Palpitations, syncope, and arrhythmias are common reasons for children to be referred to as a paediatric cardiologist. In many cases, the aetiology of these episodes can be determined at an outpatient appointment using an appropriate history, physical examination, and ECG. When these simple, non-invasive tools are unable to elucidate the cause of a patient’s symptoms, short-term non-invasive heart rhythm monitoring, such as a Holter, is often employed. If this 24-hour, non-invasive step does not yield a diagnosis, and/or if the symptoms are rare, an insertable cardiac monitor may be used to discern its aetiology.Reference Giada, Michele and Maura1
Insertable cardiac monitors are implanted subcutaneously into the patient’s chest wall to record heart rates and rhythms, while correlating them with symptoms over prolonged periods. With the availability of Bluetooth™ capable devices linked to smartphone applications, these devices allow patients and/or their families to notify physicians in real time of concerning events.2 The Jot Dx™ is the latest model of an insertable cardiac monitor (Abbott, Abbott Park, IL), which is 1.4 cc in size with new programmable features that comes in a pre-loaded delivery system to simplify implantation technique. The recommended implantation technique calls for inserting the device at the 4th intercostal space at a 45-degree angle to the sternum, along the cardiac axis. This technique presents unique challenges in small paediatric patients due to their limited chest surface area. This limitation may lead to concerns of skin tenting at the distal tip of the device, resulting in the potential of pressure-induced skin necrosis and device extrusion. We report on the insertion of a Jot Dx™ in a very young paediatric patient using a novel vertical, parasternal insertion technique to avoid post-implantation complications.
Case presentation
A 16-month-old girl with a history of complete androgen insensitivity presented to the paediatric cardiology clinic for concerns about two episodes of altered level of consciousness. These episodes were triggered when the patient was startled and were associated with paleness and brief loss of postural tone. There was no associated crying or agitation, nor was there loss of bowel or bladder control. She quickly recovered in seconds and experienced no postictal period. At her cardiology visit, she had normal vital signs, cardiovascular physical examination, and electrocardiogram. Due to a paternal history of the bicuspid aortic valve, she underwent an echocardiogram which demonstrated normal cardiac anatomy and function. She had 14 days of cardiac monitoring with a Zio™ (iRhythm Technologies, Inc., San Francisco, CA, USA) patch, which demonstrated normal sinus rhythm; however, she did not experience an episode.
Given the unusual nature of these events and negative non-invasive test results, a decision was made to proceed with the implantation of an insertable cardiac monitor. Due to the fact that the patient was 16 months old and weighed only 11.7 kg at the time of the procedure, it was decided to implant the device in a vertical orientation along the left sternal border to reduce the risk of skin tenting, pressure-induced skin necrosis, and device extrusion.
Procedure
After obtaining informed consent, the patient was brought to the electrophysiology laboratory, where a time-out was performed and roles were assigned to staff. She underwent mask anaesthesia induction, and her chest was prepped and draped in the usual fashion. A rolled blanket was placed under the patient’s shoulders to assist with elevating the chest and to position her head, with the neck extended and turned towards the patient’s right. This head position allowed for unencumbered device implantation to the left of her sternum (Fig 1).
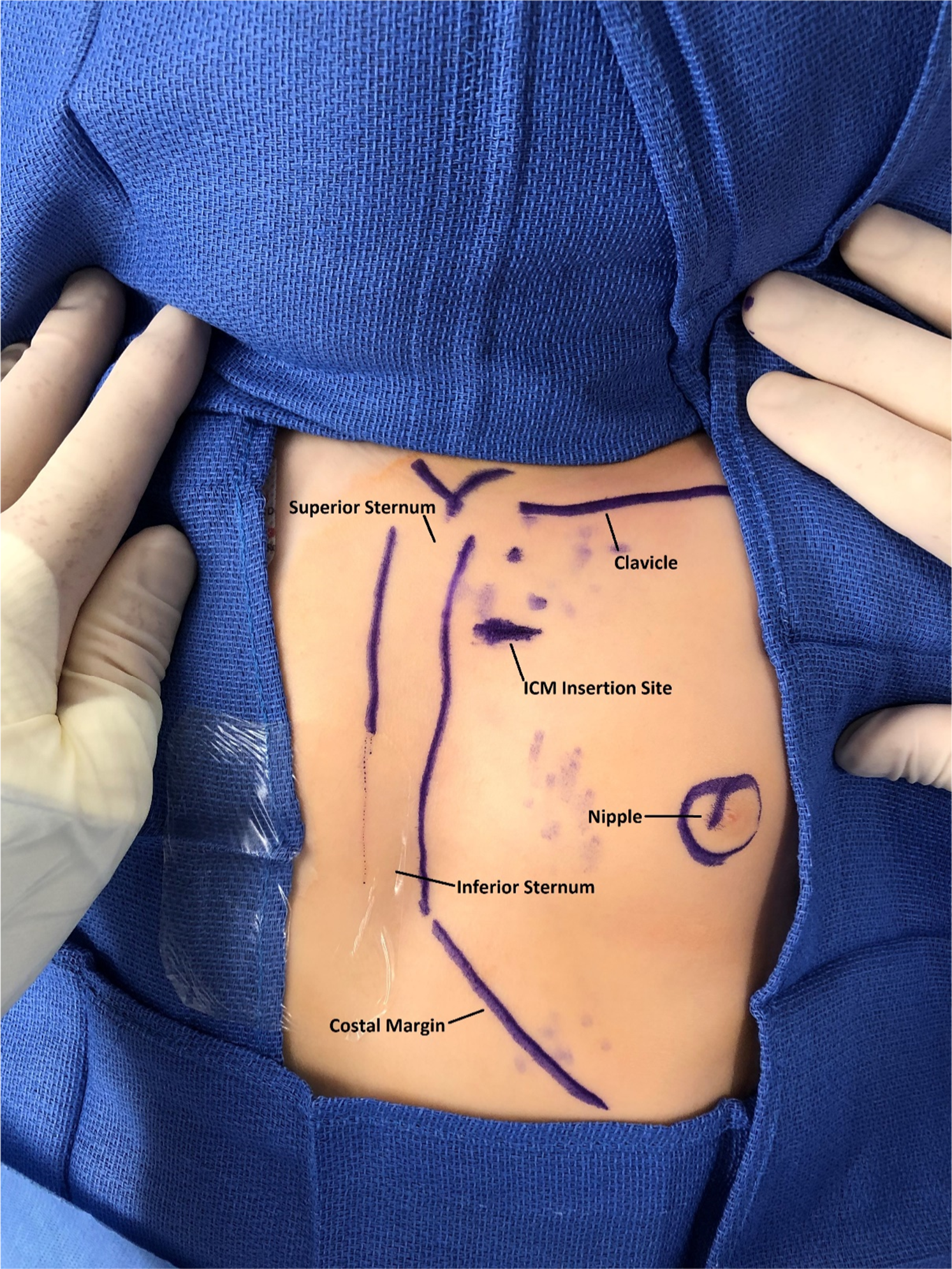
Figure 1. Anatomic landmarks and insertable cardiac monitor insertion site delineated with sterile skin marker.
3 ml of 0.5% bupivacaine was injected into the subcutaneous tissue of the 3rd intercostal space prior to the device implantation. Using the insertion tool, a horizontal 1 cm incision was performed 1.5 cm lateral to the left sternal border at the 3rd intercostal space. The device delivery system was introduced through the incision and positioned in the vertical orientation parallel to the sternum (Fig 1). The device was interrogated at the time of implantation with the Merlin™ PCS (Abbott, Abbott Park, IL). Clear P-waves were visualised on the tracings (Fig 2). Her skin was closed with 4-0 suture in a running subcuticular stitch. Anaesthesia was discontinued, the laryngeal mask airway was removed, and the patient was transferred to the cardiac step-down unit in stable condition with blow-by oxygen.

Figure 2. Interrogation of insertable cardiac monitor demonstrating good capture of P-waves.
Follow-up
The patient recovered well from the device implantation procedure and was discharged on post-operative day one. At subsequent follow-up appointments, family denied any episodes of erythema, swelling, or tenderness at the site of her device. Remote transmissions sent by the family continued to demonstrate clear P-waves, QRS complexes, and T-waves (Fig 3).

Figure 3. Remote transmission 7 months after implantation continued to demonstrate good capture of the heart rhythm.
Discussion
Insertable cardiac monitor use in paediatric patients has been found to be a useful tool in the diagnostic odyssey of patients with infrequent palpitations or atypical syncope episodes without a clear aetiology, when other non-invasive testing is inconclusive. Advantages of the Jot Dx™ include its small size allowing implantation in smaller patients, Bluetooth™ technology compatible with smartphone applications, and the ability to tailor device settings to record and transmit events pertinent to the specific clinical situation. The standard insertion technique, while acceptable in many older patients, presents some challenges when considered for younger children. Due to the small size of their chest walls and limited subcutaneous tissue, standard insertion technique raises cosmetic concerns for prominent skin bulging and risks pressure-induced necrosis of the skin or device extrusion. In this report, we have described a modified technique of elevating the sternum with a towel roll, turning the patient’s head toward the right, and inserting the device in a vertical orientation parallel to the left sternal border. This method reduces the risks of standard insertion technique and still provides excellent data capture.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.








