then assayed for VO2 at 37°C in a thermostatically controlled oxygraph apparatus equipped with a Clark electrode (Hansatech Instruments Ltd). O2 uptake in state 3 respiratory and state 4 respiratory and the respiratory control ratio (RCR) were calculated as previously reported using glutamate/malate or succinate as oxidative substrates( Reference Serviddio, Bellanti and Romano 20 ). The protein concentrations of the hepatic mitochondria were quantified according to the Bradford method( Reference Bradford 21 ).
Determination of hepatic mitochondrial citrate synthase and succinate dehydrogenase activities
The activities of citrate synthase (CS) and succinate dehydrogenase (SDH) in hepatic mitochondria were determined using commercial kits (Nanjing Jiancheng Institute of Bioengineering). Briefly, the activity of CS was determined using a spectrophotometric method based on the enzyme-catalysed reaction between acetyl-CoA and oxaloacetic acid. The CoA-SH produced by this reaction could react with 5,5'-dithio-bis (2-nitrobenzoic acid), thereby forming a mercaptide ion. The changes in absorbance were monitored at 412 nm. One unit of CS activity was defined as the amount of enzyme catalysing 1 nmol citrate at 37°C in 1 min. The activity of SDH was assayed spectrophotometrically at 600 nm. When homogenate protein catalysed the substrates, FAD was reduced to FADH, coupled with a 2,6-dichlorophenolindophenol reduction reaction. One unit of SDH activity was defined as the decrease by 0·01 absorbance units at 37°C in 1 min. All results were normalised to total protein concentration in each sample for inter-sample comparison. The protein concentrations of hepatic mitochondria were quantified according to the Bradford method( Reference Bradford 21 ).
Measurement of hepatic ATP concentration
ATP was determined using the ATP assay kit (Beyotime Institute of Biotechnology) based on firefly luciferase by a chemiluminescence counter Lumat LB9507 (Berthold Technologies). Approximately 50 mg of frozen liver was removed quickly and placed in 1:9 (w/v) lysis buffer (10 mm-dithiothreitol, 1 mm-MgSO4, 0·5 mm-EDTA and 1 mg/l-bovine serum albumin, pH 7·8), and homogenised using an Ultra-Turrax homogenizer (Tekmar Co.). Then, the homogenate was centrifuged at 12 000 g for 5 min at 4°C, and the supernatant was analysed rapidly. The protein concentrations in liver homogenate were quantified by following the Bradford method( Reference Bradford 21 ).
Assay of hepatic hexokinase and pyruvate kinase activities
Approximately 0·3 g of frozen liver was removed quickly and placed in 1:9 (w/v) sterile saline solution (8·6 g/l, pH 7·4). The liver specimen was homogenised using an Ultra-Turrax homogenizer at 13 500 rpm for 1 min. Then, the homogenate was centrifuged at 4500 g for 20 min at 4°C, and the supernatant was analysed rapidly. The activities of hexokinase (HK) and pyruvate kinase (PK) were determined using colorimetric kits (Nanjing Jiancheng Institute of Bioengineering). Briefly, HK activity was measured spectrophotometrically at 340 nm with glucose-6-phosphate dehydrogenase glucose, ATP and NADP+ in the presence of 1 μmol/l rotenone to inhibit the mitochondrial respiration chain. One unit of HK activity was expressed as the amount of enzyme producing 1 μmol of NADPH at 37°C in 1 min. The activity of PK was assayed spectrophotometrically at 340 nm. When homogenate protein catalysed the substrates, pyruvic acid was formed by phosphoenolpyruvate, coupled with an NADH oxidation reaction. One unit of PK activity was defined as the amount of enzyme producing 1 μmol of pyruvic acid at 37°C in 1 min. All results were normalised to total protein concentration in each sample for inter-sample comparison. The protein concentrations in the homogenate were quantified according to the Bradford method( Reference Bradford 21 ).
Determination of hepatic mitochondrial DNA content
Total DNA was extracted from snap-frozen liver using a QIAmp tissue kit (Qiagen). The relative mitochondrial DNA (mtDNA) content was measured by co-amplifying the mt D-loop (accession number AF276923) and the nuclear-encoded β-actin (accession number DQ452569) gene using real-time PCR assay according to the method described by Liu et al.
(
Reference Liu, Yao and Yu
22
). The mtDNA was amplified using the following set of primers: forward 5'-GATCGTACATAGCACATATCATGTC-3', reverse 5'-GGTCCTGAAGTAAGAACCAGATG-3', yielding a 198-bp product. The β-actin was amplified using the following set of primers: forward 5'-CCCCTCCTCTCTTGCCTCTC-3', reverse 5'-AAAAGTCCTAGGAAAATGGCAGAAG-3', yielding a 74-bp product. The PCR amplification was performed on an ABI StepOnePlusTM Real-Time PCR System (Applied Biosystems) under the following conditions: 95°C for 10 s, fifty cycles involving a combination of 95°C for 5 s and 60°C for 25 s and 95°C for 10 s. The conditions of the melting curve analysis were as follows: one cycle of denaturation at 95°C for 10 s, followed by an increase in temperature from 65 to 95°C at a rate of 0·5°C/s. The reaction volume was 20 μl, consisting of 1 μl of DNA, 1 μl of probes (TaKaRa Biotechnology), 8 μl of TaqMan Universal Master mix (TaKaRa Biotechnology), 1 μl of each of forward and reverse primers, 1 μl of enhance solution (TaKaRa Biotechnology) and 7 μl of double-distilled H2O. Each sample was amplified in duplicate. The relative quantification values were calculated according to the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Liu, Yao and Yu
22
). The data were plotted using Prism 5.0 (GraphPad Software Inc.), as shown in Fig. 1.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Liu, Yao and Yu
22
). The data were plotted using Prism 5.0 (GraphPad Software Inc.), as shown in Fig. 1.

Fig. 1 Effect of soyabean oil (SO) and medium-chain TAG (MCT) on hepatic mitochondrial DNA (mtDNA) content in intra-uterine growth-retarded (IUGR) and normal-birth-weight (NBW) piglets. Values are means (n 4), with their standard errors represented by vertical bars. Piglets were fed SO or MCT diet for 28 d and were sampled at an age of 49 d. BW, birth weight.
Total RNA isolation and mRNA quantification
Total RNA was isolated using Trizol Reagent (TaKaRa Biotechnology) from a snap-frozen liver sample using the manufacturer’s protocol. The RNA integrity was checked on 1 % agarose gel with ethidium bromide staining. The RNA concentration and purity were determined from OD260/280 readings (ratio>1·8) using a NanoDrop ND-1000 UV spectrophotometer (NanoDrop Technologies). After determining the RNA concentration, 1 μg of total RNA was reverse-transcribed into complementary DNA (cDNA) using the PrimeScriptTM RT Reagent Kit (TaKaRa Biotechnology) according to the manufacturer’s guidelines. Real-time PCR was performed on an ABI StepOnePlusTM Real-Time PCR System according to the manufacturer’s instructions. The primer sequences for the target and reference genes (nuclear respiratory factor 1 (NRF1), nuclear respiratory factor 2 (NRF2), PPARα, mitochondrial transcription factor A (TFAM), PPARγ coactivator-1α (PGC1α), glucokinase (GCK), CS, NADH dehydrogenase 1α subcomplex, 8 (NDUFA8), NADH dehydrogenase 1α subcomplex, 13 (NDUFA13), NADH dehydrogenase 1 subcomplex unknown, 2 (NDUFC2), SDH complex, subunit A (SDHA), ubiquinol cytochrome c reductase binding protein (UQCRB), cytochrome c oxidase subunit IV (Cox IV), ATP synthase, H+ transporting, mitochondrial F0 complex, subunit C1 (ATP5G1), ATP synthase, H+ transporting, mitochondrial F1 complex, β polypeptide (ATP5B) and β-actin) are given in the online Supplementary Material S2. Briefly, the reaction mixture was prepared using 2 μl of cDNA, 0·4 μl each of forward and reverse primers, 10 μl of SYBR Premix Ex TaqTM (TaKaRa Biotechnology), 0·4 μl of ROX Reference Dye (TaKaRa Biotechnology) and 6·8 μl of double-distilled water. Each sample was tested in duplicate. PCR consisted of a pre-run at 95°C for 30 s and forty cycles of denaturation at 95°C for 5 s, followed by a 60°C annealing step for 30 s. The conditions of the melting curve analysis were as follows: one cycle of denaturation at 95°C for 10 s, followed by an increase in temperature from 65 to 95°C at a rate of 0·5°C/s. The relative levels of mRNA expression were calculated using the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method after normalisation against the reference gene β-actin(
Reference Livak and Schmittgen
23
). The values of NBW-SO group were used as a calibrator, and the results were plotted using Prism 5.0, as shown in Fig. 2.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method after normalisation against the reference gene β-actin(
Reference Livak and Schmittgen
23
). The values of NBW-SO group were used as a calibrator, and the results were plotted using Prism 5.0, as shown in Fig. 2.

Fig. 2 Effect of soyabean oil (SO) and medium-chain TAG (MCT) on hepatic gene expression related to energy metabolism and mitochondrial biogenesis in intra-uterine growth-retarded (IUGR) and normal-birth-weight (NBW) piglets. Values are means (n 4), with their standard errors represented by vertical bars. Piglets were fed a SO or MCT diet for 28 d and were sampled at an age of 49 d. a,b Mean values within a row with unlike letters were significantly different (P<0·05) between groups. BW, birth weight; NRF1, nuclear respiratory factor 1; NRF2, nuclear respiratory factor 2; TFAM, mitochondrial transcription factor A; PGC1α, PPARγ coactivator-1α; GCK, glucokinase; CS, citrate synthase; NDUFA8, NADH dehydrogenase 1α subcomplex, 8; NDUFA13, NADH dehydrogenase 1α subcomplex, 13; NDUFC2, NADH dehydrogenase 1 subcomplex unknown, 2; SDHA, succinate dehydrogenase complex, subunit A; UQCRB, ubiquinol cytochrome c reductase binding protein; Cox IV, cytochrome c oxidase subunit IV; ATP5G1, ATP synthase, H+ transporting, mitochondrial F0 complex, subunit C1; ATP5B, ATP synthase, H+ transporting, mitochondrial F1 complex, β polypeptide; ![]() , NBW-SO;
, NBW-SO; ![]() , NBW-MCT;
, NBW-MCT; ![]() , IUGR-SO;
, IUGR-SO; ![]() , IUGR-MCT.
, IUGR-MCT.
Statistical analysis
Two-way ANOVA was used to determine the main effects (BW and Diet) and their interaction using the general linear model procedure of SPSS software (version 16.0; SPSS Inc.). When P values of interaction of main effects were <0·05, differences among the treatments were examined by one-way ANOVA using Duncan’s multiple range test, which were considered significant at P<0·05, and P values between 0·05 and 0·10 were considered a trend. Data are presented as means with their pooled standard errors.
Results
Growth performance
Both decreased (P<0·05) initial and final body weights were observed for IUGR piglets compared with NBW piglets (Table 2). The MCT diet tended to partially increase (P=0·063) the final body weight of weaned piglets.
Table 2 Effects of soyabean oil (SO) and medium-chain TAG (MCT) on growth performance in intra-uterine growth-retarded (IUGR) and normal-birth-weight (NBW) pigletsFootnote * (Mean values with their standard errors, n 4)
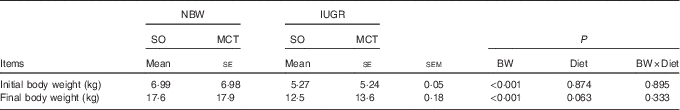
BW, birth weight.
* Piglets were fed a SO or MCT diet for 28 d and were sampled at an age of 49 d.
Plasma hormone concentrations
The concentrations of plasma hormones for the IUGR piglets were relatively similar to the NBW piglets (P>0·10; Table 3). However, increased (P<0·05) contents of circulating ghrelin were observed in piglets fed an MCT diet compared with those fed an SO diet. A tendency towards an increased (P=0·066) concentration of plasma adiponectin was observed in the piglets fed an MCT diet. In addition, the BW and Diet had significant interaction effects on plasma T4 concentration (P<0·05); MCT diet ameliorated the decreased plasma T4 concentrations in the IUGR piglets rather than in NBW piglets.
Table 3 Effects of soyabean oil (SO) and medium-chain TAG (MCT) on plasma hormone concentrations in intra-uterine growth-retarded (IUGR) and normal-birth-weight (NBW) pigletsFootnote * (Mean values with their standard errors, n 4)
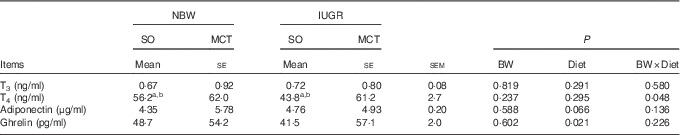
BW, birth weight; T3, 3,3',5-triiodo-l-thyronine; T4, 3,3',5,5'-tetraiodo-l-thyronine.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Piglets were fed an SO or MCT diet for 28 d and were sampled at an age of 49 d.
Hepatic metabolic status
IUGR induced an obvious reduction (P<0·05) in ATP contents in the liver of piglets compared with NBW (Table 4). IUGR piglets had decreased activity of hepatic mitochondrial CS (P<0·05) than that in NBW piglets. A similar trend (P=0·061) was observed for mitochondrial SDH activity in the liver of IUGR piglets. Feeding an MCT diet to piglets increased (P<0·05) hepatic mitochondrial CS and SDH activities, but decreased (P<0·05) hepatic PK activity. We found a trend to increased (P=0·095) contents of hepatic ATP in the piglets given an MCT diet. Moreover, there is no interaction between BW and Diet on these parameters (P>0·10).
Table 4 Effects of soyabean oil (SO) and medium-chain TAG (MCT) on hepatic metabolic status in intra-uterine growth-retarded (IUGR) and normal-birth-weight (NBW) pigletsFootnote * (Mean values with their standard errors, n 4)
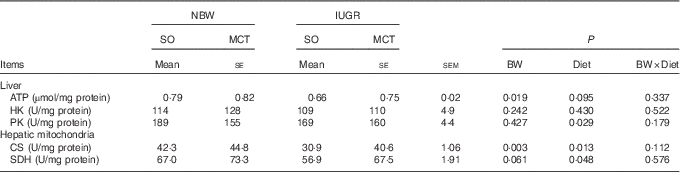
BW, birth weight; HK, hexokinase; PK, pyruvate kinase; CS, citrate synthase; SDH, succinate dehydrogenase.
* Piglets were fed an SO or MCT diet for 28 d and were sampled at an age of 49 d.
Mitochondrial VO2
The oxidation rates of succinate in the IUGR piglets were significantly lower (P<0·05) than those in NBW piglets (Table 5). Rates of ADP-stimulated VO2 (i.e. state 3 respiratory) were increased (P<0·05) for succinate in the piglets fed an MCT diet and for malate and glutamine. Treatment with MCT tended to partially recover (P=0·095) the compromised RCR induced by IUGR, when succinate was used as an oxidative substrate. However, there were no significant differences in state 4 respiratory and RCR among the groups when malate and glutamine were added into the medium (P>0·10).
Table 5 Effects of soyabean oil (SO) and medium-chain TAG (MCT) on mitochondrial VO2 in intra-uterine growth-retarded (IUGR) and normal-birth-weight (NBW) pigletsFootnote * (Mean values with their standard errors, n 4)

BW, birth weight; RCR, respiratory control ratio.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Piglets were fed an SO or MCT diet for 28 d and were sampled at an age of 49 d.
Hepatic mitochondrial DNA content
As shown in Fig. 1, hepatic mtDNA contents were decreased (P<0·05) by IUGR compared with the NBW. Dietary inclusion of MCT increased (P<0·05) the contents of mtDNA in the liver of piglets. However, the BW and Diet had no significant interaction effects on the parameter (P>0·10).
Gene expression
The mRNA expressions of genes related to mitochondrial biogenesis and energy metabolism in the liver of piglets are presented in Fig. 2. Compared with NBW, IUGR down-regulated (P<0·05) the gene expressions of NRF2 and PPARα in the liver of piglets. A similar trend exerted by IUGR was also observed for mRNA abundances of the hepatic TFAM (P=0·059) and PGC1α (P=0·092). Compared with NBW, IUGR resulted in decreased (P<0·05) expression levels of NDUFA8 and SDHA in the liver of piglets. MCT-fed piglets showed increases (P<0·05) in mRNA expressions of the hepatic PGC1α, SDHA and ATP5B, when compared with their SO-fed counterparts. A tendency for increased (P=0·097) expression of hepatic TFAM was seen in the piglets fed an MCT diet. In addition, treatment with MCT alleviated (P<0·05) the decrease in hepatic PPARα expression induced by the IUGR. Moreover, there were no significant differences in mRNA expressions of GCK, CS, NDUFA13, NDUFC2, UQCRB, Cox IV and ATP5G1 (P>0·10).
Discussion
In the present study, MCT treatment improved energy metabolism and mitochondrial biogenesis in the liver of weanling piglets. MCT like carbohydrates are hydrolysed quickly and usually completely, and then they are absorbed rapidly as medium-chain fatty acids (MCFA) travel directly to the liver via the portal vein, bypassing the lymphatic system, and are burned for energy( Reference Zentek, Buchheit-Renko and Ferrara 10 , Reference Bach and Babayan 24 , Reference Johnson and Cotter 25 ). Our previous study showed that feeding of MCT to piglets significantly improved average daily gain (NBW-MCT: 391 (se 14) g/d, IUGR-MCT: 299 (se 8) g/d; NBW-SO: 377 (se 11) g/d, IUGR-SO: 259 (se 14) g/d) and feed efficiency (NBW-MCT: 0·67 (se 0·01) g/g, IUGR-MCT: 0·67 (se 0·01) g/g; NBW-SO: 0·65 (se 0·02) g/g, IUGR-SO: 0·62 (se 0·02) g/g) but did not affect feed intake (NBW-MCT: 586 (se 25) g/d, IUGR-MCT: 450 (se 13) g/d; NBW-SO: 582 (se 19) g/d, IUGR-SO: 419 (se 10) g/d) when compared with the SO group( Reference Zhang, Chen and Li 15 ). Therefore, the beneficial effects that MCT have on growth performance of weanling piglets were most likely to result from the increased efficiency of energy metabolism, which might be associated with the shorter carbon-chain length of MCFA.
In the present study, a lower concentration of ATP was observed in the liver of IUGR piglets. Similar results were obtained by Ogata et al. ( Reference Ogata, Swanson and Collins 26 ), who reported that IUGR decreased ATP:ADP and adenylate charge ratios in the liver, which resulted from the perturbation of gaseous exchange and glucose availability. The reduction in ATP contents indicated that the processes of energy metabolism may be impaired. Cianfarani et al. ( Reference Cianfarani, Agostoni and Bedogni 27 ) documented that IUGR down-regulated mRNA expressions of glycerol-3-phosphate dehydrogenase 1 and PK in rats. The defects in the expression abundances of GCK and CS were also observed in the liver of weanling IUGR piglets( Reference Liu, Yao and Yu 22 ), which determine the rate of the glycolysis and the tricarboxylic acid cycle, respectively. Similarly, our finding of a decreased CS activity in hepatic mitochondria of the IUGR piglets may be a contributing factor to the lower efficiency of energy metabolism. Moreover, IUGR-induced decrease in SDH was observed to occur at both the activity and the transcriptional levels. A possible explanation for this result has been given by Selak et al.( Reference Selak, Storey and Peterside 28 ). They found that IUGR inhibited the activity of pyruvate dehydrogenase, which could subsequently lead to secondary decrease in SDH activity. SDH is a reliable marker of the mitochondrial capability to produce ATP, as it is part of both the tricarboxylic acid cycle and the respiratory chain (complex II). The activity of SDH may be suppressed by the accumulation of oxaloacetate in IUGR offspring, because acetyl-CoA from the pyruvate oxidation is inadequate to promote the rapid removal of oxaloacetate catalysed by CS( Reference Williamson and Cooper 29 ). The reasons behind energy deficiency induced by IUGR are complicated, and precise details of the underlying mechanisms are not fully clear, but at least two possibilities exist. First, the fetus adapts to limited energy environment by modifying mRNA expression of the genes coding for key enzyme involved in the glycolysis and the tricarboxylic acid cycle, and thereby diverts the limited substrate supply to favour the survival of vital organs such as brain. The similar effects that IUGR exerted in hepatic energy metabolism were observed for weaned piglets( Reference Liu, Yao and Yu 22 ). Second, IUGR impairs the digestive function of fully weaned piglets( Reference Michiels, De Vos and Missotten 30 ), which decreases the availability of metabolic substrate that may lead to a reduction in enzyme activity.
Ooyama et al.( Reference Ooyama, Kojima and Aoyama 31 ) reported that rats after MCT ingestion display significantly increased ATP contents in the liver than that of the LCT group. Studies conducted by Balietti et al. ( Reference Balietti, Fattoretti and Giorgetti 32 , Reference Balietti, Giorgetti and Di Stefano 33 ) also indicated that the MCT diet was capable of counteracting the age-related decrease of SDH activity and preventing the reduction in the mitochondrial area involved in energy supply in the late-adult rats. In the present study, feeding an MCT diet recovered IUGR-induced decrease in the activity of the hepatic CS. MCT-fed piglets showed a significantly increased activity of the hepatic SDH compared with their SO-fed counterparts, which was observed alongside up-regulated expression of the hepatic SDHA. Furthermore, the inhibition of SDH induced by oxaloacetate accumulation may be removed with increased levels of the hepatic acetyl-CoA from the MCFA oxidation. It is interesting to note that IUGR led to the offspring preference for fats as energy sources by increasing expression levels of the genes related to intracellular trafficking oxidation of fatty acids( Reference Morris, Vickers and Gluckman 34 ). Thus, feeding the IUGR offspring an MCT-rich diet may be an effective approach to recovering IUGR-induced deficiency in metabolic substrate.
An observation of this study was the impaired OXPHOS system in the IUGR piglets, which likely resulted from the dysfunction of electron respiratory chain. Compared with NBW piglets, the maximal respiration rates of hepatic mitochondria in the IUGR piglets decreased obviously when succinate (complex II-dependent) was used as an oxidative substrate, indicating a block to the electron flow in the respiratory chain at the enzymatic level downstream of complex II, which might have a certain relationship with the reduction in hepatic SDH activity. Furthermore, the disturbances of the OXPHOS system can be caused by numerous genetic defects. The array analysis conducted by Morris et al.( Reference Morris, Vickers and Gluckman 34 ) described a number of decreases in the transcriptions of NADH dehydrogenase subunits of complex I in the liver of IUGR rats, including NDUFC2, NDUFB6 and NDUFA8. Similarly, ATP synthase, H+ transporting, mitochondrial F1 complex, O subunit was down-regulated by IUGR, which generates ATP from the electron gradient( Reference Morris, Vickers and Gluckman 34 ). Liu et al. ( Reference Liu, Yao and Yu 22 ) found obviously decreased expressions of Cox IV in the liver of IUGR piglets, and mRNA abundance of the ATP synthase was reduced by a similar amount.
Turner et al. ( Reference Turner, Hariharan and TidAng 35 ) found that MCFA-fed mice displayed a more potent stimulation of mitochondrial biogenic pathways in the muscle and a significant increase in the expression of subunits from complex II and complex IV in the liver, when compared with their long-chain fatty acid-fed counterparts. The present study indicated that MCT partially counteracted IUGR-induced decrease in the RCR when succinate (Complex II-dependent) was added into the medium, which appeared to result from a higher VO2 in state 3 respiration in hepatic mitochondria after MCT treatment. The enhanced flux of substrates through oxidative metabolism may offer a major contribution to the MCT-induced changes in the OXPHOS system and energy metabolism. In addition, MCT increased mRNA abundance of the hepatic ATP5B, which might be responsible for the increased ATP concentration in the MCT-fed piglets. The coupling step in mitochondrial ATP synthesis is generally known to be an energy-requiring release of ATP bound to ATP5B. Molecular descriptions by mutational analysis on ATP-binding sites in ATP5B have revealed its critical role in the energy metabolism process, and ATP5B is usually considered a significant parameter of ATP synthesis( Reference Thomas, Garboczi and Pedersen 36 ).
In keeping with previous studies( Reference Park, Kim and Kim 9 , Reference Theys, Bouckenooghe and Ahn 37 ), our results indicated that IUGR piglets had lower contents of hepatic mtDNA than that in NBW piglets, which was consistent with the reduced influence of multiple transcriptional factors involved in mitochondrial biogenesis. Studies regarding the regulation of mitochondrial number and function have drawn increasing attention on the nuclear transcription factors, such as NRF1 and NRF2( Reference Huss and Kelly 38 , Reference Ekstrand, Falkenberg and Rantanen 39 ). In this study, IUGR piglets exhibited a down-regulated mRNA expression of hepatic NRF2, which governs several mitochondrial genes involved in OXPHOS, mitochondrial protein import and mitochondrial translation( Reference Huss and Kelly 38 ). A concomitant reduction in TFAM expression was observed in the liver of IUGR piglets. TFAM is critical to initiate mitochondrial biogenesis and has an additional role in packaging of mtDNA( Reference Ekstrand, Falkenberg and Rantanen 39 ). A similar result was obtained by Liu et al. ( Reference Liu, Yao and Yu 22 ), who reported a decrease in hepatic TFAM at the transcriptional level in the IUGR piglets. In addition, the lack of hepatic PPARα expression seen in the IUGR piglets might provide a major contribution to the poor capacity of fatty acid oxidation, which affects intracellular lipid and carbohydrate metabolism through direct transcriptional control of the genes related to fatty acid uptake and mitochondrial β-oxidation pathways( Reference Lefebvre, Chinetti and Fruchart 40 ). Similarly, Magee et al. ( Reference Magee, Han and Cherian 41 ) found that IUGR resulted in a decreased protein expression of hepatic PPARα that persisted until adult life.
The above-mentioned pathways regulating expression of the nuclear-encoded mitochondrial factors will converge in PGC1α ( Reference Cannino, Di Liegro and Rinaldi 42 ), which is preferentially expressed in the tissues with a higher energy demand and serves as a master regulator of mitochondrial biogenesis( Reference Kim, Fillmore and Chen 43 ). In fact, there is a simultaneously decreased expression of hepatic PGC1α in the IUGR piglets, suggesting a key mechanism involved in IUGR-induced energy deficiency. This finding is in accordance with a previous study by Liu et al. ( Reference Liu, Yao and Yu 22 ). In addition, Pinney et al. ( Reference Pinney, Han and Simmons 44 ) demonstrated that PGC1α gene expression was decreased in the liver of IUGR adult rats, resulting from silencing histone modifications at the proximal promoter of PGC1α. Thus, IUGR may cause an eventual inhibition in hepatic PGC1α expression that leads to a primary defect in mitochondrial function.
In this trial, MCT alleviated the decrease in the hepatic PPARα expression induced by IUGR. Xu et al. ( Reference Xu, Lambert and Montana 45 ) reported that MCFA have low binding affinity for PPAR. Nevertheless, PPARα could also be activated by interaction with the adiponectin( Reference Okamoto, Kihara and Funahshi 46 ), which was increased in the plasma of MCT-fed piglets. Higher levels of plasma and adipocyte adiponectin, as well as its mRNA expression in perirenal adipose tissue, have also been documented in the MCT-treated rats( Reference Takeuchi, Noguchi and Sekine 47 ). Furthermore, the up-regulated PGC1α expression in the liver of MCT-fed piglets might contribute to the increased expression of hepatic PPARα, as PGC1α lies upstream from PPARα. The observations indicated that a possible mechanism related to the improved energy metabolism may involve activation of the PGC1α/PPARα complex at the transcriptional level, which is associated with an increased hepatic reliance on fatty acids as the primary energy substrate( Reference Rodgers, Lerin and Haas 48 ). The up-regulated expression of hepatic PGC1α in the MCT-fed piglets may be explained, in part, by the increased concentrations of total ghrelin in the plasma. Nishi et al. ( Reference Nishi, Hiejima and Hosoda 49 ) found that ingested MCFA or MCT could serve as a source of fatty acids in the acyl modification of ghrelin, which is important in the regulation of gherlin production. The role that ghrelin had in increasing PGC1α expression has been described in previous papers( Reference Barazzoni, Zanetti and Cattin 50 , Reference Bayliss and Andrews 51 ). At the same time, treatment with MCT counteracted IUGR-induced decreased T4 concentrations in the plasma of piglets, which plays a positive role in activating PGC1α expression because the PGC1α gene carries thyroid response elements in the gene promoter( Reference Weitzel and Iwen 52 ).
A depressed activity of hepatic PK seen in the present study suggested a reduction in the metabolic efficiency of the glycolysis, which might be dependent on substrate availability. We have previously shown that feeding an MCT diet to piglets increased the activity of the hepatic glucose-6-phosphate dehydrogenase, the first and rate-limiting enzyme of PPP( Reference Zhang, Chen and Li 15 ). Therefore, the results obtained herein, in conjunction with the known effects of PGC1α/PPARα complex on energy substrate switch, may explain the mechanisms behind the enhanced channelling of glucose metabolites through the PPP in the MCT-fed piglets. This diversion serves to generate the reducing potential in the form of NADPH that is essential for restoring the redox balance of the cell. In addition, the MCT diet alleviated IUGR-induced decreased activity of hepatic SDH. Considering its unique redox properties, SDH offers a key contribution to the redox balance of the ubiquinone pool that is an important antioxidant reservoir in the inner membrane of mitochondria and exerts a major role in the control of O2 toxicity( Reference Rustin, Munnich and Rotig 53 ). A previous study also found that SDH deterioration increased reactive oxygen species generation and activated apoptotic process( Reference Ishii, Ishii and Hartman 54 ). Thus, the study may further support the findings obtained in our previous work that the MCT diet has auxiliary therapeutic potential to attenuate hepatic oxidative damage in the IUGR piglets( Reference Zhang, Chen and Li 15 ).
Moreover, the beneficial effect that MCT exerted on piglets’ growth depends on the intake dose of MCT and the age of piglets. Lepine et al. ( Reference Lepine, Boyd and Welch 55 ) reported that newborn piglets received 15 ml of MCT at 6 and 16 h after birth had no significant positive effects on their survival possibly due to an excessive increase in blood ketone body by even-MCFA (newborn piglets have low hepatic β-oxidation capacity) that caused metabolic acidosis, narcotic effects, vomiting, lethargy and death. Casellas et al. ( Reference Casellas, Casas and Piedrafita 56 ) demonstrated that small piglets (BW <1·25 kg) received a reduced dose of MCT (2 g/24 h) during the first 3 d of life and exhibited an increased survivability compared with the control group. The dose used in the present study was slightly higher than that in the study by Yen et al. ( Reference Yen, Lai and Lin 57 ) who reported that dietary supplementation with 3 % MCT improved the gain:feed ratio of weanling piglets during the first 2 weeks post weaning, but it was far less than the recommended dose for the newborn piglets (6 g/body weight (kg)0·75) provided by Chiang et al. ( Reference Chiang, Pettigrew and Clarke 58 ).
In conclusion, our results indicate a potential benefit for early nutritional intervention with MCT to improve hepatic energy metabolism and mitochondrial biogenesis for weanling piglets. The study also broadens our understanding regarding the alterations in hepatic oxidative status of the MCT-treated piglets.
Acknowledgements
The authors are grateful to the colleagues of free radical regulation research centre of Fudan University for their dedicated and skillful technical support.
This study was supported by the National Basic Research Program of China (973) (no. 2012CB124703) and the National Natural Science Foundation of China (no. 31272454). The funders had no role in the design, analysis or writing of this article.
The contributions of the authors are as follows: H. Z. and T. W. designed the study. H. Z. and Y. L. participated in the animal experiment. H. Z., Y. L., X. H. and L. Z. conducted the research. H. Z. and Y. L. analysed the data. H. Z. discussed the results and wrote the paper. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516000404










