Introduction
The early evolution of animals, or metazoans (Metazoa), can be traced back to the Ediacaran Period (635–541 Ma) based on the fossil record of macroscopic soft-bodied impressions, carbonaceous compressions, and subsequently mineralized and organically preserved organisms (Narbonne, Reference Narbonne2005; Moczydłowska et al., Reference Moczydłowska, Westall and Foucher2014; Wan et al., Reference Wan, Yuan, Chen, Guan, Pang, Tang and Xiao2016; Xiao et al., Reference Xiao, Narbonne, Zhou, Laflamme, Grazhdankin, Moczydłowska-Vidal and Cui2016; Schiffbauer et al., Reference Schiffbauer, Selly, Jacquet, Merz, Nelson, Srange, Cai and Smith2020). However, a more ancient metazoan origination event has been inferred using molecular clocks, which suggests emergence times ranging from between 833–650 Ma (dos Reis et al., Reference dos Reis, Thawornwattana, Angelis, Telford, Donoghue and Yang2015), to a more specific point estimate of up to 780 Ma (Erwin et al., Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011). The affinities of the various Ediacaran metazoan taxa are likewise unresolved and are often contested because of their unusual morphologies and lack of unambiguous diagnostic features in comparison to modern phyla; these have resulted in assorted identifications including sponges, placozoans, cnidarians, lophophorates, and putative bilaterian metazoans (Narbonne, Reference Narbonne2005; Fedonkin et al., Reference Fedonkin, Simonetta, Ivantsov, Vickers-Rich and Komarower2007a, Reference Fedonkin, Gehling, Grey, Narbonne and Vickers-Richb; Xiao and Laflamme, Reference Xiao and Laflamme2009; Sperling and Vinther, Reference Sperling and Vinther2010; Erwin et al., Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011; Sperling et al., Reference Sperling, Peterson and Laflamme2011; Zhuravlev et al., Reference Zhuravlev, Wood and Penny2015; Hoyal Cuthill and Han, Reference Hoyal Cuthill and Han2018; Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019; Schiffbauer et al., Reference Schiffbauer, Selly, Jacquet, Merz, Nelson, Srange, Cai and Smith2020; Shore et al., Reference Shore, Wood, Curtis and Bowyer2020).
The preserved remains of Ediacaran metazoans seem to represent body tissues that were largely composed of soft organic matter (Moczydłowska et al., Reference Moczydłowska, Westall and Foucher2014; Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014; Yin et al., Reference Yin, Zhu, Davidson, Bottjer, Zhao and Tafforeau2015; Wan et al., Reference Wan, Yuan, Chen, Guan, Pang, Tang and Xiao2016; Bobrovskiy et al., Reference Bobrovskiy, Krasnova, Ivantsov, Luzhnaya (Serezhnikova) and Brocks2019; Schiffbauer et al., Reference Schiffbauer, Selly, Jacquet, Merz, Nelson, Srange, Cai and Smith2020), although calcified skeletons had appeared by ca. 550 Ma (Germs, Reference Germs1972; Grant, Reference Grant1990; Grotzinger et al., Reference Grotzinger, Watters and Knoll2000; Wood et al., Reference Wood, Grotzinger and Dickson2002, Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019; but see Schiffbauer et al., Reference Schiffbauer, Selly, Jacquet, Merz, Nelson, Srange, Cai and Smith2020, and Yang et al., Reference Yang, Steiner, Schiffbauer, Selly, Wu, Zhang and Liu2020). The onset of metazoan mineralization is a precursor to the independent acquisition of biomineralized skeletons and of different mineralogies by multiple metazoan clades, an important feature of the Cambrian Explosion (541–485 Ma; Erwin et al., Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011; Erwin and Valentine, Reference Erwin and Valentine2013; Murdock, Reference Murdock2020).
Here we report an assemblage of three-dimensionally (3-D) preserved macroscopic tubular and conical fossils from marine siliciclastic strata of Ediacaran age on the Digermulen Peninsula of Arctic Norway. These specimens represent three distinct morphotypes, which we recognize as distinct taxa, and are thus interpreted as components of a coeval assemblage that is stratigraphically restricted to the basal succession of the Stáhpogieddi Formation. This interval lies 40–42 m above the Mortensneset Diamictite—an uppermost marker of the Varangerian glaciations in Scandinavia (Føyn, Reference Føyn, Gee and Sturt1985; Nystuen, Reference Nystuen1985) and correlated with the regional Ediacaran Gaskiers glaciation at ca. 580 Ma (Halverson et al., Reference Halverson, Hoffman, Schrag, Maloof and Rice2005, Reference Halverson, Wade, Hurtgen and Barovich2010; Rice et al., Reference Rice, Edwards, Hansen, Arnaud, Halverson, Arnaud, Halverson and Shields-Zhou2011; Pu et al., Reference Pu, Bowring, Ramezani, Myrow, Raub and Landing2016). The affinities of the new taxa remain uncertain, but collectively all features suggest eumetazoans. We apply a range of microscopic, computed micro-tomographic, petrographic, and geochemical techniques to determine their morphology and mineralogy. This reveals evidence of primary siliceous biomineralization, which to our knowledge, constitutes the earliest examples of silica-based animal skeletons yet documented from the fossil record. The global stratigraphic ranges of the Ediacaran impression fossils, including Dickinsonia Sprigg, Reference Sprigg1947, Swartpuntia Narbonne et al., Reference Narbonne, Saylor and Grotzinger1997, Hiemalora Fedonkin, Reference Fedonkin1982, and Aspidella Billings, Reference Billings1872, and 3-D modular Palaeopascichnus Palij, Reference Palij and Ryabenko1976 occurring in the Stáhpogieddi Formation (Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013, Reference Högström, Jensen, Ebbestad, Taylor, Høyberget, Agić, Meinhold and Palacios2017; Høyberget et al., Reference Høyberget, Högström, Ebbestad and Jensen2017; McIlroy and Brasier, Reference McIlroy, Brasier, Brasier, McIlroy and McLoughlin2017; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018; additional new data on Dickinsonia and Swartpuntia per A. Högström, personal communication, 2018; Fig. 2) are compiled and the sedimentary continuity of this formation is discussed with the purpose of estimating the age of the new taxa.
Geological setting, stratigraphy, and age
Tectonic setting
The Stáhpogieddi Formation is well exposed across the Digermulen Peninsula in far northeastern Finnmark, Arctic Norway. Structurally, the region forms part of the Gaissa Thrust Belt (or Gaissa Nappe Complex) within the Lower Allochthon of the eastern Scandinavian Caledonides. The Digermulen Peninsula is in a continuous transition with the parautochtnonous and autochthonous areas around Tanafjorden and on the Varanger Peninsula (Gee and Sturt, Reference Gee and Sturt1985; Gayer et al., Reference Gayer, Rice, Roberts, Townsend and Welbon1987; Siedlecka et al., Reference Siedlecka, Reading, Williams and Roberts2006; Rice, Reference Rice, Corfu, Gasser and Chew2014; Fig. 1).

Figure 1. Sketch-map of Scandinavia with studied area marked in northern Norway by arrow in (1), pointing to geological map of the Ediacaran-Cambrian strata on the Digermulen Peninsula coastal outcrop in (2), showing the new fossils locality. The outline of the Varanger and Digermulen peninsulas and the Tanafjorden area is in (3) with Gaissa Thrust Belt shaded gray.
Geologically, it has been subjected to limited tectonic, diagenetic, and thermal modifications in comparison to the highly metamorphosed nappes in the central Caledonides, which have a complex history of folding and décollement (Roberts and Gee, Reference Roberts, Gee, Gee and Sturt1985; Gayer et al., Reference Gayer, Rice, Roberts, Townsend and Welbon1987; Dallmeyer et al., Reference Dallmeyer, Reuter, Clauer, Liewig and Gayer1989; Roberts, Reference Roberts2003; Rice, Reference Rice, Corfu, Gasser and Chew2014; Meinhold et al., Reference Meinhold, Wemmer, Högström, Ebbestad, Jensen, Palacios, Høyberget, Agić and Taylor2019a). The Gaissa Thrust Belt has experienced low angle thrusting over a relatively short distance (Rice, Reference Rice, Corfu, Gasser and Chew2014) and is sequentially sub-divided into the Vadsø, Ekkerøya (elsewhere considered a formation-level unit of the Vadsø Group; Føyn, Reference Føyn, Gee and Sturt1985), Tanafjorden, Vestertana, and Digermulen groups, with a total succession thickness of ~5 km.
The Vestertana Group, including the studied Stáhpogieddi Formation (Fig. 2), was deposited in a foreland basin under shallow marine to deep outer shelf sedimentary conditions on a passive continental margin (Roberts and Siedlecka, Reference Roberts and Siedlecka2002; Rice et al., Reference Rice, Edwards and Hansen2012; Rice, Reference Rice, Corfu, Gasser and Chew2014; W. Zhang et al., Reference Zhang, Roberts and Pease2015). This basin developed along the western Baltica paleocontinent at ~50°S (orientation at the time; Torsvik and Cocks, Reference Torsvik and Cocks2017).
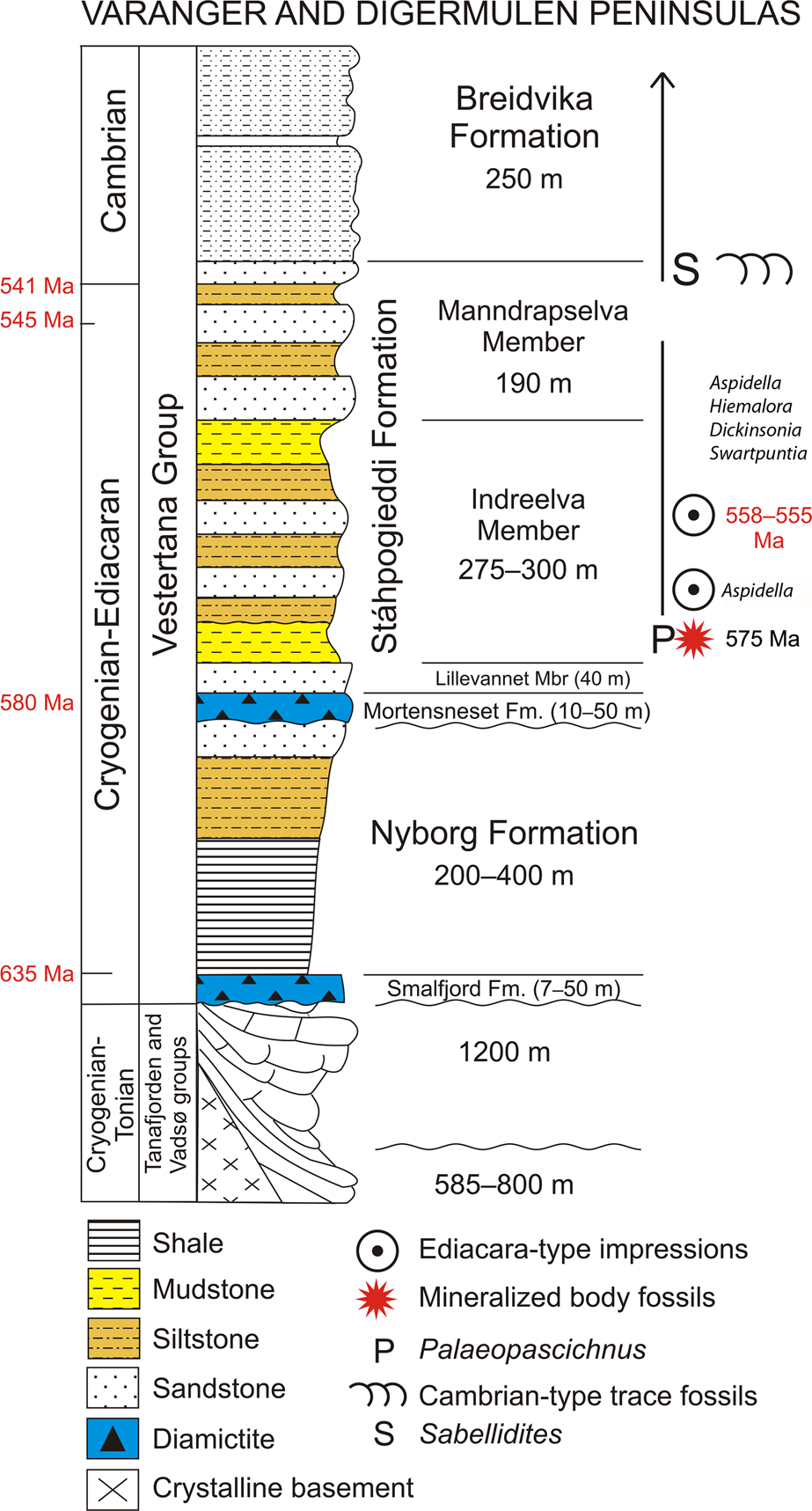
Figure 2. Ediacaran sedimentary succession on the Digermulen Peninsula showing the stratigraphic distribution of fossils and new mineralized body fossils. In the left column, the isotopic ages of the Marinoan and Gaskiers glaciations at 635 Ma and 580 Ma, respectively, and their time-equivalent diamictites are marked; the base of the Cambrian at 541 Ma, and the age of the topmost Manndrapselva Member at 545 Ma are all published data (Peng et al., Reference Peng, Babcock, Cooper, Gradstein, Ogg, Schmitz and Ogg2012; Zhang et al., Reference Zhang, Hua and Zhang2015; Pu et al., Reference Pu, Bowring, Ramezani, Myrow, Raub and Landing2016; Xiao et al., Reference Xiao, Narbonne, Zhou, Laflamme, Grazhdankin, Moczydłowska-Vidal and Cui2016). Note the Cryogenian age of the Smalfjord Formation at the base of the succession. In the right column, the age of the Ediacara-type impression fossils is chronostratigraphically recognized from their global ranges at 558–555 Ma and at the lower occurrence of only Aspidella (referenced in the text). The age of new mineralized body fossils is interpolated herein at ca. 575 Ma.
Stratigraphic information
The Vestertana Group extends stratigraphically from the upper Cryogenian Smalfjord Formation, which incorporates mostly terrestrial fluvioglacial diamictite and glaciomarine sediments correlated with the Marinoan Glaciation at 635.5 ± 0.6 Ma (Edwards, Reference Edwards1984; Gayer and Rice, Reference Gayer, Rice and Gayer1989; Condon et al., Reference Condon, Zhu, Bowring, Wang, Yang and Jin2005; Rice et al., Reference Rice, Edwards, Hansen, Arnaud, Halverson, Arnaud, Halverson and Shields-Zhou2011, Reference Rice, Edwards and Hansen2012; see Bechstädt et al., Reference Bechstädt, Jäger, Rittersbacher, Schweisfurth, Spence, Werner and Boni2018 for a recent review; further referred to 635 Ma) to the Breidvika Formation of the lower Cambrian Terreneuvian Series (Reading and Walker, Reference Reading and Walker1966; Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018; Fig. 2). The age range of the Vestertana Group is chemo- and biochronologically constrained to 635–521 Ma (Rice et al., Reference Rice, Edwards, Hansen, Arnaud, Halverson, Arnaud, Halverson and Shields-Zhou2011; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). The Vestertana Group sequentially comprises the Smalfjord, Nyborg, Mortensneset, Stáhpogieddi, and Breidvika formations and is 1120–2025 m thick, depending on the lateral facies distribution across the depositional basin (Reading, Reference Reading1965; Reading and Walker, Reference Reading and Walker1966; Banks et al., Reference Banks, Edwards, Geddes, Hobday and Reading1971; Banks, Reference Banks1973; Edwards, Reference Edwards1984; Føyn, Reference Føyn, Gee and Sturt1985; Rice et al., Reference Rice, Edwards and Hansen2012). The Mortensneset Formation contains an upper glaciomarine diamictite correlated with the Gaskiers Glaciation at 580 Ma (Halverson et al., Reference Halverson, Hoffman, Schrag, Maloof and Rice2005; Halverson and Shields-Zhou, Reference Halverson, Shields-Zhou, Arnaud, Halverson and Shields-Zhou2011; Rice et al., Reference Rice, Edwards, Hansen, Arnaud, Halverson, Arnaud, Halverson and Shields-Zhou2011; Pu et al., Reference Pu, Bowring, Ramezani, Myrow, Raub and Landing2016; see also discussion by Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). The two glacigenic formations were previously attributed to the Lower and Upper Tillites of the late Proterozoic Varanger Ice Age, respectively (Føyn, Reference Føyn, Gee and Sturt1985; Nystuen, Reference Nystuen1985).
The post-glacial Stáhpogieddi and Breidvika formations are marine siliciclastic successions that preserve age-diagnostic fossils (Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; McIlroy and Brasier, Reference McIlroy, Brasier, Brasier, McIlroy and McLoughlin2017; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018) and constitute a conformable succession with the underlying Mortensneset Formation (Reading, Reference Reading1965; Reading and Walker, Reference Reading and Walker1966; Banks et al., Reference Banks, Edwards, Geddes, Hobday and Reading1971; Banks, Reference Banks1973; Edwards, Reference Edwards1984; Føyn, Reference Føyn, Gee and Sturt1985; Gayer and Rice, Reference Gayer, Rice and Gayer1989; Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Rice et al., Reference Rice, Edwards and Hansen2012; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; Rice, Reference Rice, Corfu, Gasser and Chew2014; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). Regional unconformities have been recognized at the bases of the Smalfjord and Mortensneset diamictites (Edwards, Reference Edwards1984; Rice et al., Reference Rice, Edwards and Hansen2012; see compilation by Rice, Reference Rice, Corfu, Gasser and Chew2014; Fig. 2), and thus have implications for determining the relative age of the Stáhpogieddi Formation.
The Stáhpogieddi Formation is a 530-m-thick succession of alternating mudstones, siltstones, and sandstones, with a few thin calcified siliciclastic beds in the uppermost part, and is undeformed, other than being tilted and dipped at 19–28°W, cleaved, and affected by diagenesis and low-grade thermal overprinting (Dallmeyer et al., Reference Dallmeyer, Reuter, Clauer, Liewig and Gayer1989; Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Meinhold et al., Reference Meinhold, Wemmer, Högström, Ebbestad, Jensen, Palacios, Høyberget, Agić and Taylor2019a, Reference Meinhold, Jensen, Høyberget, Arslan, Ebbestad, Högström, Palacios, Agić and Taylorb; Fig. 2). The formation has been subdivided into the Lillevannet, Indreelva, and Manndrapselva members, which reflect transgressive-regressive parasequences within a continuous succession; shallowing events mark the sedimentary parasequences, including two distinct upward-coarsening turbidite sandstone intervals occurring within the Indreelva Member and three within the Manndrapselva Member (Reading, Reference Reading1965; Reading and Walker, Reference Reading and Walker1966; Banks et al., Reference Banks, Edwards, Geddes, Hobday and Reading1971; Banks, Reference Banks1973; Siedlecka, Reference Siedlecka1985; Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Rice, et al., Reference Rice, Edwards and Hansen2012; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). The boundaries between the members of the Stáhpogieddi Formation are gradual or at sharp lithologic contacts but conformable.
The basalmost Lillevannet Member is a 40-m-thick quartzitic sandstone with cross-bedding and ripple cross-lamination. It is also interbedded with mudstone and occasional conglomerate layers that reflect a transition from fluvial to shallow marine, sub-tidal environments (Reading and Walker, Reference Reading and Walker1966; Banks et al., Reference Banks, Edwards, Geddes, Hobday and Reading1971; Edwards, Reference Edwards1984; Rice et al., Reference Rice, Edwards and Hansen2012).
The overlying Indreelva Member, which has yielded the new fossils, is up to 300-m-thick succession and consists of red and green, thin-bedded mudstone with subsequent alternating siltstone, mudstone, and sandstone beds (Fig. 2). A rapid transition from sub-tidal mudstone into a deeper, sub-wave-base facies occurs in the basal portion of the member. The succession was deposited in quiet water environments on an offshore deeper shelf (characterized by mudstone, siltstone) with two regressive intervals (sandstone) tracking a gradual change into shallow marine shelf conditions (Reading, Reference Reading1965; Banks et al., Reference Banks, Edwards, Geddes, Hobday and Reading1971; Banks Reference Banks1973; Edwards, Reference Edwards1984; Rice et al., Reference Rice, Edwards and Hansen2012).
The uppermost Manndrapselva Member is a 190-m-thick succession of alternating coarse-grained quartzitic sandstone interbedded with greywacke and thin-bedded siltstone. It incorporates three shallowing-upward parasequences consisting of fine-grained distal to proximal turbidite sediments deposited on an outer marine shelf; the quartzitic sandstone intervals reflect shallower marine conditions (Reading, Reference Reading1965; Reading and Walker, Reference Reading and Walker1966; Banks et al., Reference Banks, Edwards, Geddes, Hobday and Reading1971; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018).
The only occurrence of carbonate sediments within the Stáhpogieddi Formation includes a series of discontinuous calcified siliciclastic beds up to 15 cm thick. These are randomly distributed as calcareous lenses and concretions over a 23-m-thick interval of the upper Manndrapselva Member, and are observable over a limited outcrop on the Digermulen Peninsula (Meinhold et al., Reference Meinhold, Jensen, Høyberget, Arslan, Ebbestad, Högström, Palacios, Agić and Taylor2019b). These upper Manndrapselva Member siliciclastic sediments formed during the late Ediacaran and were locally early diagenetically calcified and altered into concretions through the late Cambrian–Ordovician time (Meinhold et al., Reference Meinhold, Jensen, Høyberget, Arslan, Ebbestad, Högström, Palacios, Agić and Taylor2019b). The stratigraphic interval bearing these carbonates lies 40 m below the base of the Cambrian recognized in the succession, is of latest Ediacaran age and a rather insignificant sedimentary component within the 530-m-thick siliciclastic Stáhpogieddi Formation The upper boundary of the Stáhpogieddi Formation with the succeeding Breidvika Formation is lithologically abrupt but without any depositional break (Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018).
The Stáhpogieddi Formation contains the diagnostic Cambrian-type trace fossils and organic tubular metazoan Sabellidites Yanishevsky, Reference Yanishevsky1926 in its uppermost part, the Ediacara-type impression fossils in the middle, the new body fossils in the lowermost part, and casts and molds of modular Palaeopascichnus macrofossils extending throughout the formation (Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; McIlroy and Brasier, Reference McIlroy, Brasier, Brasier, McIlroy and McLoughlin2017; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). The Ediacaran-Cambrian boundary is recognized in the uppermost Manndrapselva Member (Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; McIlroy and Brasier, Reference McIlroy, Brasier, Brasier, McIlroy and McLoughlin2017; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018; Fig. 2).
Sedimentary continuity within the Stáhpogieddi Formation
Regionally, in a scale of the marine depositional basin extending across the Digermulen and Varanger peninsulas, the entire succession, including the Mortnesneset Diamictite Formation, the Lillevannet Member, and the succeeding members of Stáhpogieddi Formation, is sedimentologically continuous. This has been examined by numerous studies and synthesized by Rice et al. (Reference Rice, Edwards and Hansen2012) and Rice (Reference Rice, Corfu, Gasser and Chew2014), see also discussion by Jensen et al. (Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). On the Digermulen Peninsula, the contact between the Mortensneset Formation and the Lillevannet Member is lithologically abrupt between diamictite and mudstone or sandstone, but without erosion and is conformable (Reading and Walker, Reference Reading and Walker1966; Edwards, Reference Edwards1984; Gayer and Rice, Reference Gayer, Rice and Gayer1989; Rice et al., Reference Rice, Edwards, Hansen, Arnaud, Halverson, Arnaud, Halverson and Shields-Zhou2011, Reference Rice, Edwards and Hansen2012; Rice, Reference Rice, Corfu, Gasser and Chew2014; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). This contact was suggested to be a sharp erosional unconformity (McIlroy and Brasier, Reference McIlroy, Brasier, Brasier, McIlroy and McLoughlin2017, p. 355), but without indicating any sedimentary structures for erosion. The conglomerate layers in the middle of the Lillevannet Member, which occur only on the Varanger Peninsula, show a shallowing, regressive interval with channels cutting sub-surface and fluvial influx from the nearby continental margin of a high relief into marine basin, but these layers do not extend farther into shelf on the Digermulen Peninsula (Banks et al., Reference Banks, Edwards, Geddes, Hobday and Reading1971; Edwards, Reference Edwards1984). These conglomerates are not considered to represent an erosional unconformity involving time hiatus, but rather show lateral facies distribution within the Lillevannet Member.
McIlroy and Brasier (Reference McIlroy, Brasier, Brasier, McIlroy and McLoughlin2017, p. 353) suggested that a significant depositional hiatus occurred within either the Lillevannet or Indereelva members based on their comparison of the 530-m-thick upper Ediacaran (ca. 580–541 Ma) succession of the Stáhpogieddi Formation with the ~2500 m thick succession between the Gaskiers Formation and the Cambrian GSSP horizon in southeastern Newfoundland (McIlroy and Brasier, Reference McIlroy, Brasier, Brasier, McIlroy and McLoughlin2017 cited as Dalrymple et al., Reference Dalrymple, Ichaso and Narbonne2006, but Ichaso et al., Reference Ichaso, Dalrymple and Narbonne2007 is the correct citation for this data) corresponding to the same time interval. However, this is misleading because the Newfoundland strata (including the Conception and St. John's groups in west Conception Bay, the Avalon Zone) comprise deep-water, siliciclastic-volcanoclastic turbidites, contourites, and volcanic ash beds deposited in marine slope-to-basin settings. Such fore-arc, pull-apart depositional basins within the tectonic subduction zone of a spreading center (Ichaso et al., Reference Ichaso, Dalrymple and Narbonne2007) typically experience very fast sedimentation rates (Stow, Reference Stow and Reading1986; Leeder, Reference Leeder1994). This clearly conflicts with the Stáhpogieddi Formation, which was otherwise deposited on a passive margin, and therefore is substantially thinner, but continuous with no demonstrable sedimentological unconformities (Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Rice et al., Reference Rice, Edwards and Hansen2012; Rice, Reference Rice, Corfu, Gasser and Chew2014; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018; Fig. 2). We therefore favor a more feasible depositional comparison with the 500-m-thick upper Ediacaran succession in the Adelaide Rift Complex in South Australia; this fine-grained siliciclastic succession comprises minor dolomites and accumulated on carbonate shelf to delta front with some basinal sedimentation (Grey, Reference Grey2005, p. 38, fig. 13) and has been bracketed at 580–541 Ma based on isotopic dating of the Acraman ejecta layer versus the Ediacaran-Cambrian boundary (Grey, Reference Grey2005, p. 54).
The Ediacaran fossil ranges and chronostratigraphy
The Ediacaran soft-bodied macroscopic impression taxa (some also known from 3-D casts and molds in other occurrences), including Dickinsonia, Swartpuntia, Hiemalora, and Aspidella, and 3-D preserved Palaeopascichnus are recorded in the Stáhpogieddi Formation (Fig. 2). Their first appearance datum (FAD), last appearance datum (LAD), and stratigraphic ranges are established from global records and used for our estimation of the age of the assemblage containing them on the Digermulen Peninsula. Furthermore, this age provides a biochronologic time frame for the new fossils, which underlie ~130 m below in the rock succession (Fig. 2). The FADs and LADs of individual taxa may be approximately recognized from isotopic dates of the rock levels that are close to their records, and we follow those well-dated ranges.
Biostratigraphic range of Dickinsonia.––The impression fossil total grouping of Dickinsoniomorpha has been constrained to either 559–550 Ma (Fedonkin and Vickers-Rich, Reference Fedonkin, Vickers-Rich, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007; Grazhdankin, Reference Grazhdankin2014) or 556–541 Ma time intervals (Narbonne et al., Reference Narbonne, Xiao, Shields, Gradstein, Ogg, Schmitz and Ogg2012; Xiao et al., Reference Xiao, Narbonne, Zhou, Laflamme, Grazhdankin, Moczydłowska-Vidal and Cui2016). The nominal genus Dickinsonia ranges between 558–555 Ma (Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019), with the FAD and LAD following isotopic calibrations from the White Sea assemblage in Russia (Martin et al., Reference Martin, Grazhdankin, Bowring, Evens, Fedonkin and Kirschvink2000; Fedonkin and Vickers-Rich, Reference Fedonkin, Vickers-Rich, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007).
Biostratigraphic range of Swartpuntia.––The monotypic genus Swartpuntia is isotopically dated to between 545.1 ± 1 Ma and 543.3 ± 1 Ma and up to 539.4 ± 1 Ma or 538 ± 1 Ma in the Nama Group of Namibia (Narbonne et al., Reference Narbonne, Saylor and Grotzinger1997; Vickers-Rich, Reference Vickers-Rich, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007), and recently updated to 538.99 ± 0.21 Ma (Linnemann et al., Reference Linnemann, Ovtcharova, Schaltegger, Gärtner, Hautmann, Geyer, Vickers-Rich, Rich, Plessen, Hofmann, Zieger, Krause, Kriesfeld and Smith2019). In the White Sea assemblage, Swartpuntia germsi Narbonne, Saylor, and Grotzinger, Reference Narbonne, Saylor and Grotzinger1997 is recorded in the middle of the Verkhov Formation, which is isotopically dated to between 558.3 ± 1 Ma and 555.3 ± 0.3 Ma (Martin et al., Reference Martin, Grazhdankin, Bowring, Evens, Fedonkin and Kirschvink2000; Fedonkin and Vickers-Rich, Reference Fedonkin, Vickers-Rich, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007). Swartpuntia cf. S. germsi occurs together with the cloudiniids, which range at 550–541 Ma, in the Wood Canyon Formation of Nevada, USA, and shortly below the appearance of the Cambrian Treptichnus Miller, Reference Miller1889 trace fossil, thus confirming its uppermost Ediacaran extension (Hagadorn and Waggoner, Reference Hagadorn and Waggoner2000) as known in Namibia. The Swartpuntia-like molds similarly occur alongside Treptichnus in the lowermost Cambrian Uratanna Formation in the Flinders Ranges of South Australia (Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019) and support a LAD of the genus that accords with the more reliably dated Nama assemblage (Linnemann et al., Reference Linnemann, Ovtcharova, Schaltegger, Gärtner, Hautmann, Geyer, Vickers-Rich, Rich, Plessen, Hofmann, Zieger, Krause, Kriesfeld and Smith2019). The range of Swartpuntia is 558–539 Ma and its FAD coincides with that of Dickinsonia (558–555 Ma).
Biostratigraphic range of Hiemalora.––The range of Hiemalora is within the time interval of 565–550 Ma (Hofmann et al., Reference Hofmann, O'Brien and King2008; Chen et al., Reference Chen, Zhou, Xiao, Wang, Guan, Hua and Yuan2014; Grazhdankin, Reference Grazhdankin2014). The LAD is within the Shibantan Member of the Dengying Formation in China, whose deposition is bracketed to between 551–541 Ma, with a precisely dated horizon of the formation basal age at 551.09 ± 1.02 Ma (Schmitz, Reference Schmitz, Gradstein, Ogg, Schmitz and Ogg2012). Chen et al. (Reference Chen, Zhou, Xiao, Wang, Guan, Hua and Yuan2014) alternatively listed a LAD of 550 Ma. The range of Hiemalora could be substantially wider, if one would accept this taxon to be synonymous with Aspidella and both to represent the preservation states of holdfasts of frondose taxa like Charniodiscus Ford, 1958 rather than discrete species (Burzynski and Narbonne, Reference Burzynski and Narbonne2015; Burzynski et al., Reference Burzynski, Dececchi, Narbonne and Dalrymple2017a, Reference Burzynski, Narbonne, Dececchi and Dalrympleb). This synonymy has not yet been supported by the record of Aspidella and Hiemalora being attached to specimens of the same frondose taxon. On the other hand, the connection between Hiemalora as being the holdfast of Primocandelabrum hiemaloranum Hofmann, O'Brien, and King, Reference Hofmann, O'Brien and King2008 is documented in a fossil specimen preserving those two taxa together (Hofmann et al., Reference Hofmann, O'Brien and King2008). Additionally, Primocandelabrum Hofmann et al., Reference Hofmann, O'Brien and King2008 is not attributed to any recognized Ediacaran clade, whereas Charniodiscus is associated with Arboreomorphs (Burzynski and Narbonne, Reference Burzynski and Narbonne2015), suggesting that Hiemalora may not be the preservation state of Aspidella.
Biostratigraphic range of Aspidella.––The type species Aspidella terranovica Billings, Reference Billings1872 has a global stratigraphic range of 579–550 Ma (Grazhdankin, Reference Grazhdankin2014), with slightly narrower of 571–560 Ma range in Avalon Zone type area of SE Newfoundland (Liu et al., Reference Liu, Kenchington and Mitchell2015; Pu et al., Reference Pu, Bowring, Ramezani, Myrow, Raub and Landing2016), or 580–560 Ma in the Mackenzie Mountains of NW Canada (Burzynski et al., Reference Burzynski, Dececchi, Narbonne and Dalrymple2017a). Wood et al. (Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019) listed Aspidella within a simplified category of “discs” at 571–540 Ma, but without including Hiemalora and seemingly without its older part in NW Canada and globally. Two specimens of Aspidella from the Mackenzie Mountains among numerous “discs” are reported from Cryogenian strata and suggest extension this taxon's stratigraphic range by ca. 80 Ma (Hofmann et al., Reference Hofmann, Narbonne and Aitken1990; Burzynski et al., Reference Burzynski, Dececchi, Narbonne and Dalrymple2017a). Regardless of the Cryogenian record and the late Ediacaran acme range of Aspidella at ca. 571–540 Ma, the taxon is less stratigraphically significant. We treat the two taxa, Aspidella and Hiemalora, as individual genera and their ranges are only partially overlapping at 565–550 Ma (i.e., coinciding with the range of Hiemalora).
Biostratigraphic range of Palaeopascichnus.––This modular fossil, also broadly treated within the group of palaeopascichnids, has been taxonomically synonymized with several taxa and its range suggested to span the entire Ediacaran Period (Kolesnikov et al., Reference Kolesnikov, Rogov, Bykova, Danelian, Clausen, Maslov and Grazhdankin2018). The junior synonym Orbisiana Sokolov, Reference Sokolov1976, emend. Wan et al., Reference Wan, Xiao, Yuan, Chen, Pang, Tang, Guan and Maisano2014 occurs in the lowermost Ediacaran Lantian Formation of South China, which is estimated at 635–590 Ma (Yuan et al., Reference Yuan, Chen, Xiao, Zhou and Hua2011; Wan et al., Reference Wan, Xiao, Yuan, Chen, Pang, Tang, Guan and Maisano2014, Reference Wan, Yuan, Chen, Guan, Pang, Tang and Xiao2016; Wang et al., Reference Wang, Guan, Zhou, Peng, Pratt, Chen, Chen, Chen, Yuan and Xiao2017; Kolesnikov et al., Reference Kolesnikov, Rogov, Bykova, Danelian, Clausen, Maslov and Grazhdankin2018), but with re-evaluation to ca. 620–580 Ma based on correlation with the member II of the Doushantuo Formation (Yuan et al., Reference Yuan, Chen, Xiao, Zhou and Hua2011; Wang et al., Reference Wang, Guan, Zhou, Peng, Pratt, Chen, Chen, Chen, Yuan and Xiao2017), for which the cited age is estimated (Liu and Moczydłowska, Reference Liu and Moczydłowska2019). Palaeopascichnus has been identified in Baltica and Avalonia at 565–541 Ma (Grazhdankin and Maslov, Reference Grazhdankin and Maslov2009, Reference Grazhdankin and Maslov2015; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018) and in Australia at 560 Ma (Haines, Reference Haines2000; Bowring et al., Reference Bowring, Grotzinger, Condon, Ramezani, Newall and Allen2007). The overall global range of paleopascichnids is estimated at 580–540 Ma (Grazhdankin, Reference Grazhdankin2014). We follow the range of Palaeopascichnus to span most of the Ediacaran Period, ca. 620–540 Ma (not all), and the taxon has limited stratigraphic significance.
Based on the current recognition of stratigraphic ranges in isotopically dated sedimentary intervals containing the fossils, the overlapping ranges of Aspidella, Hiemalora, Dickinsonia, and Swartpuntia at 558–555 Ma provide the closest available age that is inferred for the strata recording these taxa in the Stáhpogieddi Formation (Fig. 2).
Age of the Stáhpogieddi Formation and recorded fossils
The base of the Stáhpogieddi Formation correlates with the top of the Mortensneset Formation diamictite at 580 Ma (Halverson et al., Reference Halverson, Hoffman, Schrag, Maloof and Rice2005; Halverson and Shields-Zhou, Reference Halverson, Shields-Zhou, Arnaud, Halverson and Shields-Zhou2011; Rice et al., Reference Rice, Edwards, Hansen, Arnaud, Halverson, Arnaud, Halverson and Shields-Zhou2011, Reference Rice, Edwards and Hansen2012; Pu et al., Reference Pu, Bowring, Ramezani, Myrow, Raub and Landing2016). Detrital zircon grains of Baltican provenance recovered from the medium-grained turbiditic sandstone in the upper Manndrapselva Member of the Tanafjorden area, which extends laterally to the Digermulen Peninsula, have provided ages of 542.1 ± 2.8, 542.4 ± 4.8 and 544.9 ± 5.1 Ma (W. Zhang et al., Reference Zhang, Roberts and Pease2015). The maximum age of this sediment interval is thus 545 Ma (W. Zhang et al., Reference Zhang, Roberts and Pease2015; Fig. 2), which concurs with the 541 Ma age estimate based on trace fossils from the uppermost horizons of the Manndrapselva Member (Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). These ages bracket the Stáhpogieddi Formation to between 580–541 Ma and slightly younger where it extends into the Cambrian (Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018; Fig. 2). The uppermost Manndrapselva Member records the first appearance of Treptichnus pedum (Seilacher, Reference Seilacher1955) and other Cambrian-type trace fossils (Jensen, Reference Jensen1997; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; McIlroy and Brasier, Reference McIlroy, Brasier, Brasier, McIlroy and McLoughlin2017; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). Treptichnus pedum is also the index taxon for the base of the Cambrian System at the GSSP in Newfoundland (Narbonne et al., Reference Narbonne, Myrow, Landing and Anderson1987; Brasier et al., Reference Brasier, Cowie and Taylor1994; Landing, Reference Landing1994). U-Pb dates from the Ediacaran-Cambrian transitional strata (Grotzinger et al., Reference Grotzinger, Bowring, Saylor and Kaufman1995; Bowring and Schmitz, Reference Bowring and Schmitz2003; Bowring et al., Reference Bowring, Grotzinger, Condon, Ramezani, Newall and Allen2007) have refined the lower Cambrian boundary to 541 ± 0.63 Ma (Peng et al., Reference Peng, Babcock, Cooper, Gradstein, Ogg, Schmitz and Ogg2012; further referred to 541 Ma; Fig. 2) or possibly to ca. 539 Ma (Linnemann et al., Reference Linnemann, Ovtcharova, Schaltegger, Gärtner, Hautmann, Geyer, Vickers-Rich, Rich, Plessen, Hofmann, Zieger, Krause, Kriesfeld and Smith2019). The latter age of the stratigraphic level suggested for the Ediacaran-Cambrian boundary in the Nama Group of Namibia (in a composite section) is within 538.99 ± 0.21 Ma to 538.58 ± 0.19 Ma (Linnemann et al., Reference Linnemann, Ovtcharova, Schaltegger, Gärtner, Hautmann, Geyer, Vickers-Rich, Rich, Plessen, Hofmann, Zieger, Krause, Kriesfeld and Smith2019; further referred to 539 Ma). Before the new age may be accepted by the International Commission of Stratigraphy (ICS), we will use the age at 541 Ma for the lower Cambrian boundary and the age at 539 Ma for the ranges of certain fossil taxa recognized in Namibia (Linnemann et al., Reference Linnemann, Ovtcharova, Schaltegger, Gärtner, Hautmann, Geyer, Vickers-Rich, Rich, Plessen, Hofmann, Zieger, Krause, Kriesfeld and Smith2019) and extending from the Ediacaran.
Classic Ediacaran impression fossils, including Aspidella, Hiemalora, Dickinsonia, and Swartpuntia, all occur in the middle Indreelva Member of the Stáhpogieddi Formation (Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013, Reference Högström, Jensen, Ebbestad, Taylor, Høyberget, Agić, Meinhold and Palacios2017; Høyberget et al., Reference Høyberget, Högström, Ebbestad and Jensen2017; new identification of Dickinsonia and Swartpuntia; Fig. 2) and provide a demonstrable age range of 558–555 Ma (Fig. 2), and certainly not younger relying on the LAD of Dickinsonia. The FAD of Dickinsonia and Swartpuntia, which is isotopically dated (Martin et al., Reference Martin, Grazhdankin, Bowring, Evens, Fedonkin and Kirschvink2000; Fedonkin and Vickers-Rich, Reference Fedonkin, Vickers-Rich, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007), constrains the maximum age. The time interval 558–555 Ma is the biochronologically estimated age of this stratigraphic horizon in the Stáhpogieddi Formation The lowermost occurrence of Aspidella in the succession (Högström et al., Reference Högström, Jensen, Ebbestad, Taylor, Høyberget, Agić, Meinhold and Palacios2017; Høyberget et al., Reference Høyberget, Högström, Ebbestad and Jensen2017) may be close to this taxon acme occurrence known from 571 Ma (Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019), and is shortly above the new fossils (Fig. 2).
Our new Indreelva Member assemblage co-occurs with Palaeopascichnus, which prompted a specific age correlation for this interval at ca. 565 Ma (Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018). As mentioned, this taxon has wide stratigraphic range spanning 620–540 Ma and we decline from using this taxon for age determination in the Stáhpogieddi Formation.
The age of the new fossil assemblage may also be estimated from its relative stratigraphic position within the Stáhpogieddi Formation and calculated rate of deposition within the succession. Examples and arguments exist that the rates of deposition may be very variable and change in marine environments. Variables that are considered in estimating the rate of deposition include record fidelity, complex and incomplete nature of stratigraphic record owing to changing dynamics of the depositional system, variation in rate of deposition for elements in the basin, time and length scales, and stochastic processes (“noise”) (Tipper, Reference Tipper2016; Davies et al., Reference Davies, Shillito and McMahon2019; Holbrook and Miall, Reference Holbrook and Miall2020; Straub et al., Reference Straub, Duller, Foreman and Hajek2020). However, in long timescales, the rates of deposition are statistically buffered over periodicity (Straub et al., Reference Straub, Duller, Foreman and Hajek2020) and cyclic variability sums produce the average rate of deposition, which can be plausibly estimated from measured parameters of sediment thickness over the time span of the section (Holbrook and Miall, Reference Holbrook and Miall2020; Straub et al., Reference Straub, Duller, Foreman and Hajek2020). The accuracy of average rates of deposition depends on the vertical thickness and longer durations of time (Holbrook and Miall, Reference Holbrook and Miall2020). The measured rates of deposition are balanced among states of deposition, erosion, and stasis (Davies et al., Reference Davies, Shillito and McMahon2019) and are normalized for longer measurement duration (Straub et al., Reference Straub, Duller, Foreman and Hajek2020). Despite the limitations of the incomplete records and variation of rates of deposition, the estimation of rate of deposition is worthwhile and possible (Tipper, Reference Tipper2016).
Depositional environments of the Stáhpogieddi Formation in the lowland basin were relatively stable on the passive continental margin, and cyclic shallowing and deepening events due to sea-level changes were of low amplitudes within shelf settings without tectonic disturbance or erosional unconformities (Gayer and Rice, Reference Gayer, Rice and Gayer1989; Rice et al., Reference Rice, Edwards and Hansen2012; Rice, Reference Rice, Corfu, Gasser and Chew2014). This formation consists of transgressive-regressive parasequences, in which the rate of deposition between alternating units of mudstone, siltstone, and sandstone certainly varied in small-scale intervals. However, in a substantial thickness (530 m) of sedimentologically continuous succession it is rather uniform. In a cyclic repetition of fine- to coarser-grained siliciclastic rocks, the average rate of deposition is normalized and similar throughout the succession. Plausibly therefore, the long deposition time within 39 Ma (580–541 Ma and slightly younger; Fig. 2), together with its continuous sedimentation at similar average rate of 13.59 m/Ma, allows the interpolation of the ages within various portions of the formation. Most likely, the basal Indreelva Member, containing the new body fossils and overlying 40–42 m above the Mortensneset Formation, can be estimated at as little as 3 Ma, and rounded to as much as 5 Ma younger than the base of the formation assuming a degree of error (Fig. 2). Notably, this estimated ca. 575 Ma age limit is consistent with the FAD for typical Ediacara-type impression fossils in the middle Indreelva Member, which occur ~130 m above, and their overlapping ranges at 558–555 Ma. Calculating independently from the rate of deposition in the narrower sediment interval limited by the age-controlled horizons between 558–555 Ma and 580 Ma (Fig. 2), this 172 m of continuously deposited sediments in 22–25 Ma allow estimation of the time horizon of new fossils to be 5.46–6.10 Ma younger than 580 Ma. This conforms to the age estimated from the entire succession at ca. 575 Ma. Therefore, the average rate of deposition may be considered here as reliable.
Materials and methods
Twenty-two individual fossil specimens were collected from natural exposures along bedding plains in mudstone of the lowermost Indreelva Member in the Stáhpogieddi Formation (Fig. 2). The host sediment laminae bent plastically around the skeletons, which were slightly distorted by tectonic compression but retained hollow lumen or were mostly post-mortem infilled with the host sediment. Three different morphotypes are recognized at the rank of monotypic genera (of single species) and taxonomically described.
The fossils were studied under a reflected light microscope (RLM), a transmitted and polarized light microscope (PLM), an environmental scanning-transmission electron microscope (STEM) equipped with energy-dispersive X-ray detector for energy dispersive spectroscopy (STEM EDS), and a field emission electron probe microanalyzer (EMP) also equipped with EDS detector. Three-dimensional rendering and visualization of internal structures was also undertaken using a Nikon H225 ST Micro-Computed Tomography (CT) at the University of Oslo (Supplementary Data). Petrographic thin-sectioning was also carried out with geochemical and acid tests, as well as STEM EDS, EMP EDS, and Laser-Raman spectroscopy to determine mineralization (Supplementary Data). The mineral composition of the surrounding mudstone matrix was examined using Laser-Raman spectroscopy and X-ray diffraction (XRD; Supplementary Data).
Repository and institutional abbreviation
The material is reposited in the paleontological collections of the Museum of Evolution at Uppsala University (PMU). The collection is assigned as the Digermulen Peninsula fossils, Finnmark, Norway, and carry the catalogue numbers PMU 34736–34757, in which the last two digits refer to the individual specimen number.
Systematic paleontology
Terminology
Although the higher taxonomic affinities of the fossils described herein are unresolved, they are hierarchically assigned to Metazoa and Eumetazoa based on the eukaryote classification of Adl et al. (Reference Adl, Simpson and Lane2012, Reference Adl, Bass and Lane2019) and dos Reis et al. (Reference dos Reis, Thawornwattana, Angelis, Telford, Donoghue and Yang2015) (see also Cunningham et al., Reference Cunningham, Vargas, Yin, Bengtson and Donoghue2017). The term “animal” is used as an informal equivalent of metazoans in accordance with Blair (Reference Blair, Hedges and and Kumar2009). Morphologic terminology (Fig. 3) follows the recommendations of the International Zoological Code of Nomenclature (www.iczn.org). For example, we use “tube,” “tubular wall,” or “tubular skeleton” to describe cylindrical structures and “conus,” “conical wall,” or “conical skeleton” for conical structures. We have therefore avoided the otherwise ambiguous terms “conotubular” or “conical tube.” Similarly, we prefer “funnel-shaped segments or elements” (sensu Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012) instead of “funnel-shaped cylinders,” and have refrained from using “apex,” “apical end,” or “basal apex” (sensu Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010, Reference Cai, Schiffbauer, Hua and Xiao2011, Reference Cai, Cortijo, Schiffbauer and Hua2017; Cortijo et al., Reference Cortijo, Cai, Hua, Schiffbauer and Xiao2015) because of possible conflicting interpretation as embryonic segments that may have been embedded in the substrate. Finally, the term “apical flaring” was consistently used for defining portions of segments in Cloudina (Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012), which are oriented towards the distal end of the body.

Figure 3. Sketch-drawings and morphologic terminology of new fossil taxa drawn proportionally to their dimensions; interrupted lines define the lumen within walls. (1, 2) Holotype of Anulitubus n. gen. Moczydłowska in Moczydłowska et al.; (3) holotype of Coniculus n. gen. Moczydłowska in Moczydłowska et al.; (4) representative specimen of Fistula n. gen. Moczydłowska in Moczydłowska et al. Thick black outline of aperture and body surface in (3, 4) marks the wall outer veneer layer.
Type locality
All the taxa described in this study are from the coastal rock exposure at Árasulluokta Cove between the Árasulluokta and Manndrapselva rivulets on the southeastern coast of Tanafjorden, Digermulen Penninsula, Finnmark County, northeastern Norway. Mapsheet Langfjorden 223611, coordinates 420 303.
Type stratum
All taxa described in this study are from the red-brown, thin-bedded slaty mudstone in the lowermost part of the Indreelva Member, Stáhpogieddi Formation. The type horizon is ~40–42 m above the base of the Stáhpogieddi Formation coinciding with the top of the underlying Mortensneset Diamictite Formation; Ediacaran ca. 575 Ma.
Domain Eukarya Woese, Kandler, and Wheelis, Reference Woese, Kandler and Wheelis1990
Clade Metazoa Haeckel, Reference Haeckel1874
Clade Eumetazoa Bütschli, Reference Bütschli1910
Phylum uncertain
Genus Anulitubus new genus Moczydłowska in Moczydłowska et al.
Type species
Anulitubus formosus n. gen. n. sp. Moczydłowska in Moczydłowska et al.
Diagnosis
As for the type species.
Etymology
From Latin ānulāt-us, -a, -um, annulate, wearing a ring; ānul-us, -i, m., a ring; tub-us, -i, m., tube, pipe. Referring to the annulate tube or tube composed of rings.
Remarks
There are several terminal Ediacaran (550–541 Ma) macroscopic annulated body fossils known, but none of them could be fully compared with the newly described taxon. Anulitubus n. gen. is similar in an overall appearance and size to the annulate tapering fossil Conotubus Zhang and Lin in Lin et al., Reference Lin, Zhang and Zhang1986, which Cai et al. (Reference Cai, Schiffbauer, Hua and Xiao2011) considered to be a monotypic genus. The type species, Conotubus hemiannulatus Zhang and Lin in Lin et al., Reference Lin, Zhang and Zhang1986, emend. Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2011, has transverse annulations on the wall (Lin et al., Reference Lin, Zhang and Zhang1986; Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2011). Other diagnostic features include a “conical tube” structure composed of “nested funnel-shaped cylinders” with some transverse annulation (Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2011, p. 48); note that “annulation” is not explicitly mentioned in this revised diagnosis. Conotubus is preserved as carbonaceous films and compressions or by pyritization (pyritized tube), replication or coating by clay minerals, and glauconitic casting of the tube (Cai and Hua, Reference Cai and Hua2007; Hua et al., Reference Hua, Chen and Yuan2007; Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010, fig. 4C; Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2011, Reference Cai, Schiffbauer, Hua and Xiao2012; Schiffbauer et al., Reference Schiffbauer, Xiao, Cai, Wallace, Hua, Hunter, Xu, Peng and Kaufman2014). The original composition was organic, as indicated by geochemical analyses (Hua et al., Reference Hua, Chen and Yuan2007; Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2011; Schiffbauer et al., Reference Schiffbauer, Xiao, Cai, Wallace, Hua, Hunter, Xu, Peng and Kaufman2014).
Unlike Conotubus, Anulitubus n. gen. consists of a cylindrical annulate tube of uniform diameter. Its individual ring-shaped segments are vertically stacked in contrast to the nested conical segments in Conotubus. The segments in Anulitubus n. gen. are defined and separated by grooves. An incomplete septum also occurs within the tube lumen, whereas Conotubus lacks septa and has flaring rims around the apertures on its funnel-shaped segments.
Another annulate taxon, Cloudina Germs, Reference Germs1972, emend. Cai et al., Reference Cai, Cortijo, Schiffbauer and Hua2017, differs from Anulitubus n. gen. in being conical with multiple, nested elements forming a funnel-in-funnel tube construction. Cloudina also has transverse and/or oblique annulations that are distributed differently along the funnels in the various species (Cai et al., Reference Cai, Cortijo, Schiffbauer and Hua2017). This fossil wall was thought to have a calcareous mineral composition (Germs, Reference Germs1972; Grant, Reference Grant1990; Cortijo et al., Reference Cortijo, Martí Mus, Jensen and Palacios2010, Reference Cortijo, Cai, Hua, Schiffbauer and Xiao2015; Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012; Penny et al., Reference Penny, Wood, Curtis, Bowyer, Tostevin and Hoffman2014; Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017), but it has been revealed to be originally organic and only taphonomically mineralized (Yang et al., Reference Yang, Steiner, Schiffbauer, Selly, Wu, Zhang and Liu2020).
Sinotubulites Chen et al., Reference Chen, Chen and Qian1981, emend. Cai et al., Reference Cai, Xiao, Hua and Yuan2015 is distinguished by a cylindrical tube that is opened at both ends and variously circular, polygonal, or triangular in cross-section. The wall is multilayered with a tube-in-tube structure and transversely corrugated surface (Cai et al., Reference Cai, Xiao, Hua and Yuan2015). The tube composition is considered to be organic and secondarily phosphatized (Hua et al., Reference Hua, Chen and Yuan2007), or primarily organic with lightly biomineralized (possibly calcareous or aragonitic) lamellae and forming exoskeleton (M. Chen et al., Reference Chen, Chen and Qian1981; Hua et al., Reference Hua, Chen and Yuan2007; Z. Chen et al., Reference Chen, Bengtson, Zhou, Hua and Yue2008; Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010). Although not supported by geochemical analyses and without recognizing the original composition, Sinotubulites is maintained to be biomineralized and having shell (Cai et al., Reference Cai, Xiao, Hua and Yuan2015; Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019).
Gaojiashania Yang et al., 1986 (in Liu et al., Reference Lin, Zhang and Zhang1986; Chen et al., Reference Chen, Sun and Hua2002; Cai and Hua, Reference Cai and Hua2011) is another tubular fossil with external circular rings on the tube surface that may have been articulated (Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010). Gaojiashania is preserved as carbonaceous compressions, or pyritized or glauconized compressions, that appear to have organic or weakly mineralized skeleton (Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010) yet without reference to primary mineral.
Shaanxilithes Xiang et al., Reference Xiang, Ding, Luo, He and Wang1984, forms ribbon-like compression fossils with closely spaced annulations (Hua et al., Reference Hua, Chen and Zhang2004; Shen et al., Reference Shen, Xiao, Dong, Zhou and Liu2007; Z. Zhang et al., Reference Zhang, Hua and Zhang2015). The original structure may have been a cylindrical tube (Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010) that was originally organic (Meyer et al., Reference Meyer, Schiffbauer, Xiao, Cai and Hua2012; Tarhan et al., Reference Tarhan, Hughes, Myrow, Bhargava, Ahluwalia and Kudryavtsev2014), but often found coated with clay minerals (Hua et al., Reference Hua, Chen and Zhang2004, Reference Hua, Chen and Yuan2007). Regardless of the uncertainty as to the morphology of elements forming the annulation (individual elements or thickenings of the wall, nested or stacked), this taxon may resemble Anulitubus n. gen. only in having the original cylindrical shape.
Holotype
Specimen PMU 34736; illustrated in Figs. 4, 5.

Figure 4. Macroscopic, mineralized tubular annulate Anulitubus formosus n. gen. n. sp. Moczydłowska in Moczydłowska et al., holotype specimen PMU 34736. Images from RLM (1–4) showing (1) front side view, (2) opposite side view, (3, 4) apertural views with free lumen and visible incomplete septum. Images from STEM (5–7) showing (5) front side view, (6) opposite side, and (7) aperture. Scale bars = 1 mm.

Figure 5. Macroscopic, mineralized tubular annulate Anulitubus formosus n. gen. n. sp. Moczydłowska in Moczydłowska et al. holotype specimen PMU 34736. Images from RLM in (1), and CT in (2–7); (1, 2) front view with three annuli, two grooves visible on the outer surface, and one groove on the inner surface within the lumen; (3) apertural view showing incomplete septum with central opening; (4) front view with lower portion that is embedded in the sediment (marked in gray color), showing the first annular segment together with the basal cup-shaped element; (5) mirror orientation of image in (4) with sediment entombing the specimen, but clearly showing the central knob at the tube base; (6, 7) mirror images of the cup-shaped element displaying the concave surface facing the tube lumen and radial symmetry. Scale bars in (1–7) are 1 mm.
Diagnosis
Macroscopic, cylindrical tubular, annulate body fossil having rigid, siliceous biomineralized and thick wall, circular cross section and open aperture at its distal portion. The proximal portion is closed and rounded. The tube consists of ring-shaped segments (annuli) that are of the same diameter and stacked vertically one upon the other. The annuli are defined by incised sutures (grooves) on the outer and inner surfaces of the wall. The sutures are transverse to the tube long axis and parallel. Incomplete septa within the lumen are located between the annuli and possess small opening in the center.
Description
The tubular body fossil is 7.5–8.0 mm in total length and the portion exposed from the sediment is 4.0–5.0 mm, whereas the additional portion visible in the CT image is 2.5–3.0 mm in length (Figs. 4.1, 4.2, 4.5, 4.6, 5.4). Circular in cross section, the tube has an outer diameter of 4.2 × 5.2 mm and its wall thickness around the aperture is 0.5–0.7 mm including the outer edge of wall ~100–135 μm in thickness (Figs. 4.4, 7). The wall has a constant thickness along the length of tube and the wall is single-layered. Three annuli are separated by grooves that correspond to one another on inside and outside of the wall, but the wall is continuous (Figs. 4.1–4.4, 5.1, 5.2). The grooves are slightly undulate around the wall circumference, causing the length of individual annuli to vary insignificantly, but retaining overall similarity in size range (Figs. 4.1, 4.2, 4.5, 4.6, 5.1, 5.4). Each individual annular segment length is 1.0–1.5 mm, and the groove length is 0.2 mm (N = 1). The diameter of the tube lumen (empty cavity embraced by the wall) is 3.2–4.0 mm. An incomplete septum with a central opening is seen within the lumen through the tube aperture and is located between the annuli (Figs. 4.3, 4.4, 4.7, 5.3). The incomplete septum divides the tube lumen into compartments or chambers, which are equivalent to the space embraced by the individual segment and are interconnected by the septal opening. The proximal portion of the tube is rounded and formed by a shallow, solid, cup-shaped element with a central knob directed outwards, while the inwards-oriented surface of this element is concave and meniscus-like (Fig. 5.4–5.7). The cup element diameter is 4.6 mm, and its height is 2.5–3.0 mm.
The tubular wall symmetry is radial and there is no differentiation in its morphology and construction with regard to tube sides that might be interpreted as the ventral or dorsal side. The position of the distal open circular aperture relative to the closed proximal end with a central knob indicates the distinctive axis and the longitudinal or even posterior-anterior symmetry of the tube and its polarity.
Etymology
From Latin forma, f., shape, figure, model, beauty; formosus, beautifully formed. Referring to “beautifully shaped annulate tube.”
Material
A single 3-D preserved specimen.
Preservation
The holotype of Anulitubus formosus n. gen. n. sp. has been exposed through natural weathering, although the proximal extremity is still entombed in rock matrix (Fig. 5.1, 5.2, 5.4, 5.5). It was preserved on the bedding plane with the sediment laminae plastically bending around it (Fig. 4.1, 4.3). The oval cross-section of the aperture suggests limited compaction (Figs. 4.7, 5.3). The specimen is relatively short, consisting of three segments, and it could be considered as incomplete. However, the open distal portion of the rigid wall is very regular, without any breakage scars (Figs. 4.3, 4.4, 5.1–5.3), and is assumed to be the original aperture of the tube. The rounded portion that is entombed in the sediment is the original, closed proximal end of the body (Fig. 5.2, 5.4–5.7).
The shagrinate wall surface around the aperture (Fig. 4.1, 4.2) is covered by clay grains that include chlorite laths and some quartz microcrystals. These derive from clay fabric in the surrounding sediment and/or are diagenetic precipitates. The presence of aluminosilicate minerals is also confirmed by STEM EDS spectra (Fig. 6) and by the lathe-like crystallographic habit of chlorite and cubic and prismatic quartz microcrystals in our STEM images (not illustrated). However, the wall surface was originally smooth, as observed in CT images in which the material of the wall is distinguished from the sediment particles (Fig. 5), and geochemical analyses demonstrate that it is siliceous (see below).

Figure 6. Wall composition of Anulitubus formosus n. gen. n. sp. Moczydłowska in Moczydłowska et al., holotype specimen PMU 34736, shown by elemental content analyzed by STEM EDS on the wall surface in (1) and the incomplete septum in (2). Measured points are marked by red asterisks. The point spectra are characteristic of silica forming the wall with high content of element carbon indigenous to the wall and remnant of organic matter.
The almost un-deformed and non-fractured tubular wall with an empty lumen indicates that the wall must have been originally robust—strong enough to resist burial conditions and retain its original 3-D form.
Wall construction and composition
Having just a single specimen, it was inappropriate to section the tube wall to examine its internal construction and material composition, yet the available information from analyses on its surface and notable from the CT images is fairly conclusive. The CT images proved to be indispensable in revealing the wall construction of the tube portion that is concealed by the entombing sediment. They show not only the shape and closed proximal end, but also its internal construction, which allows inferences on the initial life history of the organism. The wall structure depicted in CT images (Fig. 5.2–5.7) is certainly single-layered and sharply differentiated into annular segments by grooves that show consistently their shape, dimensions, and 3-D special arrangement. Wall thickness and the diameter are constant (Fig. 5.2, 5.3). The annular segments have a smooth surface and appear solid in texture, whereas the grooves are more scabrous in texture, but clearly the wall is continuous (Fig. 5.2, 5.4). The proximal rounded portion of the tube is formed by the same material as the tube wall (Fig. 5.4–5.7). The cup-shaped element at the base of tube has circular cross section, concave surface, and pronounced central knob with its positive relief directed downwards along the tube (Fig. 5.4, 5.6, 5.7). This element is an integral part of the tube, of almost the same diameter as the overlying stacked ring-shaped segments (annuli); it was the initial element in the tube construction and its robust base.
As observed in RLM, STEM, and CT images, and inferred from the state of preservation and wall mechanical properties, the material forming the wall was not morphoplastic (flexible), as it would be if it were organic, but rigid and originally biomineralized, as confirmed by geochemical analyses.
The STEM EDS analyses performed on the outer surface of the wall below the aperture and on the incomplete septum (Fig. 6.1, 6.2) show the elemental content by the total weight percentage (Wt %). The point spectrum of the wall quantified the very high content of carbon (C, 41.06%), oxygen (O, 13.37%), silicon (Si, 16.18%), and iron (Fe, 10.96%) (Fig. 6.1). Accessory elements with lower contents are aluminum (Al), magnesium (Mg), chlorine (Cl), and sodium (Na), ranging from 1.14–5.37%. The presence of nitrogen (N, 5.56%) is significant because of its potential bonds with other elements in forming organic matter (Fig. 6.1). The elemental spectrum of incomplete septum shows much lower content of C (5.16%), higher of O (42.26%), and no nitrogen, whereas Si is comparable (14.24%) and metal elements are relatively higher in their contents: Fe (15.76%), Al (12.47%), and Mg (10.10%) (Fig. 6.2). The lower content of C in incomplete septum might be due to the lower organic-richness of this morphologic element or it may be preservation bias. The high content of C in the wall and remarkable lack of calcium (Ca) in the spectra, as well as the absence of carbonate minerals or calcareous cement in the host sediment, which could provide potential contamination by C, suggest that C is indigenous to the fossil wall. The elements C, O, and N are typical constituents of organic matter, and their association in the observed spectra suggests that the organic matrix of the wall is preserved. Nitrate minerals are not recorded in the studied succession and N is not diagenetically induced or a contaminant in the specimen wall. The elements Si and O are typically combined in the mineral opaline silica (silica, silicon dioxide, SiO2), which is further confirmed by the Laser-Raman spectroscopy. The elements Fe, Al, Mg, and less abundant Na and Cl are components of the wall and incomplete septum spectra and are interpreted as derived from the aluminosilicate minerals and iron oxides attached to the specimen in parts and present in the burial environment.
The STEM EDS elemental mapping of the wall transverse plane displayed predominantly Si and O, and additionally Mg, Fe, and Cl within the wall and across its thickness (Fig. 7.1–7.5). The elemental map of the incomplete septum within the tube lumen shows clearly Si and O contents (Fig. 7.6). The abundance and uniform and continuous distribution pattern of Si and O in all measured points and in different parts of the specimen (several, although not illustrated) are compelling evidence for silica forming the wall. The accessory elements (Mg, Fe, Cl) are derived from the diagenetic minerals attached to the wall. The distribution pattern of elements in the wall is homogeneous and shows that the wall was composed of material that was uniform in structure and density, without layering, and not incorporating any macrocrystals, grains, or mineral fragments.

Figure 7. The wall composition of Anulitubus formosus n. gen. n. sp. Moczydłowska in Moczydłowska et al., holotype specimen PMU 34736, shown by elemental mapping analyzed by STEM EDS on the wall aperture (1–5) and the incomplete septum surface (6). Asterisk in (5) indicates the measurement site of incomplete septum in (6). The intensity and homogenous distribution of Si and O are consistent with siliceous wall and incomplete septum, with some accessory elements derived from the host sediment. Scale bar in 1 for all images = 1 mm.
The lack of elemental Ca, phosphorus (P), and sulphur (S) in the wall spectra indicates the absence of carbonate, phosphate, and sulphate minerals, respectively, in the wall as potential constituents of the skeleton and rules-out the possibility of such primary minerals being diagenetically permineralized by silica.
The Laser-Raman spectra acquired on the wall surface at several points are recognized as quartz (crystalized SiO2) and anatase (titanium dioxide, TiO2) minerals, and as carbonaceous material by the disordered (D-) and graphitic (G-) bands characteristic for sp2-bonded, graphitic carbon (Fig. 8; Supplementary Data). The quartz signature in the fossil wall is strong and differs clearly from that of the host sediment, which was examined for comparison, and its intensity distinguishes the primary mineralogy of the fossil from the minor detrital and diagenetic quartz in the sediment (see below and under Coniculus n. gen. and Fistula n. gen.; Figs. 14, 15, Reference Butterfield21; Supplementary Data). The wall was originally composed of silica and diagenetically transformed into a microcrystalline quartz phase, while anatase was derived from the depositional environments. The carbonaceous material preserved within the mineralized wall is confirmed to be the wall's integral component, because it was detected by STEM EDS analysis, and constituted the organic matrix for precipitation of silica in the process of biomineralization (see under Skeletal biomineralization).

Figure 8. The wall composition of Anulitubus formosus n. gen. n. sp. Moczydłowska in Moczydłowska et al. revealed by the Laser-Raman spectra acquired on the wall surface in several points and showing the signatures of quartz and anatase minerals, and spectral disordered D-band and graphitic G-band of carbonaceous material that is admixed in the mineralized wall. Holotype specimen PMU 34736 illustrated in Figure 4. The specimen wall is composed of quartz that is recrystallized from the original amorphous opaline silica into microcrystalline phase during diagenesis. The anatase derived from the burial environment and is a contaminant from microcrystals attached on the specimen wall surface. The green and orange color tick marks are the positions of strong Raman peaks of anatase (TiO2, RRUFF ID: R060277) and quartz (SiO2, RRUFF ID: R040031), respectively.
Anatase is a common accessory mineral in siliciclastic rocks and it likely has a diagenetic origin from muscovite alteration in the Indreelva sediment. Anatase that is recorded on the fossils’ surface is a contaminant from the entombing sediment, but it is also possible that it could derive from the biogenically mediated crystallization on the decaying organic matter substrate during burial (Supplementary Data).
Diagenetic alteration via calcification, phosphatization, pyritization, and aluminosilicification, which is typical of the terminal Ediacaran body fossils (compare Cai and Hua, Reference Cai and Hua2007; Cortijo et al., Reference Cortijo, Martí Mus, Jensen and Palacios2010, Reference Cortijo, Cai, Hua, Schiffbauer and Xiao2015; Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2012, Reference Cai, Hua, Schiffbauer, Sun and Yuan2014; Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012; Schiffbauer et al., Reference Schiffbauer, Xiao, Cai, Wallace, Hua, Hunter, Xu, Peng and Kaufman2014), does not occur in the Digermulen Peninsula succession. Silica was the original mineral in the wall of studied fossils and the host sediment cement is only insignificantly silicified (Meinhold et al., Reference Meinhold, Wemmer, Högström, Ebbestad, Jensen, Palacios, Høyberget, Agić and Taylor2019a; Supplementary Data).
Remarks
As aforementioned for the new monotypic genus, its type species Anulitubus formosus n. gen. n. sp. is dissimilar to any known Ediacaran body fossil by the spatial arrangement of annular segments and by the presence of a cup-shaped element at the proximal part of the tube.
Genus Coniculus new genus Moczydłowska in Moczydłowska et al.
Type species
Coniculus elegantis n. gen. n. sp. Moczydłowska in Moczydłowska et al.
Diagnosis
As for the type species.
Etymology
From Latin conus-i, m.; cuniculus, m., cone. Referring to conical shape of the wall.
Remarks
The new monotypic genus differs from other Ediacaran macroscopic conical fossils, either organic or biomineralized, by the morphologic simplicity of its conical, smooth on the surface, and biomineralized wall that is double-layered and siliceous. Coniculus n. sp. may resemble superficially Multiconotubus Cai et al., Reference Cai, Cortijo, Schiffbauer and Hua2017, which is a multilayered, “conical tube” with open aperture and closed “apical” end (it is proximal) and consists of fully nested cone-shaped layers that are biomineralized (Cai et al., Reference Cai, Cortijo, Schiffbauer and Hua2017). The funnel-shaped and tapering specimens of Multiconotubus are smooth on the surface, and has cone-in-cone structure (Cai et al., Reference Cai, Cortijo, Schiffbauer and Hua2017, fig. 8). However, the phosphatized specimens are fragmentarily preserved and without their terminations, and thus document neither the apertural and proximal ends, nor the proximal end being closed and interpreted as anchoring apparatus by Cai et al. (Reference Cai, Cortijo, Schiffbauer and Hua2017). The morphology of the entire body remains uncertain, as is the primary mineralogical composition in assumed biomineralized wall.
Coniculus elegantis new species Moczydłowska in Moczydłowska et al.
Figures 9–11
Holotype
Specimen PMU 34737; illustrated in Figures 9, 10.

Figure 9. Macroscopic, mineralized conical Coniculus elegantis n. gen. n. sp. Moczydłowska in Moczydłowska et al., holotype specimen PMU 34737. (1, 2) Images from RLM in a front side view; (3) opposite side view; and (4, 5) apertural view. Distinct outer veneer layer of the wall is seen as a sharp outline in the apertural, circular cross sections (4, 5) and can be chipped off at the edge (1, 5). The thicker inner wall layer is seen around the aperture (1, 4, 5) and the lumen is infilled with sediment in the lower portion (5). The end of cone is entombed in the sediment at approximate level marked by white arrow in (2) and recognized by the relief and by comparison with CT images. Scale bars for (1–3) = 6 mm, and for (4, 5) = 1 mm.

Figure 10. Coniculus elegantis n. gen. n. sp. Moczydłowska in Moczydłowska et al. CT images in a front side view of the specimen (1) and a mirror and turned side view (2) showing constant wall thickness and sharp edges, and the lumen lower part infilled with the sediment (yellow color in images). Scale bar = 5 mm.
Diagnosis
Macroscopic, elongate conical, thick-walled siliceous biomineralized skeleton with open circular aperture and narrow, closed proximal portion. The cone wall is double-layered and consists of an outer, very thin compact layer (veneer layer) with distinct smooth, glossy surface, and an inner, less-dense and thicker layer. Both layers are of constant thicknesses. The cone lumen is hollow.
Description
Conical body fossil is 12–13 mm in total length and its widely open distal aperture is at maximum 4.0 x 5.0 mm in diameter. The cone tapers sharply to the narrow proximal end that is 1.5–2.0 mm in width, as seen in CT images (Fig. 10), but is also recognizable by topological relief from the entombing sediment (Figs. 9.1, 9.2, 11.2). The smooth-surfaced conical wall is stiff, solid, mineralized, and slightly concave at the aperture; it embraces a hollow central lumen (Fig. 9.4, 9.5).

Figure 11. Coniculus elegantis n. gen. n. sp. Moczydłowska in Moczydłowska et al. Images from RLM (1–5) and STEM (6). Conical wall with tapered closed proximal ends that are entombed in sediment (1, 2) or broken apart (3), and apertural ends showing sharp-edged outer veneer layer (4, 5). Lumen is infilled with host sediment (4, 5). The surface of specimens may be partly coated by the sediment aluminosilicates, including chlorite (6, enlarged from 1, right side). White arrow in (2) indicates position of proximal end. Specimens PMU 34738 (1), PMU 34740 (2), PMU 34739 (3–5). Scale bars in (1–5) = 2 mm, in (6) = 3 μm.
The wall total thickness is 0.5–1.0 mm, including the distinct outer veneer layer of the wall that is 0.1–0.3 mm in thickness and is observed in cross sections of apertural or broken portions in all specimens (Figs. 9.1, 9.4, 9.5, 10, 11.1, 11.2, 11.4, 11.5). The veneer layer forms a sharp outline of the wall at the aperture or sharp edge when broken, and covers the cone. The wall inner layer appears as a narrow circular belt in the apertural end of the cone and in sections alongside the cone (Figs. 9.1, 9.4, 9.5, 11.4).
Observed in the CT vertical transect image (Fig. 10), the upper portion of the cone lumen, ~5.7 mm in length, has a rounded bottom and is the sediment-infilled surface seen also through the aperture of the specimen. The sediment infilling continues from this surface to a narrower conical extension of the lumen, ~2 mm in length, and continues towards the proximal end of the cone. The wall section is sharp in appearance and of constant thickness.
The holotype dimensions are: conical wall length 12.0–13.0 mm; outer diameter at aperture 4.0 x 5.0 mm; diameter at proximal portion 2.0 mm; wall total thickness 0.6–0.8 mm (600–800 μm); wall outer veneer layer thickness 0.1–0.2 mm (100–200 μm); lumen diameter at aperture 3.5 × 4.0 mm. Dimensions of other, fragmentary preserved specimens are: wall length is 10.0–13.0 mm; outer apertural diameter 2.5 × 3.2 mm to 3.0 × 4.0 mm; diameter at proximal portion 1.5 × 2.0 mm to 2.0 × 3.0 mm; wall total thickness 0.5–1.0 mm; wall outer veneer layer thickness 0.2–0.3 mm (N = 3).
Etymology
From Latin elegans, -antis, adj., fine, elegant. Referring to very regular shape of the conical wall (“a little elegant cone”).
Materials
Four 3-D preserved specimens. Additional to the holotype are specimens: PMU 34738, 34739, and 34740, illustrated in Figure 11.
Preservation
The holotype is very well preserved, only minimally flattened, and preserving a hollow central lumen (Fig. 9.1, 9.4, 9.5). The lumen is filled in with host sediment only in its lower portion (Figs. 9.4, 10). This proximal portion of the specimen is entombed in the red-brownish host sediment (Fig. 9.1–9.3), but is distinguished by the relief of dark gray color and glossy surface of the cone wall forming the narrow end (Fig. 9.1, 9.2). The cone surface is encrusted in small patches by chlorite, the early diagenetic clay mineral recognized in STEM images (Fig. 11.6).
Three other specimens are preserved with sediment chips attached to their surface or covering part of the cone (Fig. 11.1–11.3), and one specimen has a broken proximal portion (Fig. 11.3). They have tapering proximal portions (Fig. 11.1, 11.2), and their diameters are in the same range. The lengths of the specimens are comparable, although uncertain in a total range because of the fragmentary preservation. The specimens have their lumina filled with host sediment. The broken proximal portion shows the section of the mineralized wall in contrast to the lumen infilling by sediment and recrystallized quartz, and demonstrates that the lumen extended down from the aperture throughout the entire cone (Fig. 11.3–11.5). The constant wall thickness is visible in cross-sections of all specimens. The outer veneer layer of the wall is well preserved under the peeled-off sediment that covered the specimen surface (Fig. 11.1, upper part) as a smooth surface and continued into a sharp edge. When fractured on the side of the cone, the veneer layer is exposed as a sharply outlined scar, in contrast to the underlying inner layer of the wall with a less-dense texture (Fig. 11.2). The texture of the inner layer is poorly observed because the specimens’ cross sections are mostly covered by clay minerals, but this layer is recognizable by its constant thickness around the aperture, and clear delimitation from the veneer layer (Figs. 9.1, 9.4, 9.5, 11.1, 11.2).
All specimens are slightly compressed parallel to the bedding and cleavage planes. Their apertures are oval (4.0 × 5.0 mm, 3.7 × 4.0 mm, 3.0 × 4.0 mm, 2.5 × 3.2 mm) and their conical walls are only slightly deformed. The specimens were originally circular in cross-section and regularly conical, and show brittle fracturing of the wall (Figs. 10, 11.1, 11.2).
Wall construction and composition
Morphological features and the state of preservation reveal a double-layered wall in which the layers are distinguished by their texture and constant thicknesses. The glossy veneer layer with a sharp edge at the aperture formed a compact surface layer, and the inner thicker layer provided substantial robustness to the wall. Both layers constructed the resistant skeleton of the organism.
For identification of the wall composition, a simple chemical test by controlled dropping of concentrated (45%) hydrofluoric acid (HF) on the exposed wall (Fig. 11.3) resulted in rapid corrosion of the surface, which is consistent with dissolving silica from the wall. The same treatment of the sediment was almost non-reactive. The reaction to hydrochloric acid (10% HCl) applied on the specimen and the entombing sediment was negative (not dissolving minerals) and excludes the presence of carbonate minerals, which is independently proven by the lack of Ca in elemental spectra of the tube wall (see below). The siliceous composition of the wall is affirmed by the STEM EDS and EMP EDS analyses and the Laser-Raman spectroscopy.
The STEM EDS elemental content was measured on the wall surface and the wall apertural cross section of the holotype (Fig. 9.1, 9.2) by the total weight percentage (Wt %). The point spectrum on the wall section shows the high content of C (33.31%), O (37.88%), Si (13.60%), and N (8.20%) (Fig. 12.1). Additional elements, such as Cl, manganese (Mn), Al, and Mg are in lower contents, between <1.00% and <3.00%, and the sulphur (S) content is only 0.33% (Fig. 12.1). The point measured on the wall surface also shows high content of C (19.06%), O (40.02%), and Si (9.59%) but not N (Fig. 12.2). The elements Fe (19.48%), Al (6.81%), and Mg (4.47%) are also significant components. The elemental spectrum acquired on another point of the wall surface showed similar content of C (16.99%), O (43.97%), and silicon (10.91%), and less abundant Fe (14.95%), Al (8.95%), Mg (3.10%) and potassium (K; 1.12%) (not illustrated).

Figure 12. The wall composition of the Coniculus elegantis n. gen. n. sp. Moczydłowska in Moczydłowska et al., holotype specimen PMU 3434737 analyzed by STEM EDS on the cross section of the wall inner layer (1) and wall surface veneer layer (2). The highest elemental content of Si and O is indicative of siliceous composition with admixture of C and N from organic matrix. Red asterisks mark the measurement points on the insets of STEM images. Scale bars in STEM images = 1 mm.
The high content of Si and O in all measured points is conclusive evidence of the siliceous composition of the wall. Carbonate minerals are absent in the specimen wall and in the sediment (HCl test; Ca absence in the wall elemental EDS spectra; XRD spectra of the sediment; Figs. 12, 21; Supplementary Data). Because of this absence, the abundance of C in the wall is inferred to be indigenous. The abundance and elemental association of C, O, and N indicate an organic matrix within the siliceous wall, comparable to what is inferred for Anulitubus n. gen. The substantially higher content of C observed in the wall inner layer (apertural section; Fig. 12.1) than in the veneer layer (wall surface; Fig. 12.2) may not be a random or biased occurrence, but may be explained by a predominant occurrence of organic matrix in the inner layer. This layer has a less dense texture and might have had higher content of organic matrix in comparison with the compact veneer layer. The substantial amounts of the metal elements, including Fe, Al, Mg, Mn, and sodium (Na), in the wall are inferred to be derived from the aluminosilicate minerals and iron oxides present in the burial environments.
The Laser-Raman spectra acquired in three points on the wall of holotype indicate quartz and anatase signatures (Fig. 13; Supplementary Data), and prove that the wall (Figs. 9, 10) was composed of silica (presently in quartz phase), whereas anatase originated from the host sediment or precipitated from the depositional environments (see also under Anulitubus n. gen.).

Figure 13. The wall composition of the Coniculus elegantis n. gen. n. sp. Moczydłowska in Moczydłowska et al., holotype specimen PMU 34737, illustrated in Figure 9, shown by the Laser-Raman point spectra from the wall surface in the distal and proximal portions and characteristic signatures of quartz and anatase minerals. The wall was siliceous and anatase derived from the burial environment. The blue and orange tick marks are the positions of strong Raman peaks of anatase (TiO2, RRUFF ID: R060277) and quartz (SiO2, RRUFF ID: R040031), respectively.
The wall of Coniculus n. gen. examined in petrographic thin section in light microscopy parallel light and with crossed polarizers (Fig. 14.1, 14.2, respectively) appears to be composed of a fine-grained mixture dominated by opal (amorphous silica), quartz, and seldom sheet silicates. The outer veneer layer is compact and sharp in line, as seen in the vertical section, and the underlying inner layer is a mixture of opal and very fine microcrystalline quartz and silicates. The lumen is infilled by the host sediment and is fractured by secondary chalcedony veins; the broken apertural portion is massively covered by elongated crystals of secondary chalcedony (Fig. 14.1, 14.2, upper edge of specimen). There is no sign of any pseudomorphs, replication by other minerals, or surface encrusting. Subsequent EDS survey using EMP of the same thin section confirmed that the wall is mainly composed of pure silica with admixture of a clay-like phase. The infill of the lumen is dominated by the crystallized secondary silica (chalcedony).

Figure 14. Petrographic thin section images of Coniculus n. gen. Moczydłowska in Moczydłowska et al. (PMU 34739), (1) parallel light, (2) crossed polarizers, and Fistula n. gen. Moczydłowska in Moczydłowska et al. (PMU 34744), (3, 5) parallel light and (4, 6) crossed polarizers, in longitudinal sections showing two layers of the wall and internal lumen infilled by host sediment. The wall layers are composed of microcrystalline phases dominated by silica (quartz, opal) and minor sheet silicates. The infilling of lumen is penetrated by coarse chalcedony veins. The outer veneer layer (dark line) is thin and compact and the inner layer (light gray band) is wide and microcrystalline. The aperture of Coniculus n. gen. Moczydłowska in Moczydłowska et al. (1, 2) shown in the upper edge of the specimen is infilled by elongated crystals of chalcedony. In Fistula n. gen. Moczydłowska in Moczydłowska et al. (3, 4) lumen is shown at the right side, to the left is wall with internal and veneer layers, and a chip of host sediment attached on the surface, respectively. The opposite side of specimen in (5, 6), the lumen with chalcedony veins is at left side, then inner layer and veneer layers to the right. Numbers with arrows in yellow color in (6) refer to EMP EDS analyses shown in Figure 15.
Remarks
The new species is dissimilar to any previously described Ediacaran body fossils by its very regular shape of the conical wall, being straight along the long axis, and its original siliceous mineralogy of the cone.
Genus Fistula new genus Moczydłowska in Moczydłowska et al.
Type species
Fistula crenulata n. gen. n. sp. Moczydłowska in Moczydłowska et al.
Diagnosis
As for the type species.
Etymology
From Latin fistula-ae, f., tube, pipe. Referring to the shape of tubular fossil that is formed by a cylindrical wall of a constant thickness and enclosing a free lumen.
Remarks
This new monotypic genus differs from other Ediacaran macroscopic tubular fossils by having a genuinely cylindrical shape and a remarkably thick wall that is additionally double-layered and siliceous. The tube surface bears wrinkles and sutures marking segments, but is not annulated.
The new genus differs from Coniculus n. gen., which also has a double-layered wall with conspicuous veneer layer and is siliceous, by its cylindrical habit, crenulated surface, and segment sutures in contrast to the conical and smooth surface on the latter taxon.
Fistula crenulata new species Moczydłowska in Moczydłowska et al.
Figures 15–17
Holotype
Specimen PMU 34743, illustrated in Figure 16.1, 16.2; Paratype PMU 34748, illustrated in Figure 17.2, 17.4.

Figure 15. Analyses of elemental composition and mineralogy of Fistula n. gen. Moczydłowska in Moczydłowska et al. wall and lumen infilling minerals by a field emission electron probe microanalyzer equipped with energy-dispersive spectroscopy detector (EMP EDS) (1–6). (1–4) Minerals infilling the fossil lumen are diagenetic and comprise the first generation of Ca-carbonate (calcite) associated with Mn-oxide (1, 2) and silica that are, in turn, partially replaced by the second generation of silica (chalcedony) (4), Ca-carbonate (calcite) and barite (3). (5, 6) The fossil wall is composed of pure silica (5) with an admixture of a Mg-Fe aluminosilicate, probably mixed-layer clay mineral (5, 6).

Figure 16. Macroscopic, mineralized cylindrical and segmented Fistula crenulata n. gen. n. sp. Moczydłowska in Moczydłowska et al. All are RLM images. (1, 2) Holotype specimen PMU 34743, side view in (1) with a sediment chip at left lower portion, and apertural view in (2) showing the sharp edge of the wall outer veneer layer and the thick inner layer around free lumen. (3, 5) Specimen PMU 34744, showing the faint crenulation on wall surface and two wall layers; the sediment chip is attached to the wall surface on the right side and is clearly distinguishable from the veneer layer; white arrows point to the surface wrinkles. (4, 6) Specimen PMU 34741 showing the groove between the segments on the wall surface (6) and the two wall layers in cross section (4); the sediment is attached to the left side of specimen (black triangles) and easily recognizable from the specimen surface by peeling off (4, 6); black asterisks indicate outer veneer layer, and black dots the wall inner layer. Specimen cross sections demonstrate the radial symmetry of cylindrical exoskeletons. Scale bars = 5 mm.

Figure 17. Macroscopic, mineralized, cylindrical, and segmented Fistula crenulata n. gen. n. sp. Moczydłowska in Moczydłowska et al. All are RLM images with the exception of CT image in (6). (1) Specimen PMU 34749, in major part exposed from the reddish sediment; the largest specimen with surface crenulation (faint wrinkles indicated by white arrows); circular cross section on the left end; covered by diagenetically crystallized quartz (white color). (2, 4) Paratype specimen PMU 34748 lying on the bedding plane and demonstrating longitudinal polarity; wall segments delimited by incised sutures (4); proximal end is rounded (2, bottom), and aperture is open (2, upper part). (3, 5, 6) Specimen PMU 34742, showing cylindrical exoskeleton with segments (5, 6), circular cross section (3), and constant wall thickness at fractures between the segments (6); free lumen is infilled with sediment and visible under the fractured wall (6) and at the apertural end covered by diagenetic quartz (5, left end, white in color). (7, 8) Specimen PMU 34745, undeformed and circular in cross section demonstrating its radial symmetry (8), lying on the bedding plane and partly exposed from the host sediment, with sediment layers bent around specimen (8); both wall layers are visible and lumen is infilled with sediment. Scale bars = 5 mm for all images.
Diagnosis
Macroscopic, cylindrical in shape body fossil with siliceous biomineralized, thick, double-layered wall, bearing faint wrinkles and narrow incised sutures (grooves) on the surface, which define segments, and having a hollow lumen that is circular in cross section. The wall surface is smooth, glossy, and slightly crenulate; the wrinkles and sutures are transverse to the tube long axis and relatively regularly distributed. The proximal portion of the tube is rounded and closed, but its apertural portion is open and circular in outline. The tube wall consists of an outer, very thin, veneer-like layer that has a distinctive compact texture; the inner layer is thicker and less dense in substance. The two layers of the wall are constant in their thicknesses along the tube length.
Description
Tubular and cylindrical body fossil of constant diameter with hollow lumen, opened aperture, and closed proximal end (Figs. 16.1, 16.2, 17.2, 18.3, 18.4). The tube wall is double-layered and its radial symmetry is demonstrated by circular cross sections in undeformed specimens (Figs. 16.2, 17.7, 17.8). The tube, along its long axis from aperture to proximal end, shows morphological polarity (Figs. 17.2, 18). The tube total length in complete specimens is 50–73 mm; diameter 6–10 mm; wall thickness 2.0–2.3 mm, including the outer veneer layer 0.1–0.3 mm in thickness; lumen diameter 3.3–4.0 mm (may be distorted up to 7.7 mm). The two layers of the wall are perfectly aligned and constant in their thickness, but the outer veneer layer has a much denser texture than the inner and thicker layer (Figs. 16.2, 16.3, 17.8, 18). The tube segments that are delimited by the surface suture grooves are of similar length (~7.0 mm) and distributed at ~7.0 mm distances (Fig. 17.2, 17.4, 17.5). Surface crenulation and wrinkles are observed in some specimens (Figs. 16.1, 16.5, 17.1), and wrinkles are 1.0–3.0 mm in length and at intervals of 2.0–3.0 mm, occasionally 7.0–8.0 mm.

Figure 18. CT images of the Fistula crenulata n. gen. n. sp. Moczydłowska in Moczydłowska et al., holotype specimen PMU 34743, revealing the internal features of the tube wall and its two layers. Sediment partly covering the specimen in front view is gray in color in (1, 2), as well as infilling the breakage of the wall, which is blue in color (4). The wall is marked by artificial colors: outer veneer layer is gray in (3, 4); wall inner layer is green in (2–4). The knob-like structure is visible at the tube bottom (3, 4) and broken portion of the veneer layer on the left side in (3). The central lumen infilled with the sediment appears as vertically stacked yellow bands in (4). Scale bars equal 5 mm.
The internal features, seen only in CT images, are the knob-like structure at the bottom of the tube and the hollow lumen extending throughout the entire tube, although infilled by sediment in the lower portion (Fig. 18.2–18.4). Another specimen (PMU 34742), studied under RLM (Fig. 16.5) and by CT (Fig. 17.6), shows the fractured wall along the suture and demonstrates its constant thickness and sharp edges reflecting its rigidity, as well as sediment infilling the lumen.
The measurements of specimens compressed from their original circular cross sections are slightly distorted and given as ranges. The dimensions of specimens are: in holotype, the tube length is 14.0–15.0 mm; tube diameter 7.0 × 8.0 mm; total wall thickness at cross section 2.0–2.25 mm; wall outer veneer layer thickness 0.1 mm (RLM) or 50–100 μm (STEM); tube lumen diameter 3.25 × 4.0 mm; individual segment length ~7.0 mm. The largest and most complete specimen (PMU 34749) has a tube length of 73 mm; tube diameter 8.0–10.0 mm; wrinkles length 1.0 mm; distance between wrinkles 2.0–3.0 mm; distance between segment sutures ~7.0 mm. In other, fragmented specimens, the tube partial length is 10.0–50.0 mm; tube width in side view 5.0–10.0 mm (compressed); tube diameter 6.0–9.0 mm; wall inner layer thickness 2.0–2.3 mm; wall outer veneer layer thickness 0.1–0.3 mm; lumen diameter 2.15–7.75 mm; wrinkles length 1.0–3.0 mm; distance between wrinkles 2.0–3.0 mm to 7.0–8.0 mm (when distorted); (N = 15).
Etymology
From Latin crenulatus-a, minutely crenate, crenulate, crenulated, and meaning of having an irregular wavy outline. Referring to a slightly crenulated cylindrical wall with perpendicular faint wrinkles on the wall surface (“minutely crenulated tube”).
Materials
Seventeen 3-D preserved specimens.
Preservation
Specimens are slightly compressed to varying degrees and may show circular cross-section on one end and more oval on the other. However, their tubular walls are rigid and sharply fractured rather than showing ductile deformation. They were buried as lying on the bedding planes or obliquely entombed within the sediment layers, but probably not transported any significant distance. There are no signs of bottom currents or wave action in the host sediment, nor any sedimentary structures indicative of transport.
The holotype specimen is preserved with its proximal portion, but the apertural portion is likely broken away (Figs. 16.1, 16.2, 18). Two specimens (PMU 34749 and 34745) are undeformed and circular in cross section with their diameters 10 mm and 9 mm, respectively (Fig. 17.1, 17.7, 17.8). The complete specimen that is preserved intact shows the maximum length and diameter known for the species (PMU 34749; Fig. 17.1).
Three preservational variants are observed among specimens attributed to Fistula crenulata n. gen. n. sp., reflecting their completeness or selective fragmentation, preservation state of being hollow inside or filled in with the host sediment, and being exposed from or partly entombed in the sediment with resultant obscuring of certain morphological features. Preservation variant 1 (Fig. 17.1, 17.2, 17.4; N = 2) is represented by complete, cylindrical specimens with well-preserved terminations and the surface crenulation, yet without clearly visible wall section and internal lumen. Preservation variant 2 (Fig. 16.1–16.6; N = 9) is represented by fragmentarily preserved specimens that have only one original end, or none, because of breakage, but displays the double-layered wall in cross sections of the cylindrical tube, the central hollow lumen, and the wall surface. Preservation variant 3 (Fig. 17.7, 17.8; N = 6) is similar in showing the specimens’ surface, but only the outer veneer layer of the wall, whereas the remaining part of the tube interior is covered by or filled in with sediment, and the specimens are fragmented.
Wall construction and composition
The wall construction differentiated into two layers by their alignment, texture, and thickness, observed in RLM and PLM, is also shown clearly in CT images. Internal feature of the knob-like element at the proximal end and possible compartments within the lumen, which could be also inferred from the presence of the surface sutures, are recognized (Fig. 17).
The wall of Fistula n. gen., observed in petrographic thin section in parallel light (Fig. 14.3, 14.5) and with crossed polarizers (Fig. 14.4, 14.6), is made of a mixture of opal and microcrystalline quartz and minor admixture of silicates. The outer veneer layer is thin and compact, as seen on both sides of a vertically sectioned specimen, and the inner layer is a mixture of opal and very fine microcrystalline quartz. The lumen is infilled by diagenetic minerals and fractured by secondary chalcedony veins (Fig. 14.3, 14.4, right side of images; Fig. 14.5, 14.6, left side of images). There is no sign of pseudomorphs, replication by other minerals, or surface encrusting. Detailed EMP EDS survey of the same thin section revealed that the wall is mostly composed of silica with an admixture of a Mg-Fe aluminosilicate, probably mixed-layer clay mineral (Fig. 15.5, 15.6). In contrast to the wall, the lumen is filled in by first generation of Ca-carbonate (calcite) associated with Mn-oxide and silica that are, in turn, partially replaced by the second generation of silica (chalcedony), Ca-carbonate (calcite), and barite (Fig. 15.1–15.4).
In CT images of the holotype seen in a front view (Fig. 18.1), the material comprising the cylindrical wall is distinguished from the gray sediment by green artificial color that partly coats the specimen surface (Fig. 18.2) and extends below the proximal end of the specimen. The sediment is also infilling the oblique fracture of the specimen wall and is visualized by gray and blue color in Figure 18.4. The wall is thick, constant in thickness, rounded, and closed at the proximal end, and the central lumen also has a constant diameter (Fig. 18.2–18.4, olive color). The substantial total wall thickness is recognized along the length of the tube and is formed in a major part by the inner layer (Fig. 18.2–18.4). The inner layer of the wall is visualized in artificial green color and is thick and embraces the lumen, which is marked in olive color (Fig. 18.2–18.3). The outer veneer layer is depicted by gray color and the sediment infilling the lumen by olive color (Fig. 18.4). The sediment infilling in the lower portion of the lumen appears as transverse or slightly oblique, vertically stacked, band-like structures (Fig. 18.4, olive color) that may reflect compartments equivalent to tube segments (Fig. 17.4). The planes separating these structures may suggest pre-existing, morphological features and remains of the cross-walls that defined the compartments (chambers). The compartments could correspond to the segments that are marked on the wall surface by sutures and partial cross-walls or semi-septa between compartments that would coincide with sutures. The oblique orientation of the band-like structures likely resulted from post-mortem collapse or taphonomic deformation.
The outer veneer layer is shown as a thin layer in both of the longitudinal sections of the specimen (Fig. 18.4, gray line) and is missing on the side of the fractured wall (Fig. 18.4, lower left corner). The fracture demonstrates the sharp, brittle edges of the veneer layer (Fig. 18.3, gray color) in contrast to the underlying inner layer of the wall that appears less dense in texture. In another specimen (PMU 34742) under RLM (Fig. 17.5) and by CT (Fig. 17.6), the fractured wall along the suture on its surface shows a section of veneer layer and its constant thickness. By removing the image of sediment from the specimen surface (Fig. 18.1, 18.2), the veneer layer is seen in green artificial color (Fig. 18.3) and gray color (Fig. 18.3, 18.4). On the side of the proximal end of the tube, this layer shows clearly its uniform thickness in an open scar (Fig. 18.3, 18.4). The veneer layer is 100 μm and the inner layer thickness in CT images transect view is 600–800 μm. The veneer layer covers the basal knob-like structure at the tube bottom (Figs. 18.3, 18.4) and confirms that this structure was a morphological entity and does not result from deformation. This structure is inferred to be the initial element in the construction of the wall by the young (or even larval) organism.
The chemical reaction to controlled dosing of HF on the exposed wall (specimen PMU 34746) was rapid corrosion of the surface, which indicates that SiO2 is dissolved from the wall. In contrast, the host sediment surrounding the specimen was not digested by HF. The chemical and mineralogical composition of the wall is revealed by Laser-Raman spectroscopy on two specimens and differs in intensity from to the quartz signatures of the host sediment.
Laser-Raman spectra were acquired at several points on the proximal, middle, and distal portions of the wall surface of specimen PMU 34752 (Fig. 19). The signatures are indicative of SiO2 in microcrystalline quartz phase. The strongest signatures were at the proximal portion where the wall surface is dark, smooth, glossy, and best preserved. An additional spectrum of TiO2 in the specimen distal portion (Fig. 19) is inferred to be derived from mineral crystals in the burial environment, as in other studied taxa.

Figure 19. Wall composition of Fistula crenulata n. gen. n. sp. Moczydłowska in Moczydłowska et al. revealed by the Laser-Raman spectra acquired on the wall surface in several points on different portions of specimen PMU 34752, and showing the signatures of quartz and anatase minerals. The wall is siliceous in the quartz microcrystalline phase, and anatase is a contaminant from the sediment. The blue and orange tick marks are the positions of strong Laser-Raman peaks of anatase (TiO2, RRUFF ID: R060277) and quartz (SiO2, RRUFF ID: R040031), respectively.
Multiple Laser-Raman point spectra were acquired on the exposed surface of the wall from another specimen (PMU 34750; Fig. 20.1). The sediment directly entombing the specimen was examined, as well as the sediment overlying this stratigraphic horizon, for independent comparison of the sediment composition and diagenetic alteration (Fig. 20.2, 20.3). The specimen fragment is preserved with its proximal end covered in part by sediment, its more distal portion sectioned by fracture, and the veneer layer chipped-off and continually extending above it. The tube lumen is infilled by host sediment, but without any sign of quartz recrystallization. The specimen length is 20 mm, diameter 4.75–6.75 mm, inner layer thickness 1.25–1.50 mm, and veneer layer thickness 0.2–0.3 mm.

Figure 20. The Laser-Raman spectra, for comparison of mineralogical composition and differentiation of primary mineralogy of the fossil, acquired (1) on the wall of Fistula crenulata n. gen. n. sp. Moczydłowska in Moczydłowska et al., specimen PMU 34750, (2) on the entombing sediment around the specimen, and (3) on another sample of host sediment a few meters above the specimen. The strong quartz spectra of the wall (N = 4) is different than the weak spectra in the entombing (N = 3) and overlying sediments (N = 5), showing the primary siliceous skeleton of the fossil in quartz microcrystalline phase. The signatures of anatase and hematite minerals are from the sediment and contaminant in the fossil. The carbonaceous material in disordered D-band indicates the organic matter admixture in the fossil wall and its presence as disseminated organic matter in the sediments. The green and orange tick marks are the positions of strong Laser-Raman peaks of anatase (TiO2, RRUFF ID: R060277) and quartz (SiO2, RRUFF ID: R040031), respectively; blue tick mark is for hematite.
Several spectra (N = 4) from the thick inner layer on the cross section of the wall show strong quartz and weak anatase signatures (Fig. 20.1), and the exposed by breakage veneer layer (N = 4) shows similar signatures of both quartz and anatase (not illustrated). Additionally, a signature of carbonaceous material in disordered band (D-band) is observed, which may suggest carbonaceous material preserved within the mineralized wall, as it was in Anulitubus n. gen.
The spectra of the oxidized, red, thin-bedded mudstone entombing the specimen shows a weak signature of quartz, strong of hematite, weak of anatase, and carbonaceous material in the D-band (N = 3; Fig. 20.2). This quartz signature is significantly weaker than in the specimen wall (Fig. 20.1). The sediment composition examined by XRD spectra of the sample from the same bed documents the content of quartz, muscovite, feldspars, kaolinite, hematite, leucite, and smectite (Fig. 21; Supplementary Data). Quartz, muscovite, and feldspars are detrital and other minerals are diagenetic, including minor diagenetic microcrystalline quartz. The carbonaceous material depicted by D-band in the spectra is inferred to be derived from kerogen preserved within the sediment.

Figure 21. Mineralogic composition of the sediment entombing the fossils examined by X-ray diffraction (XRD). Red, thin-bedded mudstone is composed of detrital quartz, muscovite, and feldspars. Other identified minerals are diagenetic.
The difference in the intensity of quartz signatures between the specimen wall (strong) and the sediment (weak signature, low intensity) supports the interpretation that the wall was primarily silicified before the sediment was diagenetically altered, and authigenic quartz, hematite, kaolinite, and other diagenetic clay minerals, as well as anatase, were formed in the rock cement. The original siliceous wall may have been diagenetically “enhanced” in its composition, but it was not replicated.
The Laser-Raman spectra acquired in several points (N = 5) from an additional sample of gray micaceous mudstone of the host sediment, which lies ~15 m above the fossiliferous interval, showed a weak signature of quartz, strong of anatase, and the carbonaceous material signature in the D-band (Fig. 20.3). The quartz signature is similar to that in the other sample of host sediment, but weaker than in the specimen wall. The presence of carbonaceous material, and the absence of carbonates or calcareous cement in the sediment of the Innereva Member as a possible source of element C, indicate a biotic remnant of the organic matter. Disseminated organic matter is expected to be preserved in this sediment because organic-walled microfossils (acritarchs and filamentous cyanobacteria) and organic macroscopic Palaeopascichnus are recorded in the Indreelva Member (Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018; Fig. 2; unpublished microfossils). In studied Laser-Raman points in both the specimen and host sediments, as in the XRD analysis, there is no signature of calcareous minerals (CaCO3), which evidently precludes the possibility of its existence in the fossil wall.
Remarks
The new species differs from other Ediacaran tubular taxa by its double-layered wall construction, the knob-like element at the proximal end, and the presence of sutures on the wall surface.
Metazoan affinity and taxonomic comparisons
Phenotypically, the new taxa described manifest a range of features consistent with metazoan affinity: macroscopic sizes (0.5–7.0 cm), cylindrical and conical shapes, annulated or segmented structure with smooth to crenulate surface ornamentation, single- or double-layered skeletal wall, radial symmetry, and longitudinal polarity, including closed proximal versus open apertural (oral) ends. There was also clearly staged skeletal accretion and increase in diameter from a cup or knob-like proximal initial skeleton in Anulitubus n. gen and Fistula n. gen., or a simple cone in Coniculus n. gen. The variation in skeletal morphology and complexity observed between these taxa is comparable with modern marine benthic organisms, such as cnidarians and lophophorates, as well as vermiform animals and tubular annelids (especially polychaetes), or even bryozoans. However, individual morphological elements observed in new taxa, their overall body habit, and some structural similarities exist with Ediacaran body fossils such as Cloudina, Sinotubulites, Conotubus, Multiconotubus, Gaojiashania, and Shaanxilithes. All the latter taxa possessed either organic integument or mineralized skeletons, and have been interpreted as possible representatives of currently extant phyla (Grant, Reference Grant1990; Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010, Reference Cai, Hua, Schiffbauer, Sun and Yuan2014, Reference Cai, Xiao, Hua and Yuan2015; Cortijo et al., Reference Cortijo, Martí Mus, Jensen and Palacios2010, Reference Cortijo, Cai, Hua, Schiffbauer and Xiao2015; Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012, Reference Zhuravlev, Wood and Penny2015; Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017, Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019; Schiffbauer et al., Reference Schiffbauer, Selly, Jacquet, Merz, Nelson, Srange, Cai and Smith2020; Shore et al., Reference Shore, Wood, Curtis and Bowyer2020; Yang et al., Reference Yang, Steiner, Schiffbauer, Selly, Wu, Zhang and Liu2020).
Cloudina has nested funnel-like elements with high Mg-calcite content (Grant, Reference Grant1990; Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012, Reference Zhuravlev, Wood and Penny2015; Cai et al., Reference Cai, Hua, Schiffbauer, Sun and Yuan2014; Cortijo et al., Reference Cortijo, Cai, Hua, Schiffbauer and Xiao2015; Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017), as preserved in carbonate rocks, and was interpreted as biomineralized exoskeleton from the total-group cnidarians, and thus crown-eumetazoans and non-bilaterian (Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019; Shore et al., Reference Shore, Wood, Curtis and Bowyer2020). This was based on a combination of morphological characteristics, growth and reproduction patterns, as well as microstructure of the tube, and inferred paleoecology. Yet, Cloudina has also been compared with serpulid worms that are polychaetes in the Phylum Annelida (Hua et al., Reference Hua, Chen, Yuan, Zhang and Xiao2005; Cai et al., Reference Cai, Hua, Schiffbauer, Sun and Yuan2014), but this affiliation has been questioned and Cloudina was considered to be a cnidarian (Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012; Warren et al., Reference Warren, Quaglio, Simões, Gaucher, Riccomini, Poiré, Tavares Freitas, Boggiani and Sial2017). This was concluded because of the microstructure of Cloudina and the pattern of budding in asexual reproduction that are characteristic of cnidarians (Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012). The multiple (polytomous) branching of tube and attachment structures in Cloudina, and cloudinomorphs in general, were supporting the cnidarian and non-bilaterian affinity (Shore et al., Reference Shore, Wood, Curtis and Bowyer2020). Alternatively, Cloudina and new cloudinid Zuunia have been further interpreted to be originally organic and non-mineralizing stem-group annelids (Yang et al., Reference Yang, Steiner, Schiffbauer, Selly, Wu, Zhang and Liu2020). Other cloudinomorphs, exemplified by Saarina Sokolov, Reference Sokolov and Sokolov1965, emend. Selly et al., Reference Selly, Schiffbauer and Jacquet2020 and Costatubus Selly et al., Reference Selly, Schiffbauer and Jacquet2020, that were inferred to have been organic in original composition and preserving internal cylinders representing guts have been placed among the stem-annelids, and thus bilaterians (Schiffbauer et al., Reference Schiffbauer, Selly, Jacquet, Merz, Nelson, Srange, Cai and Smith2020; Selly et al., Reference Selly, Schiffbauer and Jacquet2020). These opposing interpretations may be reconciled if cloudinomorphs show convergent morphology of funnel-stacked elements in tubes and represent polyphyletic origins (Shore et al., Reference Shore, Wood, Curtis and Bowyer2020). With regards to the new genus Anulitubus, the presence of morphological elements of segments, although ring-like and not funnel-like in the tubular body, is demonstrably a metazoan feature.
Sinotubulites is a multilayered cylindrical fossil interpreted to have been a biomineralizing organism with a probable animal affinity (M. Chen et al., Reference Chen, Chen and Qian1981; Z. Chen et al., Reference Chen, Bengtson, Zhou, Hua and Yue2008; Cai et al., Reference Cai, Xiao, Hua and Yuan2015) or of uncertain affinity (Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017). Its morphological similarity to organic-walled Corumbella has been recognized, and the latter taxon inferred to be a scyphozoan cnidarian (Van Iten et al., Reference Van Iten, Marques, de Moraes Leme, Forancelli Pacheco and Guimaraes Simões2014), with Wood et al. (Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019) positing that Sinotubulites is a mineralized counterpart of Corumbella Hahn et al., Reference Hahn, Hahn, Leonardos, Pflug and Walde1982, thus supposedly cnidarian. The double-layered wall in new conical Coniculus n. gen. and cylindrical Fistula n. gen. is present and is consistent with metazoan affinities.
Conotubus is tapering along its length and has nested funnel-shaped elements, and was grouped with Cloudina and Sinotubulites to constitute the family Cloudinidae (Hua et al., Reference Hua, Chen and Yuan2007; Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2011), and thus related to cnidarians as Cloudina was recognized (Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017, Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019; Shore et al., Reference Shore, Wood, Curtis and Bowyer2020), or Cloudina as newly attributed to stem-annelids (Yang et al., Reference Yang, Steiner, Schiffbauer, Selly, Wu, Zhang and Liu2020). Regardless of the closer phylogenetic relationships, Conotubus is accepted to be metazoan. Comparably, the overall conical body habit and wall layering structure of Coniculus n. gen., although without segmentation, is shared with metazoans.
Multiconotubus has a multilayered, cone-in-cone wall structure and is diagnosed as a conical biomineralizing organism with “apical” end closed and open aperture (Cai et al., Reference Cai, Cortijo, Schiffbauer and Hua2017). The fossil is 3-D preserved and diagenetically phosphatized (Cai et al., Reference Cai, Cortijo, Schiffbauer and Hua2017, Reference Cai, Xiao, Li and Hua2019), but its primary mineralogy and possible affinity have not yet been determined. This taxon resembles Coniculus n. gen. morphologically by conical shape and layered wall, and both are considered metazoans.
Gaojiashania is preserved as carbonaceous compressions of tubes with characteristic circular rings (Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010). This taxon has been variously interpreted as worm (Hua et al., Reference Hua, Chen and Zhang2004), or tubular metazoan in general (Weber et al., Reference Weber, Steiner and Zhu2007). The segmented flexible tube structure has been compared specifically with modern siboglinid polychaetes (Cai et al., Reference Cai, Hua and Zhang2013). The fossil was originally organic or lightly mineralized (Hua et al., Reference Hua, Chen and Zhang2004; Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010, Reference Cai, Schiffbauer, Hua and Xiao2012, Reference Cai, Hua and Zhang2013; Smith et al., Reference Smith, Nelson, Strange, Eyster, Rowland, Schrag and Macdonald2016), although without indication of its primary mineralogy.
The segments of the tubular body in Anulitubus n. gen. resemble the morphology of Gaojiashania, which is however non-mineralized.
Shaanxilithes is a ribbon-shaped and annulated or corrugated compression fossil (Hua et al., Reference Hua, Chen and Zhang2004; Shen et al., Reference Shen, Xiao, Dong, Zhou and Liu2007; Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010) that may be metazoan in origin (Tarhan et al., Reference Tarhan, Hughes, Myrow, Bhargava, Ahluwalia and Kudryavtsev2014). Its organic composition has been established by environmental SEM EDS spectra and elemental mapping and Laser-Raman spectroscopy from specimens preserved in a variety of sediments, including dolostone, siltstone, and phosphatic shale (Meyer et al., Reference Meyer, Schiffbauer, Xiao, Cai and Hua2012; Tarhan et al., Reference Tarhan, Hughes, Myrow, Bhargava, Ahluwalia and Kudryavtsev2014). This taxon body annulation may resemble the surface of Fistula n. gen.
In summary, the terminal Ediacaran body fossils are recognized as metazoans based on their morphology, symmetry, wall construction and structure, and inferred benthic mode of life and paleoecology (Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2011, Reference Cai, Schiffbauer, Hua and Xiao2012; Wood, Reference Wood2011; Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012, Reference Zhuravlev, Wood and Penny2015; Wood and Curtis, Reference Wood and Curtis2015; Penny et al., Reference Penny, Wood, Zhuravlev, Curtis, Bowyer and Tostevin2017; Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017, Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019; Schiffbauer et al., Reference Schiffbauer, Selly, Jacquet, Merz, Nelson, Srange, Cai and Smith2020; Shore et al., Reference Shore, Wood, Curtis and Bowyer2020; Yang et al., Reference Yang, Steiner, Schiffbauer, Selly, Wu, Zhang and Liu2020). By the same characteristics of having complex morphology, comparable individual features, radial symmetry and polarity, and structure of mineralized wall, the new taxa represent a metazoan grade of organisms, as was their mode of life within the benthos.
Skeletal biomineralization
We considered a potential non-mineralized organic construction of the new fossils’ walls or their diagenetic mineralization versus primary biomineralization. As aforementioned under each taxon, the lack of even accessory elements Ca or P in the walls and the host sediment excluded potential diagenetic replacement by silica of calcareous or phosphatic skeletons. The only exception is the diagenetically induced, secondary calcareous mineral in the infilling of lumen of Fistula n. gen. (Figs. 14, 15). However, this is clearly distinguished from the wall mineralogy, which is siliceous with an admixture of aluminosilicate clay mineral. There is no record of any significant pyrite crystallization in the fossils, as it would be in taphonomic pyritization of organic walls by bacterial decay (seen in SEM images, but not illustrated). Most significant is the very different degree of host-rock early diagenetic silicification or admixture of silicate clay minerals, which is low and not exceeding the common content of SiO2 in typical siliciclastic rocks. To the contrary, as shown by the series of Raman spectroscopic, petrographic, and SEM- EMP EDS analyses, the fossils’ walls are siliceous and differentiated from the sediment (Figs. 14, 15, 21), thus supporting primary biomineralization. The diagenetic history of the succession that contains the fossils and in a regional scale provides no evidence for the diagenetic silicification of strata and taphonomic alteration of fossils. The lesson is learned from the interpretations of biomineralized versus primary organic construction of Cloudina and some other cloudinomorphs, which were thought to be the earliest metazoans with biomineralized calcareous skeletons (Grant, Reference Grant1990; Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012; Cai et al., Reference Cai, Hua, Schiffbauer, Sun and Yuan2014; Cortijo et al., Reference Cortijo, Cai, Hua, Schiffbauer and Xiao2015; Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017) and turned out to be originally organic (Schiffbauer et al., Reference Schiffbauer, Selly, Jacquet, Merz, Nelson, Srange, Cai and Smith2020; Yang et al., Reference Yang, Steiner, Schiffbauer, Selly, Wu, Zhang and Liu2020). Expectedly, the next finds of described fossil taxa will provide additional preservation modes to test the interpretation favored here.
Our microscopic, petrographic, spectroscopic, and geochemical analyses all infer the presence of primary biomineralized silica in the skeletal walls of the new Digermulen fossils. The additional supporting evidence is also the lack or only accessory admixture of digenetic silicate clay minerals in the host matrix, as well as the absence of any chert nodules, intrusive siliceous layers, or veins. There is likewise no indication of mineral dissolution (mobilization), solution migration, or recrystallization (Dallmeyer et al., Reference Dallmeyer, Reuter, Clauer, Liewig and Gayer1989; Rice et al., Reference Rice, Bevins, Robinson, Roberts and Gayer1989; Meinhold et al., Reference Meinhold, Wemmer, Högström, Ebbestad, Jensen, Palacios, Høyberget, Agić and Taylor2019a), and the fossils themselves are not diagenetically replaced or encrusted.
Biomineralization is biologically induced or organic matrix-mediated mineralization (Lowenstam, Reference Lowenstam1981; Lowenstam and Weiner, Reference Lowenstam and Weiner1989), and the first type is characteristic of single-celled organisms and the second of eukaryotes (Veis, Reference Veis2003). In forming of the mineral phase by organisms, the mineral is sequestered from the aqueous environment depending on temperature, pH, water chemistry, and ion saturation (Veis, Reference Veis2003). The process of silicification in organisms always proceeds in confined spaces in which organic membranes or extracellular matrices govern the precipitation of biosilicas (Perry, Reference Perry2003). The precipitated biosilicas are amorphous and silicified organisms have complex structural features (Perry, Reference Perry2003). The complexity of walls containing organic matter in studied taxa is in accord with the inferred biomineralization, which is suggested from the chemical elemental (STEM- EMP EDS) and primary mineral (petrography, Laser-Raman signatures) composition of the wall.
Laser-Raman spectra show markedly more intense quartz peaks in the fossils (a typical diagenetic transformation phase of originally precipitated opaline silica) versus host sediment samples. These spectra indicate that the fossils were silicified before the sediment was lithified and its detrital quartz grains and cement could be diagenetically altered and affect the buried fossils (Fig. 19; Supplementary Data). Differentiation between the fossil versus host sediment mineral signatures and intensity (mineral concentration) if containing the same mineral, allows recognition of the fossil primary composition (Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2012; Meyer et al., Reference Meyer, Elliot, Schiffbauer, Hall, Hoffman, Schneider, Vickers-Rich and Xiao2014; Schiffbauer et al., Reference Schiffbauer, Xiao, Cai, Wallace, Hua, Hunter, Xu, Peng and Kaufman2014; Muscente et al., Reference Muscente, Hawkins and Xiao2015; Shang et al., Reference Shang, Moczydłowska, Liu and Liu2018).
These geochemical-spectroscopic methods of determining the material composition have been applied in studies of both mineralized and organic fossils of Ediacaran and other ages and for distinguishing in-life from taphonomic mineralization (Schopf et al., Reference Schopf, Kudryavtsev, Agresti, Czaja and Wdowiak2005; Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2012; Meyer et al., Reference Meyer, Schiffbauer, Xiao, Cai and Hua2012, Reference Meyer, Elliot, Schiffbauer, Hall, Hoffman, Schneider, Vickers-Rich and Xiao2014; Moczydłowska et al., Reference Moczydłowska, Westall and Foucher2014; Schiffbauer et al., Reference Schiffbauer, Xiao, Cai, Wallace, Hua, Hunter, Xu, Peng and Kaufman2014; Tarhan et al., Reference Tarhan, Hughes, Myrow, Bhargava, Ahluwalia and Kudryavtsev2014; Muscente et al., Reference Muscente, Hawkins and Xiao2015; Pruss et al., Reference Pruss, Payne and Westacott2015, Reference Pruss, Blättler, Macdonald and Higgins2018; Shang et al., Reference Shang, Moczydłowska, Liu and Liu2018). Laser-Raman spectroscopy is a non-destructive optical method that provides the information to identify the chemical composition of fossils and allows their mineral identification (Kudryavtsev et al., Reference Kudryavtsev, Schopf, Agresti and Wdowiak2001; Schopf et al., Reference Schopf, Kudryavtsev, Agresti, Wdowiak and Czaja2002, Reference Schopf, Kudryavtsev, Agresti, Czaja and Wdowiak2005; Wacey, Reference Wacey2009; Olcott Marshall and Marshall, Reference Olcott Marshall and Marshall2015). In the present case study, this method supports biomineralization, as does the petrography.
Independently of geochemical analyses, we thoroughly reviewed the diagenetic history of the host sediment in respect to fossil taphonomy and mode of preservation. The tectonic and diagenetic changes associated with the Caledonide orogeny had relatively limited influence on the studied sedimentary succession (Dallmeyer et al., Reference Dallmeyer, Reuter, Clauer, Liewig and Gayer1989; Rice et al., Reference Rice, Bevins, Robinson, Roberts and Gayer1989; Meinhold et al., Reference Meinhold, Wemmer, Högström, Ebbestad, Jensen, Palacios, Høyberget, Agić and Taylor2019a) and the constituent fossils. Mineral composition of shales by XRD analysis determined largely quartz and muscovite, with subordinate chlorite, plagioclase, and hematite. The detrital quartz grains are modified to elongate shape produced by cleavage-forming processes associated with low-grade metamorphism and tectonothermal activity, age-constrained to 440.2 ± 9.4 Ma (Dallmeyer et al., Reference Dallmeyer, Reuter, Clauer, Liewig and Gayer1989). This tectonothermal alteration at the stage of diagenesis transitional to anchizone (~200° C), together with pressure-related dissolution of micas but without mobilizing silica, precluded silicification of the sediment. There is no evidence of recrystallization or penetrative migration of any mineral solutions. These conditions were key for preserving the primary mineralogy of the fossils and preventing its replication. The shale from the upper Indreelva Member analyzed by XRD consists of 71% phyllosilicates (mainly illite), 18% quartz, 10% feldspar, and 1% other minerals (Meinhold et al., Reference Meinhold, Wemmer, Högström, Ebbestad, Jensen, Palacios, Høyberget, Agić and Taylor2019a). This analysis shows a relatively low content of quartz and does not support the possibility of syngenetic or diagenetic silicification. Our XRD study of laminated mudstone entombing the new fossils in the lowermost Indreelva Member also documents the presence of quartz, kaolinite, muscovite, feldspars (K-feldspar), hematite, leucite, and smectite, and possibly a zeolite group of minerals (Fig. 21; Supplementary Data). This composition is typical of fine-grained siliciclastic rock comprised of detrital, diagenetic, and weathered aluminosilicates (phyllosilicates), and detrital and authigenic quartz (Supplementary Data).
The mineralogical and diagenetic features of the host sediment are congruent with the interpretation that the new fossils preserved primary mineralogy. Comparably, the organic-walled metazoan Sabellidites, macroscopic filamentous vendotaenids, and microscopic unicellular acritarchs preserved in the formation (Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Högström et al., Reference Högström, Jensen, Palacios and Ebbestad2013; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018; unpublished data on acritarchs) remained organically preserved, thus independently attesting against the sediment syngenetic or diagenetic silicification. There could not be selective permineralization of one type of fossil. The casts and molds of co-occurring Palaeopascichnus show no sign of diagenetic silicification or other re-mineralization (Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018), and this state of preservation is in contrast to, and also supports, the primary mineralogy of the new fossils.
Diagenetic to the lowermost metamorphic grade of alteration and without remineralization or recrystallization affected the sediment long after (135 and 175 Ma) the studied organisms were buried ca. 575 Ma. They were originally resistant, three-dimensional, and mineralized by primary silica. These organisms were the earliest silica biomineralizing macroscopic organisms that formed skeletons.
Environments facilitating silica biomineralization
Accepting our age estimates, biogenic skeletal silicification would appear to have been the first step in animal biomineralization at ca. 575 Ma. Previously, aragonite-calcite biomineralization was inferred to appear at ca. 550 Ma (Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017), with phosphatization emerging shortly after 541 Ma (Erwin et al., Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011; Erwin and Valentine, Reference Erwin and Valentine2013). However, the nominal taxon Cloudina and some other cloudinomorphs, among those animals interpreted to biomineralize ca. 550 Ma, have been revised as organic and non-biomineralized but taphonomically altered into various modes such as phosphatic, organic/pyritic, calcareous, limonitic/pyritic, and siliceous preservations (Schiffbauer et al., Reference Schiffbauer, Selly, Jacquet, Merz, Nelson, Srange, Cai and Smith2020; Yang et al., Reference Yang, Steiner, Schiffbauer, Selly, Wu, Zhang and Liu2020). Namacalathus Grotzinger et al., Reference Grotzinger, Watters and Knoll2000 and Namapoikia Wood et al., Reference Wood, Grotzinger and Dickson2002 remain as possible calcareous skeletal organisms in the terminal Ediacaran (Grotzinger et al., Reference Grotzinger, Watters and Knoll2000; Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019). The initial diversification of skeletonized animals then considered has been variously attributed to changing oceanic chemistry, redox conditions, and predation (Bengtson, Reference Bengtson2004; Wood, Reference Wood2011), as well as based on the record of calcareous fossils (Cui et al., Reference Cui, Grazhdankin, Xiao, Peek, Rogov, Bykova, Sievers, Liu and Kaufman2016; Schiffbauer et al., Reference Schiffbauer, Huntley, O'Neil, Darroch, Laflamme and Cai2016; Bowyer et al., Reference Bowyer, Wood and Poulton2017; Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017, Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019). No predation is evident at that time or in the Ediacaran Period (Narbonne, Reference Narbonne2005; Gibson et al., Reference Gibson, Rahman, Maloney, Racicot, Mocke, Laflamme and Darroch2019). Silica biomineralization has been posited in sponge spicules (Sperling et al., Reference Sperling, Robinson, Pisani and Peterson2010), but not yet considered a factor in integument of metazoan body fossils in this developmental transition, but it might also had been environmentally triggered by the ready availability of silica. The concentration of silica in cold seawater is higher, facilitating sequestration into siliceous skeletons (Perry, Reference Perry2003), and such conditions existed during the Gaskiers glaciation (580 Ma) and shortly after, where biomediated silica uptake may have been more efficient. Indeed, previous studies have determined that oceanic silica concentrations were unusually high in the Precambrian (Siever, Reference Siever1992; Maliva et al., Reference Maliva, Knoll and Simonson2005; Konhauser et al., Reference Konhauser, Lalonde, Amskold and Holland2007; Tarhan et al., Reference Tarhan, Hood, Droser, Gehling and Briggs2016) and promoted chert precipitation in the absence of hydrothermal input (Shen et al., Reference Shen, Lee and Xiao2011; Ramseyer et al., Reference Ramseyer, Amthor, Matter, Pettke, Wille and Falick2013; Dong et al., Reference Dong, Shen, Lee, Shu, Peng, Sun, Tang, Rong, Lang, Ma, Yang and Guo2015; Shang et al., Reference Shang, Moczydłowska, Liu and Liu2018; Penman and Rooney, Reference Penman and Rooney2019) or biogenic mediators (e.g., siliceous sponges, foraminiferans, radiolarians, diatoms, and chrysophytes, all of which appeared much later). Early Ediacaran cap chert formation in Africa and Mongolia has been linked to the global post-Marinoan deglaciation and demonstrates the oversaturation of silica in the ocean and perturbations in the silica cycle, as was in the carbon cycle (Penman and Rooney, Reference Penman and Rooney2019), at least regionally. Similar conditions might therefore have existed in the post-Gaskiers deglaciation interval, which likewise evinces carbon cycle disturbances (Halverson et al., Reference Halverson, Wade, Hurtgen and Barovich2010; Zhou et al., Reference Zhou, Xiao, Wang, Guan, Ouyang and Chen2017; Cui et al., Reference Cui, Xiao, Cai, Peek, Plummer and Kaufman2019).
High oceanic silica concentration in the Ediacaran has been further implicated as a cause of chert precipitation and enhancement of fossil preservation by syngenetic and diagenetic mineralization of organic remains particularly in reducing microenvironments, such as that reconstructed for the Doushantuo Formation of South China (Muscente et al., Reference Muscente, Hawkins and Xiao2015; Shang et al., Reference Shang, Moczydłowska, Liu and Liu2018) and Ediacara Member of South Australia, where pervasive silica precipitation cemented casts and molds of soft-bodied organisms (Tarhan et al., Reference Tarhan, Hood, Droser, Gehling and Briggs2016). We suggest that not only the preservation mode has been prompted by high silica concentration, but that metazoans invented the biochemical sequestration of silica into their walls for constructing the skeleton due to this condition, and much earlier as recorded in the Digermulen Penisula.
The cold-water ocean with high silica concentration flooded the margin of Baltica during the post-Gaskiers deglaciation, and such marine conditions are in concert with the position of Baltica in the high paleolatitudes (30–60°S; Torsvik and Cocks, Reference Torsvik and Cocks2017). Globally, the cold-water masses that are prone to silica saturation extend in high-latitude oceans (deep and shallow layers) and in the deep bottom layers in equatorial to low latitudes. Oceans have been stratified with respect to the thermal gradient throughout most of geologic history, and the surface temperature either follows climatic zones (Leeder, Reference Leeder1994; Garrison, Reference Garrison1999) or is uniformly low during the glacial periods, as in the Cryogenian (Hoffman and Schrag, Reference Hoffman and Schrag2002; Hoffman et al., Reference Hoffman, Abbot and Askenazy2017). The middle Ediacaran environmental conditions and marine silica saturation in the basin of the Digermulen Peninsula could have imposed and been opportunistically utilized by macroscopic animals, which needed support for their soft bodies by constructing skeletons.
The primary siliceous skeletons of the new taxa appear to be in contrast to exclusively calcareous skeletons of the terminal Ediacaran animals (Namacalathus, Namapoikia), given that calcite/aragonite are currently thought to be the only minerals precipitated by early biomineralizing organisms (Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019; but see Yang et al., Reference Yang, Steiner, Schiffbauer, Selly, Wu, Zhang and Liu2020). The paleogeographic position of successions bearing these calcareous skeletal animals is in, or restricted to, the intermediate to equatorial paleolatitudes (Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012; Warren et al., Reference Warren, Quaglio, Simões, Gaucher, Riccomini, Poiré, Tavares Freitas, Boggiani and Sial2017), thus both climate and marine water were warm. Widespread marine cements facilitated the calcareous mineralogy of animal skeletons and were suitable for development of reefal and biohermal ecosystems at this time (Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019). The environmental conditions, ocean water chemistry, and Ca ion concentration have been inferred to trigger skeletonization in these animals (Grotzinger et al., Reference Grotzinger, Watters and Knoll2000; Wood, Reference Wood2011; Penny et al., Reference Penny, Wood, Zhuravlev, Curtis, Bowyer and Tostevin2017; Wood et al., Reference Wood, Curtis, Penny, Zhuravlev, Curtis-Walcott, Iipinge and Bowyer2017, Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019). However, diametrically different conditions existed when (25 Ma earlier) and where (high paleolatitudes, cold climate, and deglaciation zone) the new taxa appeared, and their ecology and metabolism have to be assessed accordingly.
Paleoecological implications
Deduced from the depositional setting of the Stáhpogieddi Formation (Reading and Walker, Reference Reading and Walker1966; Banks, Reference Banks1973; Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Rice et al., Reference Rice, Edwards, Hansen, Arnaud, Halverson, Arnaud, Halverson and Shields-Zhou2011; Torsvik and Cocks, Reference Torsvik and Cocks2017) and preservation of fossils, the newly described organisms inhabited the marine offshore shelf of a marginal inland basin located in high southern paleolatitudes, in quiet and below wave-base conditions during the transgressive, cold-water flooding in the post-glacial time (Reading and Walker, Reference Reading and Walker1966; Banks, Reference Banks1973; Farmer et al., Reference Farmer, Vidal, Moczydłowska, Strauss, Ahlberg and Siedlecka1992; Rice et al., Reference Rice, Edwards, Hansen, Arnaud, Halverson, Arnaud, Halverson and Shields-Zhou2011; Torsvik and Cocks, Reference Torsvik and Cocks2017). These environments of non-agitated and light-penetrated clear, well-oxygenated water column above the siliciclastic, laminated muddy bottom hosted the sea floor-dwelling, sessile organisms, such as co-occurring Palaeopascichnus and slightly later appearing Aspidella are interpreted to have been (Gehling et al., Reference Gehling, Narbonne and Anderson2000; Gehling and Droser, Reference Gehling and Droser2009; Wan et al., Reference Wan, Xiao, Yuan, Chen, Pang, Tang, Guan and Maisano2014; Burzynski and Narbonne, Reference Burzynski and Narbonne2015; Tarhan et al., Reference Tarhan, Droser, Gehling and Dzaugis2015; Jensen et al., Reference Jensen, Högström, Høyberget, Meinhold, McIlroy, Ebbestad, Taylor, Agić and Palacios2018; Kolesnikov et al., Reference Kolesnikov, Rogov, Bykova, Danelian, Clausen, Maslov and Grazhdankin2018). The fossils of the new genera Anulitubus, Coniculus, and Fistula were preserved in situ, although not in a life position (post-mortem collapse) and without transport (autochthonous assemblage) because there is no record of sedimentary structures indicative of currents, wave activity, water flow, or dragging. The lifestyle of these organisms, based on functional morphology of their skeletons with closed proximal and opened apertural ends, distinct longitudinal polarity, but without ventral-dorsal orientation, was most likely that of a benthic, sessile organism.
The skeleton aperture functioned likely as oral opening, which was probably lined with tentacle-like organs or chaetae to actively collect or passively uptake nutrient particles during suspension or filter feeding. From the initial skeleton with probable attachment structures (cup- and knob-shaped elements) or simple proximal cone point that was formed in the nepionic larval stage and embedded in the sediment, the organism grew above the substrate and added to the skeleton upward around the apertural opening. Such reconstructions imply an upright epibenthic life position. Forming skeletons to support their sizable soft bodies in an upright position might be beneficial for the uptake of nutrients from the water column and still being firmly settled at the sea floor. These organisms were probably feeding on bacteria and unicellular algae that formed microplankton (“marine snow”) and not on the ground bacterial mats (neither recognized by the presence of organic films nor the sediment texture).
As for Anulitubus n. gen., the life history may be further suggested. This organism larva formed the cup element and the tube was secreted into adult stage and achieved the full diameter after the first annular segment either was ready or increased minimally. The tube compartments, equivalent to the annular segment spaces, were separated by incomplete septa and the organism occupied the last, distal compartment at the time while constructing the wall of the next segment, and having a widely open aperture to the environment outside. The central hole in incomplete septum provided the free connection between compartments and possibly the way for attachment to the tube bottom by a sort of organic thread or peduncle-like extension to keep the organism permanently within the tube and not risking it being expelled through the aperture and lost.
Specimens of Anulitubus n. gen., Coniculus n. gen., and Fistula n. gen. were all found in close proximity as solitary individuals, but in contrast to other Ediacaran organisms that were living in more abundant and inter-related communities (Cai et al., Reference Cai, Hua, Schiffbauer, Sun and Yuan2014; Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019), they do not appear to have been communal. For example, Cloudina, Conotubus, and Sinotubulites have been recognized as sessile, gregarious organisms living on the bottom floor covered by microbial mats and in association with thrombolitic and stromatolitic reefs or bioherms on carbonate platform ramp (Germs, Reference Germs1972; Grant, Reference Grant1990; Cai et al., Reference Cai, Hua, Schiffbauer, Sun and Yuan2014, Reference Cai, Xiao, Hua and Yuan2015). Shaanxilithes and Gaojiashania are also thought to be epibenthic organisms (Cai et al., Reference Cai, Hua, Xiao, Schiffbauer and Li2010, Reference Cai, Hua and Zhang2013; Tarhan et al., Reference Tarhan, Hughes, Myrow, Bhargava, Ahluwalia and Kudryavtsev2014).
The late Ediacaran ecosystems (ca. 570–540 Ma) of soft-bodied and biomineralizing animals alongside the Ediacara-type organisms have been reconstructed as diverse and highly integrated communities in the shoreface sandy shelf, restricted lagoonal to inner and outer ramp of carbonate platform settings, with a sea floor rich in microbial mats (Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019). These various settings were paleogeographically located in the intermediate to low latitudes (Zhuravlev et al., Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012; Torsvik and Cocks, Reference Torsvik and Cocks2017; Warren et al., Reference Warren, Quaglio, Simões, Gaucher, Riccomini, Poiré, Tavares Freitas, Boggiani and Sial2017) and in warm climate zones with intense carbonate precipitation in warm-water environments.
The early, ca. 571 Ma ecosystem reconstructed from Avalonia, Newfoundland, is dominated by rangeomorphs and consists of taxonomically rich, leaf-like, frondose morphotypes with holdfasts, that were benthic and oriented upright in the water column (Liu et al., Reference Liu, Kenchington and Mitchell2015; see Pu et al., Reference Pu, Bowring, Ramezani, Myrow, Raub and Landing2016 for age; Butterfield, Reference Butterfield2020). This biota lived in deep-water marine, aphotic environments at the time and later on (ca. 560–555 Ma) in other shallower-water environments (Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014; Liu et al., Reference Liu, Kenchington and Mitchell2015; Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019). The Avalonian deep basinal setting recorded by siliciclastic hemipelagites (mud, silt) that developed in the turbiditic facies was located in high paleolatitudes (40–60°S; Liu et al., Reference Liu, Kenchington and Mitchell2015). Similarly, the diverse frondose and modular rangeomorphs, arboreomorphs, and erniettomorphs thrived on the sea floor, in aphotic zone of the deep-water setting in the Mackenzie Mountains of Laurentia, at ca. 580–560 Ma interval (Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014). This basin was equatorially positioned at the time (Torsvik and Cocks, Reference Torsvik and Cocks2017). The deep marine environment in the aphotic zone has been inferred to be the site of the origin of the rangeomorph biota and, accordingly, their osmotic mode of feeding (Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014; Liu et al., Reference Liu, Kenchington and Mitchell2015). Recently, rangeomorphs have been revised as being not osmotrophic, but rather heterotrophic and digesting substrates, likely living in symbiosis with a resident microbiome, and within the total-group Eumetazoa (Butterfield, Reference Butterfield2020). Although the Laurentian and Avalonian basins were widely paleogeographically spread across equatorial to high paleolatitudes, they both represent deep-basinal and thus cold-water environments. Subsequently, the same taxa existed in shallow marine environments ca. 560 Ma (Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019), which were warm water and in the photic zone. The puzzle remains how the rangeomorphs and similar biota adapted without change in morphology and physiology from their initial deep, cold-water, aphotic environments to shallow, warm-water, and photic environments?
To these reconstructions, the ecosystem estimated to ca. 575 Ma from the shallower marine, offshore shelf but cold-water setting on the Digermulen Peninsula has to be added. This ecosystem comprised epibenthic macroscopic organisms and neritic microplankton. Epibenthic were the new genera Anulitubus, Coniculus, and Fistula animals, Palaeopascichnus (Gehling and Droser, Reference Gehling and Droser2009; Wan et al., Reference Wan, Xiao, Yuan, Chen, Pang, Tang, Guan and Maisano2014), which is a protistan foraminiferan (Antcliffe et al., Reference Antcliffe, Gooday and Brasier2011; Kolesnikov et al., Reference Kolesnikov, Rogov, Bykova, Danelian, Clausen, Maslov and Grazhdankin2018), and slightly above appearing Aspidella, which is a member of the Ediacara biota of unknown affinity (Gehling et al., Reference Gehling, Narbonne and Anderson2000; Burzynski and Narbonne, Reference Burzynski and Narbonne2015). The planktonic photosynthesizing microorganisms were organic-walled acritarchs that are predominantly of algal affinities (Moczydłowska, Reference Moczydłowska2016; Liu and Moczydłowska, Reference Liu and Moczydłowska2019) and filamentous cyanobacteria (unpublished data). Benthic bacterial mats are not yet recorded, but this may reflect a taphonomic bias because of the sediment oxidation, and they are more susceptible to decay in comparison with resistant cysts of acritarchs.
The Digermulen ecosystem extended in the high paleolatitude (30–60°S), cold-water marine shelf with siliciclastic sedimentation, in the photic zone, and above the oxic chemocline. During the post-Gaskiers deglaciation, the revived hydrological cycle that caused mixing of the well-oxygenated water and increased input of mineral nutrients into the marine basin from weathering of the uncovered Baltica paleocontinent created habitats for oxygen-demanding animals and favorable living conditions. The autotrophic photosynthesizers forming the marine snow and living on the sea floor generated the organic matter for the consumers. This ecosystem hosted the earliest silica biomineralizing animals described here.
We suggest that the cold-water environments, not necessarily restricted by the depth but by the temperature, were the sites of the earliest frondose-like biota expansion. If the rangeomorph biota is epifaunal (Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014) and among total-group eumetazoan (Butterfield, Reference Butterfield2020), the Avalon assemblage included candidate cnidarians (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; Wood et al., Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019) and the animal Dickinsonia (Hoyal Cuthill and Han, Reference Hoyal Cuthill and Han2018; Bobrovskiy et al., Reference Bobrovskiy, Hope, Ivantsov, Nettersheim, Hallmann and Brocks2018), and the new biomineralizing taxa were animals, therefore the records at ca. 575–571 Ma show the radiation of animals. There were both small but macroscopic skeletal animals and large soft-bodied biota occurring in shallow- and deep-marine environments, but all cold-water. The evolution of biomineralization by silica, as recorded in the Digermulen Basin, seems to be confined to cold-water and oxic environments.
The Cold Cradle hypothesis was proposed for the evolution of the Ediacara biota and facilitated by the increased O2 availability in cold water environments (Fedonkin, Reference Fedonkin2003). More recently, it has been suggested that estimates of O2 level in the ocean would be not sufficient to develop diverse ecosystems and, instead, thermal stability, not necessarily cold conditions, has been hypothesized for the deep-water origin of Ediacara biota (Boag et al., Reference Boag, Stockey, Elder, Hull and Sperling2018). We suggest the stenothermal (Boag et al., Reference Boag, Stockey, Elder, Hull and Sperling2018) but cold-water (Fedonkin, Reference Fedonkin2003; the Digermulen Basin herein) environments for the origin of the frondose Ediacara biota (deep water but expansion into shallow water) and for the earliest biomineralization (shallow, cold-water environments).
Conclusions
The three newly described monotypic genera Anulitubus, Coniculus, and Fistula of macroscopic body fossils with mineralized walls display morphologic features, radial symmetry, and longitudinal polarity that are consistent with being skeletons of animals. Their paleoecology and the paleoenvironment of a marine shelf with well-oxygenated, cold quiet water in the photic zone above the muddy sea-floor support this interpretation and, together with characteristic functional morphology of the exoskeletons, suggest epibenthic mode of life and suspension/filter feeding by these animals. The new species differ from any previously described Ediacaran body fossils by spacial arrangement of annuli and segments, and by being uniquely primarily silicified.
The single- and double-layered skeletons are siliceous and their composition and primary mineralogy are revealed by petrography and various geochemical analyses, and supported by diagenetic processes of the host sediment. Biomineralization by silica was likely facilitated by the environmental conditions of cold, marine waters with high silica concentration, which are typical of silica sequestration into organismal skeletons. Such environments of a cold-water marine shelf existed in the Digermulen Basin during the post-Gaskiers (>580 Ma) deglaciation time and contemporaneously with the appearance of the new taxa. These environments could have forced and been opportunistically utilized by the increase in size of the animals, which needed support for their soft bodies by constructing skeletons. The new species evolved biochemical sequestration of silica into their walls and preceded significantly calcareous biomineralizing sponge (Namapoikia) and putative lophophorate animals (Namacalathus) at the 550 Ma terminal Ediacaran.
The age of the new fossil taxa is broadly bracketed within 580–541 Ma, but shortly post-dating the 580 Ma underlying Mortensneset Diamictite, which is coeval with the Gaskiers Glaciation. This age is estimated at ca. 575 Ma by the age interpolation within the sedimentologically continuous succession bracketed within 580–541 Ma and further constrained by the 558–555 Ma chronostratigraphic ages of the overlying Ediacaran biota.
The reported fossils are the earliest known in the evolutionary history biomineralizing animals that secreted siliceous skeletons and the only ones known from the Ediacaran Period. The phylogenetic affinity of the new genera remains un-resolved, but the body plan, complexity of skeleton with annulate elements, and initial larval structures indicate eumetazoan grade of organismal organization. The new biomineralizing animals evince the radiation among other Ediacaran organisms that are recorded by soft-bodied rangeomorphs of unknown affinities and appearing at 571 Ma. The oldest putative animals, including cnidarians and bilaterians preserved as carbonaceous compressions, the Lantian biota, are estimated here to ca. 620–580 Ma in age. The divergence of diverse Ediacaran animals that were soft-bodied and subsequently biomineralizing by silica and calcareous minerals is recorded by fossils in the evolutionary step-wise pattern since ca. 620 Ma until the beginning of the Cambrian.
Acknowledgments
This work was funded by the Swedish Research Council (Vetenskåpsrådet) project grant Nr 621-2012-1669 to M. Moczydłowska. Analytical work by J. Majka was partly supported by AGH-UST grant no. 16.16.140.315. We thank Ø. Hammer (University of Oslo) for his guidance in tomographic analyses and the Natural History Museum, University of Oslo, for access to the Computed Tomography facilities. We thank J. O. Ebbestad (Uppsala University, Museum of Evolution) for reviewing the taxonomic descriptions of the early draft, additional tomographic analyses of Coniculus n. gen., photographing some specimens in RLM, and discussions on the early skeletal metazoans. We are also grateful to J. Schiffbauer and an anonymous reviewer, and the editors J. Jin and A. Liu for their helpful comments on this manuscript.
Author contributions
M.M. collected the fossils, conceived the study, conducted microscopic (RLM, STEM) and geochemical (chemical tests, STEM EDS) studies, and analyzed the data. M.M. and B.P.K. wrote the paper with contributions from co-authors. D.S. and B.P.K. compiled the tomographic data. L.L. and P.L. conducted the spectroscopy analyses. J.M. conducted EMP EDS analysis on thin sections and petrographic thin sections examination. All authors participated in discussions on the results.
Competing interests
The authors declare no competing interests.
Accessibility of supplementary data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.08kprr51f.
Publisher's note
Publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
























