It is well known that the absorption of non-haem Fe is affected by the composition of the diet(Reference Miret, Simpson and McKie1). The absorption of non-haem Fe is also impaired by the presence of potent inhibitors, such as dietary fibre. Dietary fibres have been shown to impair the absorption of minerals in the small intestine because of their binding and/or sequestering effect(Reference Bosscher, Van Caillie-Bertrand and Deelstra2). Pectin is a water-soluble dietary fibre containing carboxyl groups. In solution, the carboxyl groups of pectin can form complexes with polyvalent metals, such as Ca, Mg and Fe(Reference Kohn and Furda3–Reference Debon and Tester5).
Our previous study(Reference Miyada, Nakajima and Ebihara6) showed that pectin improves Fe bioavailability in gastrectomy-induced anaemic rats, suggesting that pectin increased Fe absorption in the small intestine and/or the large intestine. Our in vitro study demonstrated that although the amount of Fe released from pectin was at a maximum at pH 2·0, it decreased as the pH value increased. At the pH of the small intestine, about 6·5–7·0, ionic Fe was not released from pectin, suggesting that pectin in the diet would reduce Fe absorption in the small intestine(Reference Miyada, Nakajima and Ebihara7). Moreover, our in vivo study showed that Fe bound to pectin was effectively utilised in rats fed an Fe-deficient diet(Reference Miyada, Nakajima and Ebihara7). These results seem to support an assumption that dietary Fe in the presence of pectin was partly absorbed from the duodenum and jejunum; however, it was also effectively absorbed from the large intestine. In the large intestine, Fe bound to pectin in the small intestine might be released by bacterial degradation and subsequently made available for absorption. Very few studies have reported the effects of degradation of pectin in the large intestine on the bioavailability of Fe bound to pectin.
In the present study, we examined whether pectin affects Fe absorption in the small intestine in ileorectomised rats. In addition, we examined the influence of caecectomy on the bioavailability of Fe bound to pectin, by measuring Hb concentration, Hb gain and Hb regeneration efficiency (HRE).
Materials and methods
Pectin
Commercial citrus pectin purchased from Herbstreith & Fox GmbH (Neuenburg, Germany) was used in Expt 1. The degree of esterification of pectin was 38–40 % and Fe content was 206 mg/kg. Commercial non-amide pectin purchased from Sanofi Bio Industries (Paris, France) was used in Expt 2. The degree of esterification of pectin was 34–40 % and Fe content was 119 mg/kg. The Fe content of pectin was measured by flame atomic absorption spectrophotometry (AA 6400F; Shimadzu, Kyoto, Japan) after wet ashing in HNO3–HClO4 (3:1). To determine the Fe levels, the standard addition method was used.
Preparation of ferrous iron and oligogalacturonic acid complex
Pectinase (1·5 g, Sucrase N; Sankyo, Tokyo, Japan) was added to 1000 ml of pectin (X66B) solution (50 g/1000 ml) and then stirred at 40 °C for 4 h. To remove the enzyme and the residue, the solution was filtered with an ultrafiltration membrane (Lab module AHP1010; Asahi KASEI Chemicals, Tokyo, Japan), with a molecular weight cut-off of 50 000. The filtrate was subjected to further filtration using an ultrafiltration membrane (Lab module SEP1013; Asahi KASEI Chemicals), with a molecular weight cut-off of 3000. The main component of the filtrate fraction had a molecular weight of about 1250, which was presumed to be a six-residue oligomer of galacturonic acid (oligogalacturonic acid; OGA). The pH value of the OGA fraction measured with a pH meter (calibrated at 20°C, Model 10; Horiba, Tokyo, Japan) was 3·4. To prepare the FeII–OGA complex, 25·3 g ferrous sulphate (FeSO4.7H2O; Wako Pure Chemical Industries, Osaka, Japan) were added to 900 ml of the OGA fraction and then stirred for 30 min. By the bathophenanthroline method with and without hydroxylamine hydrochloride solution (4 %, w/v) as the reducing reagent, Fe bound to OGA was confirmed to be in the ferrous form. If necessary, soluble dextrin (painedex#6; Matsutani Chemicals, Osaka, Japan) was added to the FeII–OGA solution to produce a dextrin:FeII–OGA ratio of 10:1, which was freeze-dried for 24 h. Flame atomic absorption spectrophotometry, performed as described above, measured the Fe content of the FeII–OGA complex as 13 333 mg/kg.
Animals and diets
The Laboratory Animal Care Committee of Ehime University approved the study. Rats were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of Ehime University.
In Expt 1, a fibre-free diet (FF diet) and a pectin diet were used (Table 1). The pectin diet was prepared by adding 50 g citrus pectin to 950 g of the FF diet. The Fe contents of the FF diet and the pectin diet were 48·0 and 55·2 mg/kg, respectively.
Table 1 Composition of the experimental diets (g/kg diet)
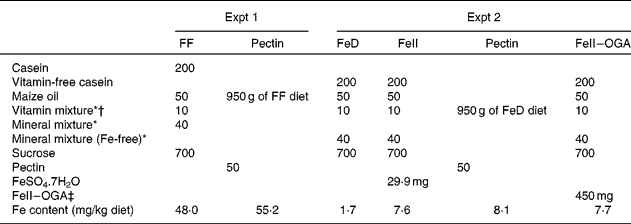
FF, fibre-free; FeD, Fe-deficient diet; FeII, ferrous iron; FeII–OGA, complex of ferrous iron and oligogalacturonic acid.
* Based on AIN-76(20).
† The vitamin mixture used in the present study contained choline chloride at 20 g/kg vitamin mixture.
‡ FeII–OGA prepared by the enzymatic degradation of pectin.
Male Wistar rats (Tokushima Laboratory Animal Research, Tokushima, Japan), weighing approximately 100 g, were used in the present study. Rats were housed individually in screen-bottomed, stainless-steel cages in a room maintained at 23 ± 1°C with a 12 h light–12 h dark cycle (lights on, 07.00–19.00 hours). Rats were given free access to water and the FF diet during the acclimatisation period of 7 d. Then, ten rats were subjected to ileorectomy under anaesthesia by an intraperitoneal injection of sodium pentobarbital (40 mg/kg body weight). The caecum and the colon were removed after ligation of blood vessels. The cut edge of the ileum was end-to-end anastomosed to the cut edge of the colon, at the position of approximately 3 cm proximal from the anus. After surgery, all rats were deprived of any nutritional supply and water for 24 h, and were then fed the FF diet for 7 d. The ileorectomised rats were randomly divided into two groups of five rats. During the experimental period of 21 d, rats were given the FF diet or the pectin diet. Rats were provided with deionised distilled water. The body weight and food intake of each rat were recorded daily in the morning before replacing the diet. Faeces were collected for examination of the apparent absorption of Fe for the last 7 d of the experimental period. After faeces were dried at 105 °C for 24 h, they were weighed and then milled.
In Expt 2, male Wistar rats weighing about 80–100 g (Japan SLC, Hamamatsu, Japan) were housed individually in screen-bottomed, stainless-steel cages in a room maintained at 23 ± 1°C with a 12 h light–12 h dark cycle (lights on, 07.00–19.00 hours). Rats were given free access to water and the FF diet during the acclimatisation period of 7 d. Then, fifteen rats were subjected to caecectomy under anaesthesia by an intraperitoneal injection of sodium pentobarbital (40 mg/kg body weight). The caecum was removed after ligation of blood vessels. After surgery, all rats were deprived of any nutritional supply and water for 24 h, and were then fed the FF diet for 7 d. The remaining fifteen rats were subjected to a sham operation; the abdominal cavity was opened and then closed in these rats. The caecectomised rats and the sham-operated rats were randomly divided into six groups of five rats and were allowed free access to distilled deionised water and one of the following diets for 3 weeks: diet containing ferrous iron (FeII) at 6 mg/kg diet, diet containing pectin at 50 g/kg diet (pectin diet) and diet containing FeII–OGA at 450 mg/kg diet (FeII–OGA diet) (Table 1). The source of FeII was ferrous sulphate (FeSO4.7H2O). The amount of Fe per kg diet of the FeII diet, the pectin diet, where the Fe bound to pectin was the only Fe source, and the Fe–OGA diet was 7·6, 8·1 and 7·7 mg, respectively. The body weight and food intake of each rat were recorded daily in the morning before replacing the diet.
Analytical methods
In Expt 1, approximately 0·5 g of the test diets and faeces were used in the determination of Fe content. Fe content was measured by atomic absorption spectrophotometry (model AA670; Shimadzu) using FeCl3 in 0·1 m-HCl solution (Fe 1000 mg/l; Wako Pure Chemical Industries) as the standard. The apparent absorption of Fe was determined using the following equation:
To determine the degradation rate of pectin, approximately 0·5 g of the pectin diet and dried faeces were added to 75 ml of 0·5 % ammonium oxalate solution and incubated for 45 min at 85°C before addition of 10 ml concentrated sulphuric acid and boiling for 1 h. After cooling, samples were made up to 100 ml with distilled water. Approximately 0·4 ml of the solution was measured at 530 nm (UltrospecII; LKB Biochrom, Cambridge, UK) after the carbazol reaction with galacturonic acid as the standard. The degradation rate of dietary pectin was determined using the following equation:
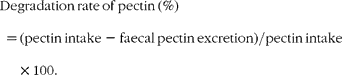
In Expt 2, Hb concentration was measured by the cyanmethaemoglobin method using a colorimetric Hb assay kit (Hb-Test; Wako Pure Chemical Industries). Blood was obtained from the tail tip every week of the experimental period. To calculate total Hb content in the blood, the mass of blood was assumed to be 67 g/kg of body mass, and Hb was assumed to contain 3·35 mg Fe/g(Reference Miller8). HRE was calculated according to the method of Mahoney & Hendricks(Reference Mahoney, Hendricks and Kies9).
Statistical analysis
All results are expressed as means with their standard errors. Data were analysed by ANOVA and significant differences between groups were determined by Student's t test. Differences were considered significant at P < 0·05. All statistical analyses were performed using the statistical software package JMP 6.0 (SAS Inc., Cary, NC, USA).
Results
Expt 1
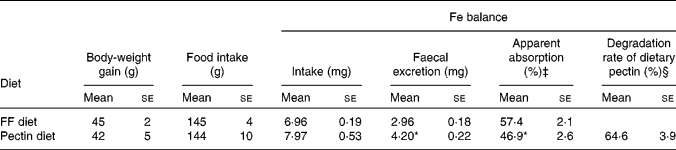
Body-weight gain and food intake for the last 7 d of the experimental period were not significantly different between rats fed the pectin diet and rats fed the FF diet. Fe excretion in the faeces was significantly increased by feeding the pectin diet compared with feeding the FF diet, though Fe intake remained the same. The apparent absorption of Fe was significantly decreased by feeding the pectin diet compared with feeding the FF diet (Table 2). Approximately 65 % of ingested pectin was degraded in ileorectomised rats.
Table 2 Effects of pectin on body-weight gain, food intake, iron balance and degradation rate of dietary pectin in ileorectomised rats (Expt 1)†
(Mean values with their standard errors, n 5)
FF, fibre-free.
* Mean values were significantly different from those of the rats fed the FF diet (P < 0·05).
† Measurements of body-weight gain, food intake and faecal collection were performed for 7 d during the experimental week 2 in Expt 1.
‡ The apparent absorption of Fe is expressed as (Fe intake − faecal Fe excretion)/Fe intake × 100.
§ The degradation rate of dietary pectin is expressed as (pectin intake − faecal pectin excretion)/pectin intake × 100.
Expt 2
Body-weight gain in rats fed the FF diet was significantly decreased by caecectomy. In the groups fed the pectin diet and the FeII–OGA diet, body-weight gain was not significantly different between the caecectomised rats and the sham-operated rats. Food intake and Fe intake were not significantly different between the caecectomised rats and the sham-operated rats (Table 3). Hb concentration at week 0 in sham-operated rats and caecectomised rats were 142 (se 3) and 144 (se 3) g/l in the group fed the FeII diet, 141 (se 2) and 136 (se 2) g/l in the group fed the pectin diet, 133 (se 2) and 131 (se 1) g/l in the group fed the FeII–OGA diet. There was no significant difference on Hb concentrations at week 0 between the caecectomised group and the sham-operated group. In groups fed the FeII diet and the FeII–OGA diet, there were no significant differences in the percentage increase in Hb concentration relative to week 0 and Hb gain throughout the experimental period between the caecectomised rats and the sham-operated rats. In rats fed the pectin diet, the percentage increase in Hb concentration relative to week 0 was significantly lower throughout the experimental period in the caecectomised rats compared with the sham-operated rats (Fig. 1). Hb gain was significantly lower throughout the experimental period in the caecectomised rats compared with the sham-operated rats (Fig. 2). In rats fed the pectin diet, HRE was significantly decreased by caecectomy only in experimental week 1. HRE in the groups fed the FeII diet and the FeII–OGA diet was not significantly different in the caecectomised rats compared with the sham-operated rats (Table 4).
Table 3 Effects of caecectomy on body-weight gain, food intake and iron intake in rats fed the ferrous iron (FeII) diet, the pectin diet or the complex of ferrous iron and oligogalacturonic acid (FeII–OGA) diet (Expt 2)
(Mean values with their standard errors, n 5)

* Mean value was significantly different from that of the sham-operated rats (P < 0·05).
† Initial body weight in sham-operated rats and caecectomised rats were 118 (se 2) and 120 (se 3) g in the group fed the FeII diet, 118 (se 2) and 119 (se 3) g in the group fed the pectin diet, 98 (se 3) and 104 (se 2) g in the group fed the FeII–OGA diet.

Fig. 1 Changes in Hb concentration over 3 weeks. Sham-operated rats (●) and caecectomised rats (■) were allowed free access to distilled deionised water and one of the following diets for 3 weeks: (a) the ferrous iron diet, (b) the pectin diet and (c) the complex of ferrous iron and oligogalacturonic acid diet. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different between the sham-operated and caecectomised rats (P < 0·05).

Fig. 2 Changes in Hb gain over 3 weeks. Sham-operated rats (●) and caecectomised rats (■) were allowed free access to distilled deionised water and one of the following diets for 3 weeks: (a) the ferrous iron diet, (b) the pectin diet and (c) the complex of ferrous iron and oligogalacturonic acid diet. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different between the sham-operated rats and caecectomised rats (P < 0·05).
Table 4 Effects of caecectomy on Hb regeneration efficiency every week in rats fed the ferrous iron (FeII) diet, the pectin diet or the complex of ferrous iron and oligogalacturonic acid (FeII–OGA) diet (Expt 2)
(Mean values with their standard errors, n 5)
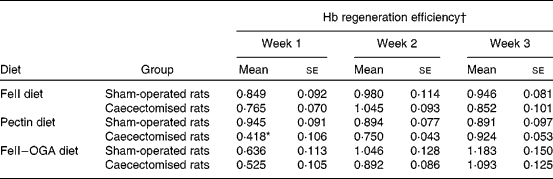
* Mean value was significantly different from that of the sham-operated rats (P < 0·05).
† Hb regeneration efficiency is expressed as Hb gain × 3·35 mg Fe/Fe intake.
Discussion
Expt 1 was designed to examine whether dietary pectin affects Fe absorption in the small intestine. In ileorectomised rats, although the Fe intake was the same in the FF diet group and the pectin diet group, faecal excretion of Fe in the latter group was significantly higher than in the former group. Apparent absorption of Fe was significantly lower in the pectin diet group compared with the FF diet group. Inhibition of Fe absorption by pectin is most probably due to pectin binding to Fe, thus making the Fe unavailable for absorption. Pectin is a polycarboxylic acid with a pK a value of about 3·5(Reference Sila, Van Buggenhout and Duvetter10). At the pH of the small intestine, about 6·5–7·0, pectin is a negatively charged polysaccharide in its ionised form that can interact with positively charged Fe. Above pH 5, ionic Fe was not released from pectin(Reference Miyada, Nakajima and Ebihara7), supporting the assumption that pectin in the diet would reduce Fe absorption. Dietary pectin, with a high degree of esterification and low molecular weight, has been found to enhance Fe solubility in the intestine and absorption of Fe in rats(Reference Kim and Atallah11, Reference Kim, Atallah and Amarsiwardena12). The degree of esterification, molecular weight and/or mode of distribution of free carboxylic groups along the polymer chain strongly affect the strength of the binding of minerals to pectin. Pectin used in the present study had a low degree of esterification. In our previous study(Reference Miyada, Nakajima and Ebihara6), pectin with a low degree of esterification increased Fe bioavailability by increasing Fe absorption in gastrectomy-induced anaemic rats.
The degradation rate of dietary pectin was approximately 65 % in ileorectomised rats. Pectin was not completely fermented in the small intestine. Any remaining pectin might bind Fe in the small intestine and then enter the large intestine. In the large intestine, Fe in pectin might be released by bacterial degradation and subsequently made available for absorption. Depolymerisation of pectin was relatively low in the ileum, suggesting that pectin passes through the small intestine as a macromolecule. In support of this, low-methoxyl pectin was fermented faster than high-methoxyl pectin in the caecum(Reference Dongowski, Lorenz and Proll13).
Expt 2 was designed to examine whether degradation of pectin in the large intestine contributes to the bioavailability of Fe in pectin. Fe absorption is responsive to the level of body Fe stores. In an Fe-deficiency state, various factors to control Fe absorption change considerably and Fe absorption increases remarkably(Reference Lombard14). To evaluate the bioavailability of Fe in the present study, rats were given test diets without making Fe deficiency. The bioavailability of Fe bound to pectin was diminished by caecectomy, although the bioavailability of FeII was not affected by caecectomy, suggesting that absorption of Fe bound to pectin takes place, to some extent, in the colon. The bioavailability of Fe in the FeII–OGA complex, which was presumed to be oligomers of galacturonic acid prepared by the enzymatic degradation of pectin, was not affected by caecectomy. These results suggest that microbial degradation of pectin in the large intestine is extremely important with regard to the bioavailability of Fe bound to pectin. Fe bioavailability may be dependent on the level of body Fe stores and Fe absorption. In rats fed the pectin diet, the differences in Hb concentration, Hb gain and HRE at week 1 between the caecectomised group and the sham-operated group might be partly due to the decrease in absorbed Fe, although the level of body Fe store was still unknown.
Pectin is almost completely fermented in the caecum of rats, resulting in the release of Fe from pectin. There are only a few reports of Fe absorption in the large intestine; however, sufficient Fe was absorbed in the large intestine for recovery from Fe-deficiency anaemia in rats(Reference Ebihara, Okano and Miyada15, Reference Ebihara and Okano16). Fe absorption from the large intestine is less efficient compared with the duodenum but it is significant, especially during Fe deficiency(Reference Yeung, Glahn and Welch17). Studies have shown that Fe is absorbed in the caecum(Reference Bougle, Vaghefi-Vaezzadeh and Roland18). The prevention of anaemia by fructo-oligosaccharides in gastrectomised rats was diminished by caecectomy(Reference Sakai, Ohta and Shiga19).
Presumably, Fe must be in a soluble form in the lumen of the gastrointestinal tract before it can be taken up by the enterocytes. Pectin is easily fermented by intestinal bacteria to produce SCFA. Propionate may form a soluble complex with Fe, thereby maintaining the solubility of Fe in the colon, as well as facilitating its transfer across the endosome membranes of the enterocytes(Reference Bougle, Vaghefi-Vaezzadeh and Roland18). Products generated by the microbial degradation of pectin, such as OGA, may form a soluble complex with Fe in the large intestine. In rats fed pectin, di- and tri-galacturonic acids were found in the contents of the colon(Reference Dongowski, Lorenz and Proll13). It is possible that Fe in the FeII–OGA complex is absorbed by a different mechanism to the Fe in pectin. Although the mechanism has not yet been clarified, the binding of Fe by pectin in the small intestine might have beneficial effects on the absorption of Fe from the large intestine.
Acknowledgements
The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors would like to thank Mr N. Nakanishi of POLA R&D Laboratories for the supply and preparation of OGA and its components. The authors declare that there are no conflicts of interests. T. M., A. N. and K. E. conducted the research; T. M. and A. N. analysed the data; T. M. and K. E. wrote the manuscript; T. M. had primary responsibility for the final content. All authors read and approved the final manuscript.








