INTRODUCTION
Acute flaccid paralysis (AFP) surveillance forms the basis for detection of poliovirus cases globally. In the Global Polio Eradication Initiative (GPEI), AFP is defined as rapid progression of weakness with loss of voluntary movement and loss of muscle tone in any part of the body in a patient aged <15 years; or paralysis in a person of any age in whom polio is suspected [1]. AFP surveillance was started in India in 1997, and includes case investigations of all persons with suspected AFP, with collection of two stool specimens for poliovirus isolation [2]. In 2004–2005, in an effort to increase the sensitivity of the AFP surveillance system, the AFP case definition was broadened to include transient weakness and facial paralysis. To further enhance sensitivity, the number of AFP case-reporting sites nationally increased from 21 403 in 2004 to 36 629 in 2012 in an effort to include health facilities serving migrant and high-risk populations [2]. As a likely consequence of changes to increase sensitivity, the non-polio AFP rate in India increased from 2·0 to 13·5 cases/100 000 population aged <15 years from 2000 to 2011, and is substantially higher than the World Health Organization (WHO) target rate of at least two non-polio AFP cases/100 000 population aged <15 years [2, 3]. However, increased reporting of AFP cases requires higher resources for both case investigations and laboratory testing [Reference Pinheiro4, 5]. While AFP surveillance accounted for <10% of the overall cost of the polio eradication programme in India in 2011 [6], AFP surveillance costs are not expected to decrease, even as India was removed from the WHO list of polio-endemic countries in January 2011, because of the need for a continued strong surveillance system [7].
Collection of two stool specimens is recommended by WHO because poliovirus is shed intermittently in stool, so testing only one sample could potentially miss an opportunity to detect the virus [Reference Lennette8]. Nonetheless, collecting one stool specimen instead of two has been proposed as one way to reduce the case investigation and laboratory testing burden, and cost of the AFP surveillance system in India and other countries. In November 2010, the India Expert Advisory Group considered the role of the second stool sample for polio diagnosis in the context of the decline in reported polio cases from 266 in 2000 to 42 in 2010 [9]. Our study aimed to assess: (1) changes in stool collection and processing performance indicators, (2) the sensitivity of stool specimens in polio diagnosis, and (3) the number of polio cases identified only by the second specimen for AFP cases reported during 2000–2010. Using these results, we provide recommendations for maintaining high quality, sensitive AFP surveillance while taking into account limited programmatic resources.
METHODS
We restricted the analysis to AFP cases in children aged <15 years reported to the National Polio Surveillance Project – India from 2000 to 2010. Stool specimens were tested at one of eight national laboratories in the country using cell culture on two poliovirus-sensitive cell lines according to WHO standards, followed by antigenic and/or molecular characterization of isolates [10]. The first and second specimens from each individual were analysed by the same methodology. Stool collection and processing performance indicators were assessed using WHO criteria [1]. Two stool specimens should be collected from each AFP case-patient within 14 days of paralysis onset and at least 24 h apart; each specimen must be of adequate volume (8–10 g), and arrive at a WHO-accredited laboratory in good condition (i.e. no desiccation, no leakage, with adequate documentation and evidence that the cold chain was maintained) [1]. Polio cases were considered to be confirmed if wild poliovirus (WPV) type 1, WPV type 3, or vaccine-derived poliovirus (VDPV) was isolated in either stool specimen.
Polio cases with two stool results reported were further analysed to estimate single specimen, combined specimen, and added sensitivity. Methods for calculating the specimen sensitivity of each individual stool, the combined sensitivity of both first and second stool specimens (i.e. person sensitivity), and the added sensitivity of the second specimen have been described previously [Reference Gary, Sanders and Pallansch11] (Fig. 1). In brief, this methodology derives maximum-likelihood estimates for the specimen sensitivities for each stool, and the approximate variances of the estimators. Each stool sample was considered to be independent of the other; therefore, each stool sample serves as the gold standard estimate for the other specimen.
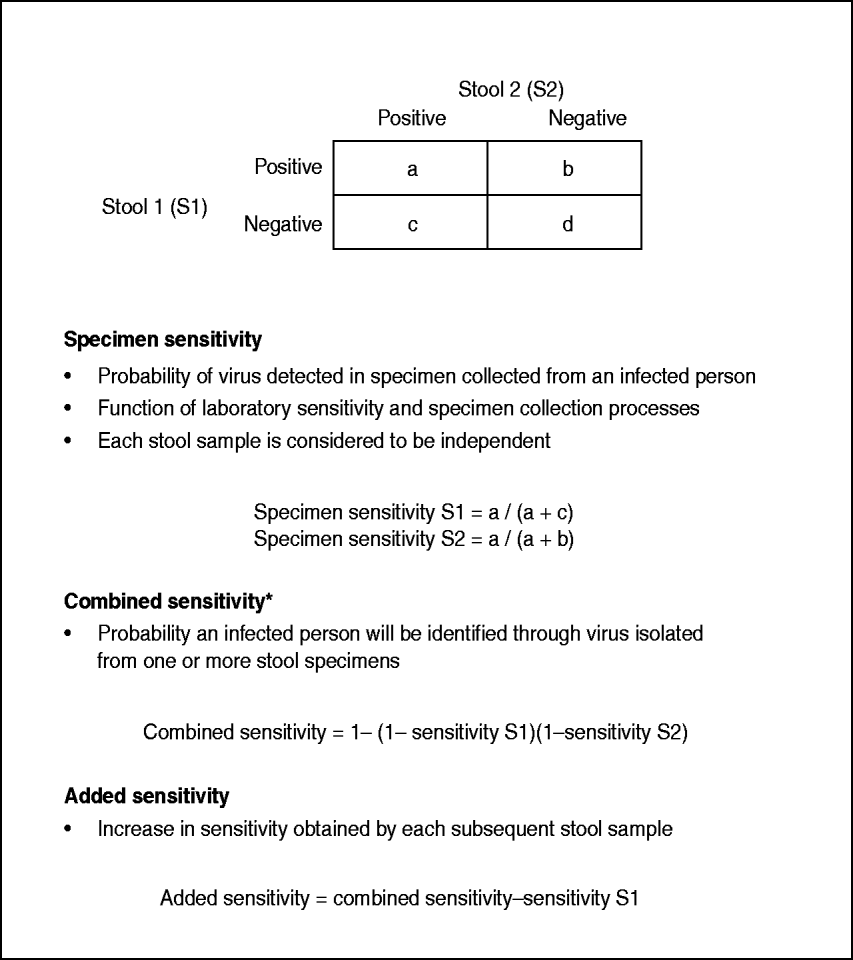
Fig. 1. Sensitivity definitions and calculations (Gary et al. [Reference Gary, Sanders and Pallansch11]). * Also known as person sensitivity.
Sensitivity estimates for confirmed polio cases were calculated for the entire period during 2000–2010 and by various factors, including stool adequacy, stool condition on arrival at the laboratory, and time interval of stool collection from onset of paralysis, patient age, and year. The Wald χ 2 test was used to test for equality of mean specimen sensitivities across categories. Results were divided into three time categories: 2000–2004 (before the AFP case definition change), 2005–2009 (after the AFP case definition change), and 2010 (a year of particularly low reported polio cases). The number of polio cases identified only by the second stool specimen was determined for the overall period 2000–2010, and by the same factors as in the sensitivity analyses. The Cochran–Armitage trend test was used to test for trends in proportions across ordinal categories. Data were analysed in SAS v. 9.2 (SAS Institute Inc., USA) and R v. 2.12.1 (R Foundation, Austria).
RESULTS
AFP cases, laboratory samples processed and stool surveillance indicators
From 2000 to 2010, 303984 children aged <15 years with AFP were identified. Of these, 290 763 (95·7%) had two stool samples with poliovirus isolation results reported, 2537 (0·8%) were missing one poliovirus isolation result, and 10 684 (3·5%) were missing both poliovirus isolation results. The median age of children with AFP was 37 months (interquartile range 22–71), 207 774 (68%) AFP cases were children aged <5 years, and 108998 (41%) were females.
During 2000–2010, reported AFP cases increased from 8095 to 55616 and stool specimens processed by the laboratory increased from 15 761 to 108 207 (Fig. 2). Confirmed polio cases fluctuated during 2000–2010, with peaks in 2002 (1603 cases), 2007 (877 cases), and 2009 (763 cases), and troughs in 2005 (66 cases) and 2010 (47 cases) (Fig. 2).
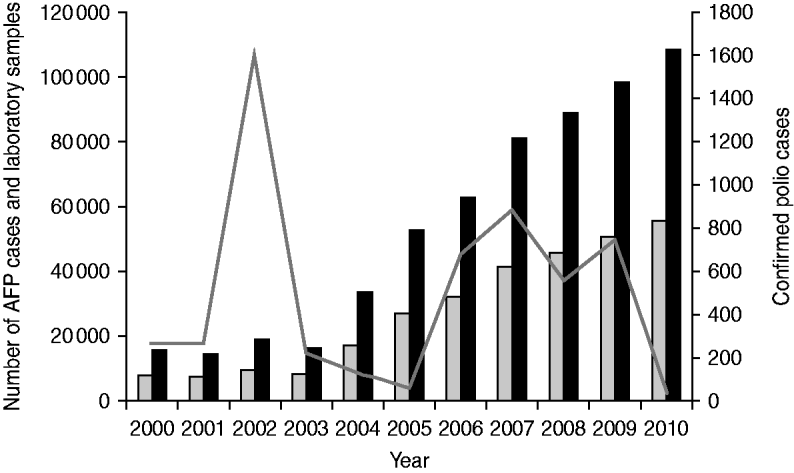
Fig. 2. Acute flaccid paralysis (AFP) cases reported, confirmed polio cases reported, and stool specimens processed, India, 2000–2010. Confirmed polio cases include wild poliovirus (WPV) type 1, WPV type 3, and vaccine-derived poliovirus. ![]() , AFP cases; ■, laboratory samples processed; –––, confirmed polio cases.
, AFP cases; ■, laboratory samples processed; –––, confirmed polio cases.
Indicators of stool adequacy, timeliness of specimen collection, condition on arrival at the laboratory, and time interval from collection of first and second stool specimens were above the WHO target level of 80% for the entire period of 2000–2010 (Table 1), with little fluctuation from year to year.
Table 1. Indicators of stool specimen adequacy, timeliness, condition, and collection for acute flaccid paralysis (AFP) cases reported, India, 2000–2010*
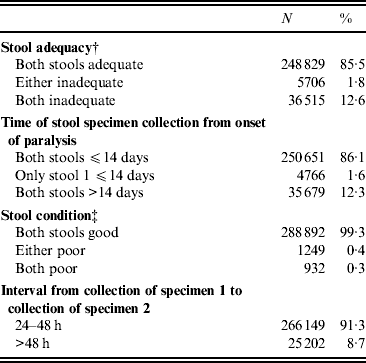
* AFP cases with missing data excluded from analysis; for all categories, <5% of all AFP cases in the database.
† Stool adequacy is defined as two stool specimens collected from each AFP case within 14 days of paralysis onset and at least 24 h apart; each specimen must be of adequate volume (8–10 g), and arrive at a WHO-accredited laboratory in good condition.
‡ A stool specimen in good condition is defined as a specimen that arrives in the laboratory with neither desiccation nor leakage, with adequate documentation and evidence that the cold chain was maintained.
The mean interval from onset of paralysis to arrival of the second stool specimen at the laboratory was 14·7 days during 2000–2004, 13·0 days during 2005–2009, and 12·2 days during 2010 (Fig. 3). The mean time interval from onset of paralysis to collection of the first stool sample accounted for the largest percentage of the total time interval in all categories, and decreased by 14% from 9·2 days during 2000–2004 to 7·9 days during 2010. The mean time interval from collection of the second stool specimen to its being sent to the laboratory decreased by 72% from 3·2 days during 2000–2004 to 1·2 days during 2010.
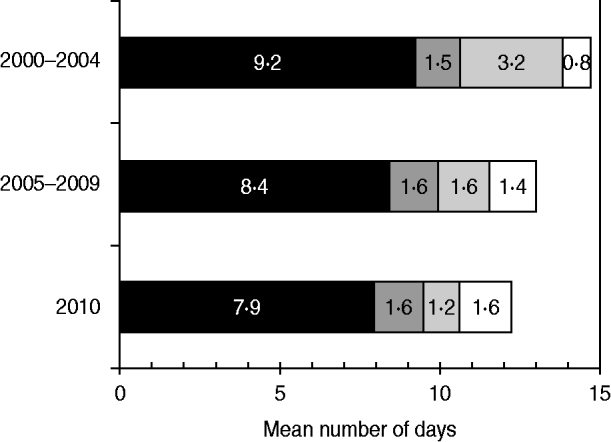
Fig. 3. Mean time interval from onset of paralysis and arrival of second specimen in laboratories, by components, India, 2000–2010. ■, Time from paralysis to collection of first stool sample (S1); ![]() , time from S1 collection to collection of second stool sample (S2);
, time from S1 collection to collection of second stool sample (S2); ![]() , time from S2 collection to sending S2 to the laboratory; □, time from sending S2 to arrival at the laboratory.
, time from S2 collection to sending S2 to the laboratory; □, time from sending S2 to arrival at the laboratory.
Stool specimen sensitivity, combined sensitivity, and added sensitivity
Estimates of stool specimen sensitivity were calculated for the 5184 polio-confirmed cases with WPV type 1, WPV type 3, or VDPV isolated from at least one stool specimen (Table 2).
Table 2. Non-polio acute flaccid paralysis (AFP) and polio-confirmed cases analysed for sensitivity, by outcome of testing
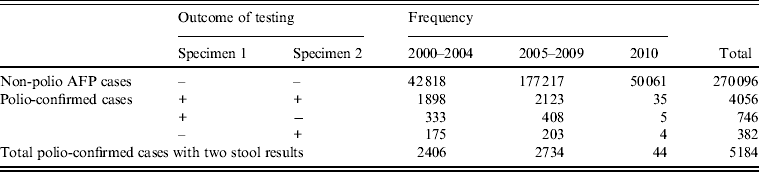
+, Indicates isolation of wild poliovirus (WPV) type 1, WPV type 3 or vaccine-derived poliovirus; –, indicates negative for all polioviruses
During 2000–2010, the specimen sensitivity of the first stool (91·4%) was significantly higher than the specimen sensitivity of the second stool (84·5%) (P < 0·0001), combined sensitivity was 98·7%, and the added sensitivity of the second stool specimen was 7·3% (Table 3). There were no differences in sensitivity estimates by time period category (P = 0·960). Specimen sensitivity was significantly higher when both stool specimens were adequate than when either of the specimens were not adequate (P = 0·037). Of 350 specimens where both specimens were not adequate, 316 (90·3%) were inadequate due to poor timeliness (both stools not collected within 14 days after the onset of paralysis) and 30 (8·6%) were inadequate due to poor condition at the time of arrival at the laboratory.
Table 3. Sensitivity of first and second poliovirus specimens from children with acute flaccid paralysis (AFP) aged <15 years with two specimen results, India, 2000–2010
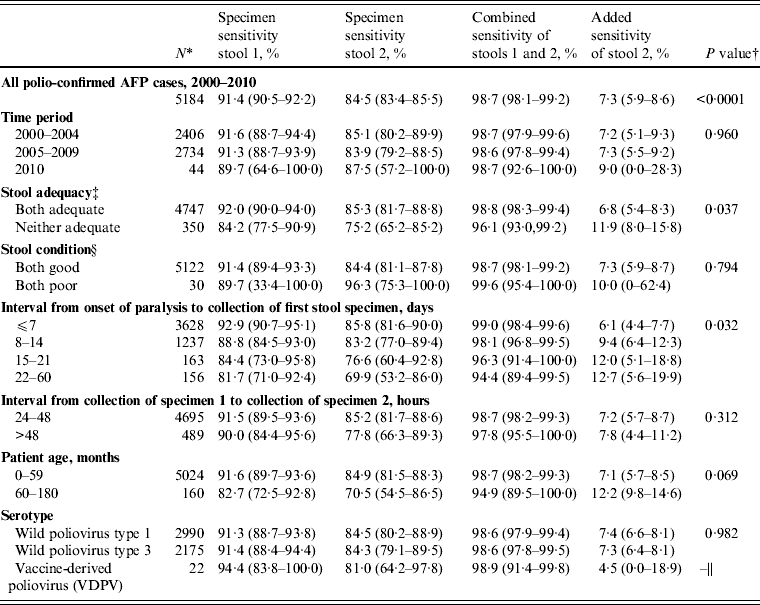
* The category denominators for stool adequacy and stool condition do not add up to the overall denominator (n = 5184) due to exclusion of certain subcategories due to small sample size (one stool adequate, n = 87; one stool in good condition, n = 32). Because serotypes are not mutually exclusive [of 5184 polio-confirmed cases nine were dually infected with wild poliovirus (WPV) types 1 and 3], it was necessary to treat WPV type 1 and type 3 cases independently for sensitivity analyses; this analysis assumes that one serotype does not influence the behaviour of the other in dually infected persons.
† P value represents the Wald χ 2 test for equality of mean specimen sensitivities across categories. For the interval from onset of paralysis to collection of first stool specimen, the P value represents the linear trend for mean specimen sensitivity across time collection intervals.
‡ Stool adequacy is defined as two stool specimens collected from each AFP case within 14 days of paralysis onset and at least 24 h apart; each specimen must be of adequate volume (8–10 g), and arrive at a WHO-accredited laboratory in good condition.
§ A stool specimen in good condition is defined as a specimen that arrives at the laboratory with neither desiccation nor leakage, with adequate documentation and evidence that the cold chain was maintained.
|| VDPV represents a separate category that is not directly comparable to other serotypes, so no statistical test was applied.
Of the factors analysed affecting adequacy (stool condition, interval from onset of paralysis to collection of stool specimen 1, interval from collection of stool specimen 1 to collection of stool specimen 2), only interval from onset of paralysis to collection of stool specimen 1 significantly affected sensitivity (P = 0·03) (Table 3). Specimens collected in the first week after the onset of paralysis had the highest specimen sensitivity (stool 1, 92·9%; stool 2, 85·8%) and combined sensitivity (99·0%), and lowest added sensitivity (6·1%) compared to later weeks. When categorized by the WHO timeliness guideline, specimens collected ⩽14 days after the onset of paralysis had significantly higher specimen sensitivity (stool 1, 91·9%; stool 2, 85·4%) than specimens collected after the first 14 days (stool 1, 82·8%; stool 2, 72·9%; P = 0·025). Specimens collected ⩽7 days after the onset of paralysis also had higher specimen and combined sensitivity, and lower added sensitivity, than those collected >7 days, although this difference was not statistically significant (P = 0·071).
Of children aged 60–180 months, specimen sensitivity was lower and added sensitivity was higher compared to specimen sensitivity and added sensitivity in children aged 0–59 months; however, the difference in mean specimen sensitivity between age groups was not statistically significant (P = 0·069). A higher percentage of stools was collected after 14 days from children aged 60–180 months (21/160, 13%) than from children aged 0–59 months (298/5024, 6%).
Cases identified by second stool specimen only
Of 5184 confirmed polio cases identified from 2000 to 2010, 382 (7·4%) were identified only by the second stool sample (Table 4). The percentage of cases identified only by the second stool sample was higher if both stools were inadequate (P = 0·0001), as the stool collection interval increased (P < 0·0001), and as patient age in months increased (P = 0·0004). Of the 382 confirmed polio cases identified by the second stool specimen only, 38 (10%) were from 33 districts and nine states that had previously been polio-free for at least 6 months (i.e. not geographically related to an ongoing outbreak).
Table 4. Percentage of polio-confirmed acute flaccid paralysis cases identified only by the second stool specimen, India, 2000–2010
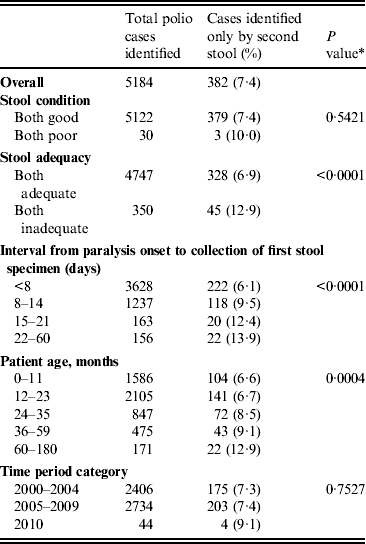
* P value represents trend test for proportions across ordinal categories.
DISCUSSION
The second stool specimen contributed an additional 7·3% sensitivity overall, resulting in a combined sensitivity of 98·7% for both specimens. Both the first and second specimens were most sensitive if collected in the first week after the onset of paralysis. The second specimen detected an additional 382 polio cases that would not otherwise have been identified, which represents 7·2% of all polio-confirmed cases reported during 2000–2010; 38 (10%) were not geographically related to an ongoing outbreak. These results highlight the substantial contribution of the second stool specimen in India in identifying polio cases.
The first stool specimen had higher specimen sensitivity (91·4%) than the second stool specimen (84·5%). Reasons for lower specimen sensitivity for the second specimen are unclear, but could be related to the later time of second stool collection after illness onset [Reference Gary, Sanders and Pallansch11, Reference Alexander, Gary and Pallansch12], viral excretion patterns, or to factors related to second stool specimen collection. Nonetheless, the second specimen increased the sensitivity of detection of confirmed polio cases as indicated by added sensitivity and the absolute number of cases identified by the second specimen only.
The lower specimen sensitivities when specimens were inadequate and in older children are due in part to the later collection time for some of these samples. This has greater implications currently as polio is historically a disease of young childhood; now, however, cases are increasingly occurring in older children and even in adults [13, 14]. As a result, healthcare providers might not recognize polio as the cause of AFP in older age groups as quickly as they do in younger children, which could lead to delayed stool collection times. Alternatively, older children who have partial immunity might have transient, asymptomatic infection with intermittent viral shedding resulting in discordant stool sample results; these discordant results would in turn decrease sensitivity.
The percentage of cases identified only by the second sample (7·2%) is similar to findings of previous studies in Latin America (8%) [Reference Pinheiro4] and from the USA (10%) [Reference Lennette8] but is lower than estimates reported previously from India (21%) [Reference Kohler15] and the Western Pacific region (31%) [Reference Yoneyama16]. The lower estimate in our analysis compared to a previous analysis of India's AFP surveillance system [Reference Kohler15] could be due to improvements in the collection, storage and transport of specimens, as well as improved laboratory performance. Indeed, by 2000, both specimen sensitivity and the percentage of cases identified by the second stool sample (12%) approached comparable levels to the current analysis from 2000 to 2010. The fact that we did not find an increase in specimen sensitivity after 2000 suggests there may be a point at which it is difficult to further increase specimen sensitivity despite improvements in field and laboratory performance.
The findings in this paper are subject to at least two limitations. First, the laboratories testing specimens changed during the analysis period and the prevalence of positive specimens going to specific laboratories also changed, making it difficult to assess laboratory-specific data over time. Second, for this analysis we must assume that isolation of poliovirus from one stool specimen is independent of the other. However, both samples are collected from the same AFP case-patient, often by the same health worker, and are usually transported together to the laboratory. Factors affecting specimen quality, including handling, packaging, and temperature, will affect both specimens equally, and may increase or decrease the laboratory's ability to isolate poliovirus. This lack of complete independence could result in overestimating or underestimating sensitivity. Nonetheless, the contribution of the second specimen is still marked, as this limitation does not affect the number of cases that would not have been detected without the second specimen.
India's AFP surveillance system is a ‘best-case’ scenario compared to other systems globally and caution should be exercised when generalizing the results to other areas with less highly functioning surveillance systems. On the other hand, some results from this study have important implications for other settings. It is a struggle to collect one stool specimen in some countries, and many specimens reach the laboratory in inadequate condition, or are collected too late after the onset of paralysis [17]. In these settings, specimen and combined sensitivity are likely to be lower because of later collection times, and the second stool sample would have greater impact on the sensitivity of AFP surveillance. But even in a well-functioning APF surveillance system, the second stool sample detected cases that otherwise would have been missed, and added sensitivity to detection.
In addition to decreasing the number of stool specimens collected, other options exist for potentially reducing the burden of collecting and testing on the surveillance system and the laboratory. Returning to the AFP case definition used in India prior to 2005, or maintaining environmental sampling at current capacity rather than expanding sites, could achieve this goal. However, these scenarios will necessarily decrease sensitivity, to some extent. In principle, efforts could be reduced in the laboratory by changing from cell culture-based detection of poliovirus to newer molecular methods. Despite advances in these methods, cell culture in RD cells remains more sensitive than real-time PCR methods for detection of poliovirus from stool specimens [18]. While these methods require less personnel time, the cost of reagents is significantly higher if they are to be applied routinely. To date, the GPEI has not been willing to trade reduced sensitivity for a faster, but more expensive, laboratory result.
In certain circumstances, ending collection and testing of the second stool specimen might be appropriate. After examination of data from the Americas, it was suggested that in endemic countries with high laboratory proficiency, collection of the second stool sample may not be necessary [Reference Pinheiro4, Reference Silveira19, Reference de Quadros20]. As a result, a recommendation to collect only one stool sample was made by the Pan American Health Organization after certification of polio eradication in the Americas. For India, consideration could be given to ending the collection and testing of the second stool specimen in the post-eradication era (following national certification 3 years after the last case is reported), if the AFP surveillance system continues to be strong. At the present time, however, when identification of every case is critical and maximum sensitivity is required to mitigate the risk of failure to detect poliovirus importations, our findings support continuing the collection of two stool specimens for AFP surveillance in India.
ACKNOWLEDGEMENTS
The authors acknowledge the World Health Organization – India for the contribution in coordinating, compiling and supporting data analysis for this study. We also thank the Government of India for making the data available for the study. This research was conducted as part of usual CDC activities and received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.











