Fish oil contains high amounts of n-3 highly unsaturated fatty acids (HUFA) such as EPA (20 : 5n-3) and DHA (22 : 6n-3), which are essential to many marine fish species because of deficiencies in the desaturation and elongation pathways necessary for their biosynthesis( Reference Sargent, Bell and McEvoy 1 , 2 ). However, fatty acids are particularly susceptible to oxidation during feed processing and storage, especially in the absence of adequate amounts of antioxidants( Reference Mourente, Bell and Tocher 3 ). Oxidised fish oil is harmful to fish( Reference Huang and Huang 4 – Reference Gao, Koshio and Ishikawa 6 ).
Vitamin E (VE) is one of the most extensively used antioxidants in foods and has been shown to reduce oxidative stress in fish( Reference Gao, Koshio and Ishikawa 6 – Reference Chen, Liu and Tian 11 ). A higher dose of VE is required in fish diets, particularly those with high levels of HUFA, because dietary lipid oxidation can reduce the VE content( Reference Baker and Davies 7 , Reference Puangkaew, Kiron and Somamoto 12 , Reference Lewis-McCrea and Lall 13 ). Moreover, several times the optimum dietary VE level is needed to enhance the non-specific immune responses of fish( Reference Lin and Shiau 14 ). However, high doses of VE may exert pro-oxidative effects on human LDL and lipids in fish tissues( Reference Bowry and Stocker 15 – Reference Ito, Murata and Tsuda 17 ).
In commercial situations, diets are often stored at room temperature for long periods of time, resulting in the oxidation of dietary lipids to varying degrees. Many previous studies have supplemented diets containing identical lipid oxidation values with graded levels of VE to evaluate optimal dietary VE concentrations under conditions of oxidative stress( Reference Huang and Huang 4 , Reference Gao, Koshio and Ishikawa 6 , Reference Tocher, Mourente and van der Eecken 8 , Reference Chen, Liu and Tian 11 , Reference Tocher, Mourente and van der Eecken 18 , Reference Gao, Koshio and Ishikawa 19 ). However, there is a lack of information with respect to the effects of VE under conditions of varying degrees of oxidative stress. The effects of the severity of oxidative stress on the role of dietary VE in marine fish are unclear.
Large yellow croaker (Larmichthys crocea) is a major commercially important marine fish in China and has been widely cultured in recent years. As a carnivorous species, its dietary lipid content is relatively high( Reference Wang, Ai and Mai 20 ). In subtropical and tropical regions such as southeast China, the climate is humid and the temperature is relatively high throughout the year, especially during the summer. Under such climatic conditions, it is probable that dietary lipids would be easily oxidised to different degrees depending upon the conditions under which the diets are stored.
Therefore, in the present study, graded oxidative stress in juvenile large yellow croaker was induced by feeding diets in which the fish oil was oxidised to various degrees. High-dose (600 mg/kg diet) VE was supplemented in the diet to determine the effects on growth performance, activities of hepatic antioxidant defence enzymes and head kidney macrophages respiratory burst activity of fish suffering various stressful conditions. The purpose of this study was to investigate the combined effects of variable degrees of lipid oxidation and high-dose VE supplementation on oxidative-stressed marine fish.
Methods
Diet preparation
White fishmeal and menhaden fish oil without antioxidant were used as the main protein and lipid sources in the diet (Table 1). The peroxide value (POV) of fresh fish oil (FFO) was 1·72 mEq/kg. FFO was oxidised to three different degrees (low, moderate and high) by heating at 50°C with vigorous aeration while monitoring the POV at 4-h intervals. The final POV values for the lowly (LO), moderately (MO) and highly oxidised (HO) fish oil were 28·29, 62·79 and 104·21 mEq/kg, respectively. After oxidisation, diets containing either fresh or oxidised fish oil were stabilised with 150 mg ethoxyquin/kg diet. The proximate composition of the experimental diets is shown in Table 2.
Table 1 Formulation of the experimental basal diet

* Fishmeal obtained from Russia AKROS Fishing Co. Ltd; soyabean meal obtained from Liulu Oli Lit; fish oil obtained from Shengda Fish Meal & Oil Co. Ltd; beer yeast obtained from Cishan Fisheries.
† Attractant: glycine and betaine.
‡ Mould inhibitor contained 50 % calcium propionic acid and 50 % fumaric acid.
§ Vitamin premix (mg or g/kg diet): thiamin, 25 mg; riboflavin, 45 mg; pyridoxine HCl, 20 mg; vitamin B12, 0·1 mg; vitamin K3, 10 mg; inositol, 800 mg; pantothenic acid, 60 mg; niacin acid, 200 mg; folic acid, 20 mg; biotin, 1·20 mg; retinyl acetate, 32 mg; cholecalciferol, 5 mg; dl-α-tocopherol, 120 mg; ascorbic acid, 2000 mg; choline chloride, 2500 mg; microcrystalline cellulose, 14·67 g.
║ Mineral premix (mg or g/kg diet): NaF, 2 mg; KI, 0·8 mg; CoCl2.6H2O (1 %), 50 mg; CuSO4.5H2O, 10 mg; FeSO4.H2O, 80 mg; ZnSO4.H2O, 50 mg; MnSO4.H2O, 60 mg; MgSO4.7H2O, 1200 mg; Ca(H2PO3)2.H2O, 3000 mg; NaCl, 100 mg; zoelite, 15·45 g.
Table 2 Proximate composition of the experimental diets (%, dry weight basis)
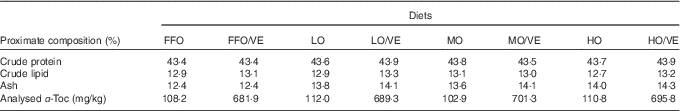
FFO, fresh fish oil; VE, vitamin E; LO, lowly oxidised fish oil; MO, moderately oxidised fish oil; HO, highly oxidised fish oil; α-Toc, dl-α-tocopheryl acetate.
A previous study in our laboratory demonstrated that approximately 60 mg dl-α-tocopheryl acetate (α-Toc)/kg diet was adequate to maintain normal physiological function in juvenile large yellow croaker( Reference Zhang 21 ). In the present study, eight experimental diets were designed to investigate the effects of 600 mg/kg VE (α-Toc) and oxidised fish oil (LO, MO and HO) on large yellow croaker: two FFO diets (FFO and FFO/VE), two LO fish oil diets (LO and LO/VE), two MO fish oil diets (MO and MO/VE) and two HO fish oil diets (HO and HO/VE). Experimental diets were sealed in plastic bags and stored indoors in wooden boxes at room temperature until use. Samples were taken from these diets and stored at −20°C for future chemical determinations.
Experimental procedures
Fish rearing and sampling were carried out according to the experimental procedures of the key laboratory of mariculture (Ministry of Education of China), Ocean University of China. Juvenile large yellow croaker L. crocea was obtained from a local commercial hatchery farm in Xiangshan Bay, Ningbo, China. Upon arrival, they were reared in open floating sea cages (3·0×3·0×3·0 m) and were fed the control diet for 2 weeks to acclimate to the experimental diet and culture conditions. At the end of the acclimatisation period, the fish were starved for 24 h, anaesthetised with eugenol (1:10 000; Shanghai Reagent) and weighed. Fish of similar sizes (7·82 (sem 0·68) g) were distributed into twenty-four sea cages (1·5×1·5×2·0 m), and each cage was stocked with sixty fish. Each diet was randomly assigned to triplicate cages. The fish were hand-fed to apparent satiation twice daily (04.30 and 16.30 hours). Fish were considered satiated when they did not exhibit feeding behaviour towards the pellets. The weight of diet for each cage before feeding was recorded and the remaining diet was weighed. Daily consumption of feed in each cage was recorded. Smaller pellets (1·5 mm) were fed to fish during weeks 1–4 and the larger pellets (2·5 mm) during weeks 5–10. The feeding trial lasted for 10 weeks. Water column characteristics were monitored weekly in the morning. During the trial, water temperature ranged from 26·5 to 29·5°C, the salinity was about 28 g/l and dissolved O2 content was approximately 7 mg/l.
Biochemical analysis
Fish were starved for 24 h at the end of the feeding trial. The total number and mean body weight of the fish in each cage were measured. Ten fish were randomly sampled from each cage for individual proximate composition analysis. Proximate composition analyses of feed ingredients, experimental diets and fish carcass were performed according to the standard methods( 22 ). Samples of the diets and fish were dried to a constant weight at 105°C to determine moisture content. Protein content was determined by measuring N content (N×6·25) using the Kjeldahl method. Body lipid levels were quantified by diethyl ether extraction using Soxhlet. Lipids in the liver were extracted as described by Folch et al.( Reference Folch, Lees and Sloane Stanley 23 ). Ash was measured by combustion at 550°C. All measurements and determinations were performed in triplicate. Fatty acid profiles were analysed using a HP6890 GC (Agilent Technologies Inc.) as described by Zuo et al.( Reference Zuo, Ai and Mai 24 ). The α-Toc concentrations of diets were determined by HPLC with a fluorescence detector according to Salo-Väänänena et al.( Reference Salo-Väänänena, Ollilainen and Mattila 25 ).
Antioxidant enzyme activity and respiratory burst activity
Blood samples were collected from the caudal vein from three fish per cage with a 27-G needle and a 1-ml syringe, allowed to clot at room temperature for 4 h and then at 4°C for further 6 h. Following centrifugation (836 g , 10 min, 4°C), serum samples were immediately frozen in liquid N2 and stored at −80°C for later analysis of antioxidant defence enzyme activities. Activities of superoxide dismutase (SOD) and catalase (CAT) were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute) following the manufacturer’s protocol. Respiratory burst activity of head kidney macrophages was measured according to a method previously described in our laboratory by Zuo et al.( Reference Zuo, Ai and Mai 24 ).
Hepatic malondialdehyde
Livers were dissected from three fish per cage, immediately frozen in liquid N2 and stored at −80°C before analysis. Malondialdehyde (MDA), one of the metabolites derived from lipid peroxidation, was measured using a thiobarbituric acid reactive substances assay kit (Nanjing Jiancheng Bioengineering Institute) following the manufacturer’s protocol.
Air-exposure mortality
After blood and tissue sampling, twenty fish were randomly collected from each cage, exposed to air at an ambient temperature of approximately 28°C for 12 min and then put back into the water. Accumulated mortality during the subsequent 20 min was monitored and was defined as air-exposure mortality (AEM).
Calculations and statistical analysis
The following variables were calculated:
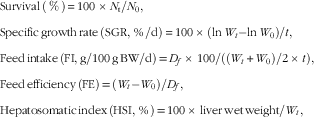 $$\eqalignno{ & {\rm Survival}\,\left( \,\%\, \right){\,\equals\,}100\,{\times}\,N_{{\rm t}} /N_{0} , \cr & {\rm Specific}\,{\rm growth}\,{\rm rate}\,{\rm (SGR,}\,{\rm \,\%\,/d)}{\,\equals}\,100\,{\times}\,({\rm ln}\,W_{t} {\minus}{\rm ln}\,W_{0} {\rm )}/t{\rm ,} \cr & {\rm Feed}\,{\rm intake}\,\left( {{\rm FI,}\,{\rm g/100}\,{\rm g}\,{\rm BW/d}} \right){\rm {\,\equals}\,}D_{f} {\rm \,{\times}\,100/((}W_{t} {\rm {\plus}}W_{0} {\rm )/2\,{\times}\,}t{\rm ),} \cr &{\rm Feed}\,{\rm efficiency}\,\left( {{\rm FE}} \right){\rm \,{\equals}\,}\left( {W_{t} {\minus}W_{0} } \right){\rm /}D_{f} , \cr & {\rm Hepatosomatic}\,{\rm index}\,\left( {{\rm HSI,}\,{\rm \,\%\,}} \right){\rm \,{\equals}\,100\,{\times}\,liver}\,{\rm wet}\,{\rm weight/}W_{t} , $$
$$\eqalignno{ & {\rm Survival}\,\left( \,\%\, \right){\,\equals\,}100\,{\times}\,N_{{\rm t}} /N_{0} , \cr & {\rm Specific}\,{\rm growth}\,{\rm rate}\,{\rm (SGR,}\,{\rm \,\%\,/d)}{\,\equals}\,100\,{\times}\,({\rm ln}\,W_{t} {\minus}{\rm ln}\,W_{0} {\rm )}/t{\rm ,} \cr & {\rm Feed}\,{\rm intake}\,\left( {{\rm FI,}\,{\rm g/100}\,{\rm g}\,{\rm BW/d}} \right){\rm {\,\equals}\,}D_{f} {\rm \,{\times}\,100/((}W_{t} {\rm {\plus}}W_{0} {\rm )/2\,{\times}\,}t{\rm ),} \cr &{\rm Feed}\,{\rm efficiency}\,\left( {{\rm FE}} \right){\rm \,{\equals}\,}\left( {W_{t} {\minus}W_{0} } \right){\rm /}D_{f} , \cr & {\rm Hepatosomatic}\,{\rm index}\,\left( {{\rm HSI,}\,{\rm \,\%\,}} \right){\rm \,{\equals}\,100\,{\times}\,liver}\,{\rm wet}\,{\rm weight/}W_{t} , $$
where N 0 and N t represent the initial and final numbers of fish in each cage, respectively. BW is the wet body weight, W t is the final mean body weight (g), W 0 is the initial mean body weight (g), t is the experimental duration in days and D f is the dry diet intake (g).
All data were subjected to a one-way ANOVA test, and differences between the means were tested by Tukey’s multiple-range test. The level of significance was set at P<0·05, and the results are presented as mean values with their standard errors. All the statistical analyses were performed using Statistica 6.0 (StatSoft).
Results
Dietary fatty acid profiles
Lipid oxidation increased the proportions of SFA and MUFA fatty acids, and decreased the proportions of EPA, DHA and total HUFA in the experimental diets (Table 3).
Table 3 Fatty acid composition (% total fatty acids) of the experimental diets containing different levels of oxidised fish oil
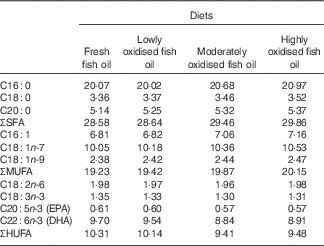
HUFA, highly unsaturated fatty acids (EPA and DHA in the present study).
Survival, growth, feed utilisation and hepatosomatic index
There were no significant differences (P>0·05) in the rate of survival, which ranged from 93·7 to 97·5 %, among the treatments (Fig. 1(A)). For the VE non-supplemented groups, the specific growth rate was significantly (P<0·05) lower in fish fed the oxidised diets than in fish fed the FFO diet. VE supplementation significantly (P<0·05) decreased the growth rate of fish fed FFO. However, VE supplementation increased the growth rate of fish fed the oxidised diets (Fig. 1(B)).

Fig. 1 Survival rate (A), specific growth rate (SGR) (B), feed intake (FI) (C), feed efficiency (FE) (D) and hepatosomatic index (HSI) (E) of juvenile large yellow croaker fed diets containing oxidised fish oil supplemented with vitamin E (VE) for 10 weeks. Values are means, with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were not significantly different (P>0·05) among treatments. BW, body weight; FFO, fresh fish oil; LO, lowly oxidised; MO, moderately oxidised; HO, highly oxidised.
FI of fish fed MO and HO were significantly (P<0·05) higher than fish fed FFO and LO. VE supplementation increased the FI of fish fed FFO and LO, but decreased the FI of fish fed MO and HO (Fig. 1(C)). No statistically significant differences (P>0·05) were detected in FE between treatments (Fig. 1(D)). The hepatosomatic index (HSI) of fish increased with increasing dietary POV and decreased with VE supplementation. The HSI of fish in the HO group was significantly higher (P<0·05) than in the HO/VE group (Fig. 1(E)).
Body composition
No statistically significant differences (P>0·05) in whole-body composition were detected among treatments, with the exception of lipid content (P<0·05). Fish fed the oxidised diets had a lower lipid content than fish fed FFO, and their lipid content increased with VE supplementation. Dietary inclusion of VE increased lipid content in the liver of fish in all the groups, with significant differences detected in fish fed FFO and LO diets (P<0·05) (Table 4).
Table 4 Body composition (% wet weight) of juvenile large yellow croaker fed diets containing oxidised fish oil supplemented with vitamin E (VE) for 10 weeksFootnote *
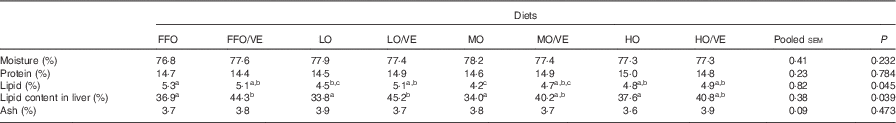
FFO, fresh fish oil; LO, lowly oxidised fish oil; MO, moderately oxidised fish oil; HO, highly oxidised fish oil.
a,b,c Mean values within the same row with unlike superscript letters were not significantly different (P>0·05, Tukey’s test).
* Data represent the mean of triplicate groups.
Antioxidant enzyme activities and respiratory burst activities
As dietary POV increased in the groups not supplemented with VE, the activities of CAT and SOD increased significantly (P<0·05). VE supplementation significantly (P<0·05) reduced the activities of CAT and SOD in fish fed the oxidised diets. In contrast, the activities of CAT and SOD were increased by VE supplementation in fish fed the FFO diet (Fig. 2(A) and (B)). Hepatic MDA levels increased significantly (P<0·05) with each increase in dietary POV (Fig. 2(C)). VE supplementation decreased the concentration of MDA in all groups, irrespective of dietary POV. No significant (P>0·05) differences in MDA concentration were observed among the VE supplementation groups (Fig. 2(C)).

Fig. 2 Hepatic catalase (CAT) activity (A), superoxide dismutase (SOD) activity (B) and malondialdehyde (MDA) content (C) of juvenile large yellow croaker fed diets containing oxidised fish oil supplemented with vitamin E (VE) for 10 weeks. Values are means, with their standard errors represented by vertical bars. a,b,c,d,e Mean values with unlike letters were not significantly different (P>0·05) among treatments. FFO, fresh fish oil; LO, lowly oxidised; MO, moderately oxidised; HO, highly oxidised.
No significant (P>0·05) difference in respiratory burst activities (optical density 630 values) was detected among treatments, although fish fed oxidised diets had lower respiratory burst activities than those fed FFO (Fig. 3).

Fig. 3 Respiratory burst activity of head kidney macrophages from juvenile large yellow croaker fed diets containing oxidised fish oil supplemented with vitamin E (VE) for 10 weeks. Values are means, with their standard errors represented by vertical bars. OD, optical density; FFO, fresh fish oil; LO, lowly oxidised; MO, moderately oxidised; HO, highly oxidised.
Air-exposure mortality
AEM increased significantly (P<0·05) with increasing dietary POV (Fig. 4). VE supplementation decreased AEM in fish fed oxidised diets and increased AEM in fish fed FFO. The positive effect of VE supplementation was most evident among fish receiving the HO diet, where a significant reduction in AEM (P<0·05) was observed.

Fig. 4 Air-exposure mortality (AEM) of juvenile large yellow croaker exposed to air for 20 min after being fed diets containing oxidised fish oil supplemented with vitamin E (VE) for 10 weeks. Values are means, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were not significantly different (P>0·05) among treatments. FFO, fresh fish oil; LO, lowly oxidised; MO, moderately oxidised; HO, highly oxidised.
Discussion
In the present study, large yellow croaker fed diets containing oxidised fish oil without supplemental VE exhibited poor growth. This is in accordance with previous studies( Reference Gao, Koshio and Ishikawa 6 , Reference Peng, Chen and Qin 26 – Reference Fontagné-Dicharry, Lataillade and Surget 28 ). It was also reported that feed oxidisation decreased FI, and consequently the growth rate, of Atlantic salmon (Salmo salar)( Reference Hamre, Kolas and Sandnes 29 ). Decreases in food intake due to the presence of oxidised lipids in the diet may be the result of altered odour or palatability of the diet( Reference Simic and Karel 30 ). However, in the present study, the FI of fish was not depressed by oxidised feed, indicating that large yellow croaker tolerate quite high levels of oxidation products before they avoid the diet because of unpleasant taste or odour. This finding is in agreement with that of Hamre et al. ( Reference Hamre, Kolas and Sandnes 29 ), who reported that FI in Atlantic salmon fed the oxidised diet was not significantly lower compared with fish fed the control diet. The FE was not significantly different among treatments, indicating that the oxidised fish oil was effectively absorbed. These results are in agreement with studies in Siberian sturgeon (Acipenser baeri)( Reference Fontagné, Bazin and Brèque 31 ). The decreased growth of fish fed oxidised diets is likely a result of increased oxidative stress caused by both the reduced nutritional value of the diet due to the loss of HUFA( Reference Lingenfelser, Blazer and Gay 32 – Reference Koven, Barr and Lutzky 34 ) and the production of toxic peroxidation products( Reference Fontagné-Dicharry, Lataillade and Surget 28 , Reference Matés, Pérez-Gómez and Castro 35 ). These mechanisms are in line with the observations from the present study, in which the activities of the two primary free-radical scavenging enzymes, CAT and SOD, were significantly increased with increasing dietary oxidised oil POV. The HSI and hepatic MDA contents were also increased with increasing dietary POV. These results clearly demonstrate that oxidised fish oil could stimulate the activities of antioxidant defence enzymes in stressed large yellow croaker and imply that fish were suffering from graded oxidative stress. Similar findings were reported in previous studies where the growth of fish fed diets containing oxidised oil was inversely proportional to the responses observed in the activities of hepatic antioxidant defence enzymes( Reference Tocher, Mourente and van der Eecken 8 , Reference Fontagné-Dicharry, Lataillade and Surget 28 , Reference Hwang, Hour and Cheng 36 ).
High-dose VE inclusion significantly enhanced the growth of large yellow croaker fed the oxidised oil diets in this study. This growth-promoting effect of VE is consistent with previous findings in tilapia( Reference Shiau and Shiau 37 ), grass carp( Reference Li, Liang and Tan 38 ), Japanese flounder( Reference Gao, Koshio and Ishikawa 6 ) and gilthead sea bream( Reference Tocher, Mourente and van der Eecken 8 ). The positive effects of VE on large yellow croaker were likely due to an alleviation of the oxidative stress induced by the oxidised oil in the diet. VE can act as a scavenger of free radicals and a singlet O2 quencher( Reference Cay and King 39 , Reference Gunstone 40 ), thereby lowering the burden placed upon antioxidant enzymes. This is consistent with the observed marked decline in the activities of hepatic antioxidant defence enzymes, which suggests significant reductions in levels of oxidative stress among large yellow croaker. In accordance, hepatic MDA concentrations, HSI values and AEM of fish were lowered by dietary VE supplementation. A similar observation was reported in grass carp where hepatic MDA content was significantly decreased following supplementation with VE( Reference Li, Liang and Tan 38 ). In the present study, VE may have also exerted beneficial effects through the enhancement of immunity in large yellow croaker. This is in accordance with the observations of significantly lower AEM of fish fed the oxidised diets supplemented with VE. Similarly, Ortuño et al. ( Reference Ortuño, Esteban and Meseguer 41 ) found that dietary administration of high doses of vitamins C and E reduced stress in sea bream (Sparus aurata) when exposed to a combination of stressors that included a 2-min air exposure.
It is noteworthy that the inclusion of a high concentration of dietary VE resulted in a significant decrease in the growth of fish fed the fresh oil diet in this study. This deleterious effect could potentially be caused by an excess dose of VE accumulated in the liver and other tissues( Reference Huang and Huang 4 , Reference Puangkaew, Kiron and Somamoto 12 , Reference Lin and Shiau 14 , Reference Peng, Chen and Qin 26 , Reference Ruff, Fitzgerald and Cross 42 , Reference Gao and Koshio 43 ). VE stored in animal tissues is partially utilised for halting the free radical cascade initiated by lipid peroxyl radicals and other assorted products of lipid peroxidation( Reference Sheehy, Morrissey and Flynn 44 ). Excess supplementation of VE to the biological system may lead to acceleration of lipid peroxidation, increasing accumulation of hydroperoxide in blood and reduced erythrocyte osmotic fragility of fish( Reference Gao, Koshio and Ishikawa 6 , Reference Tokuda and Takeuchi 16 , Reference Ito, Murata and Tsuda 17 , Reference Tokuda and Takeuchi 45 , Reference Kaewsrithong, Ohshima and Ushio 46 ). Moreover, high dietary concentrations of VE (5000 mg of α-Toc/kg of diet) have been shown to cause reduced concentrations of erythrocytes in trout blood( Reference Poston and Livingston 47 ). In addition, it was reported that an excess amount of dietary α-tocopherol could enhance lipid deposition in fish liver( Reference Tokuda and Takeuchi 16 ). Consistently, in the present study, the lipid content in the liver was increased by VE supplementation, indicating that excess VE promotes lipid accumulation, resulting in poor liver health condition in large yellow croaker. Inclusion of oxidised oil in fish diets decreased tissue α-tocopherol concentrations( Reference Baker and Davies 7 ). Therefore, it could be that these adverse effects of high-dose VE would be most evident in the FFO/VE group, due to a lack of reaction substrates in the form of oxidised oil supplied in the diet. Our results indicate that the degree of oil oxidation (i.e. the severity of oxidative stress) could be a key factor influencing the physiological effects of VE.
In conclusion, the ingestion of oxidised oil in the diet significantly decreased growth, but activated antioxidant defence enzymes, in large yellow croaker. Inclusion of high-dose VE supplementation can alleviate oxidative stress and enhance the immune response of stressed fish fed oxidised fish oil. However, high doses of VE can also exert deleterious effects on non- or less-stressed large yellow croaker.
Acknowledgements
The authors thank the participants who gave their time to this trial.
The study was supported by the National Natural Science Foundation of China (31302200), and the National Department Public Benefit Research Foundation (Ministry of Agriculture; 201003020).
J. W. and Q. A. designed the study. J. W. carried out most of experimental work and wrote the manuscript under the direction of the project leader Q. A., K. M. assisted in the experimental design and manuscript revision; H. X. and R. Z. carried out the rearing experiments. W. X. provided all fatty acid composition data.
The authors declare that there are no conflicts of interest.











