Introduction
The management of herbaceous systems is currently one of the major factors influencing habitat selection and population productivity of grassland birds (Perlut 2006, Faria et al. Reference Faria, Morales and Rabaça2016). However, in recent years, important changes have occurred in grassland systems across Europe (see Huyghe et al. Reference Huyghe, Vliegher, Gils and Peeters2014). In south-western Iberia a significant number of farms reoriented their production model to beef production, which has had a significant impact on farm management due to changes in grazing type and intensity, but also through the increase of hay/silage production and abandonment of extensive mixed sheep-cereal production (Correal et al. Reference Correal, Robledo, Ríos, Rivera, Lloveras, González-Rodríguez, Vázquez-Yáñez, Piñeiro, Santamaría, Olea and Poblaciones2006). In the Alentejo region (Portugal) for instance, cattle numbers more than doubled between 1989 and 2013 while conversely, sheep numbers decreased by about 39% (INE 2014).
One species that is more affected by grassland management in Europe is the Little Bustard Tetrax tetrax, a species of global conservation concern (SPEC 1; BirdLife International 2004), classified as “Vulnerable” on the European Red List of Birds (BirdLife International 2015), and rapidly declining in Iberia over recent years (Morales et al. Reference Morales, Traba and Arroyo2015). The Little Bustard is a sexually dimorphic species in which males do not participate in brood rearing (Cramp and Simmons Reference Cramp and Simmons1980). Although males and females tend to use different vegetation structures (short vegetation in the case of males and medium height – up to 30–40 cm – in females; Morales et al. Reference Morales, Traba, Carriles, Delgado and García de la Morena2008; Silva et al. Reference Silva, Estanque, Moreira and Palmeirim2014), recent studies demonstrated a considerable overlap in habitat requirements between males and females during the breeding season (e.g. grazing intensity and landscape features; Faria et al. Reference Faria, Rabaça and Morales2012; Tarjuelo et al. Reference Tarjuelo, Delgado, Bota, Morales, Traba, Ponjoan, Hervás and Mañosa2013). Faria et al. (Reference Faria, Rabaça and Morales2012) highlighted the selection of fields with low to moderate stocking rates by territorial males and breeding females. Wolff et al. (Reference Wolff, Dieulevent, Martin and Bretagnolle2002) documented the importance of improved pastures for the species in French Mediterranean breeding areas, although fields used for haying were described as scarcely used by the Little Bustard (Wolff et al. Reference Wolff, Paul, Martin and Bretagnolle2001). On the other hand, Lapiedra et al. (Reference Lapiedra, Ponjoan, Gamero, Bota and Mañosa2011) revealed the negative effects of early harvesting on Little Bustard productivity in intensive cereal landscapes. However, there is still no information available on the effect of management of Mediterranean dry grasslands on Little Bustard productivity.
This paper addresses the factors that contribute to population productivity and habitat selection of the Little Bustard in grassland-dominated landscapes of south-west Iberia. Specifically, we aimed to determine how grazing management and hay production during the winter and spring influence foraging behaviour and population productivity in Little Bustards. Our hypotheses are that population productivity (estimated as the number of juveniles detected per unit area and per female) and habitat selection of males, females and families during the final stage of the breeding season and the beginning of the post-breeding season are determined by (1) the type of vegetation in fields, (2) the type of livestock, (3) the rotation pattern of grazing and (4) stocking rates.
Materials and methods
Study area
The study area is located in Évora region (Alentejo province, south Portugal; 7.884902W, 38.533521N; Figure 1), in the Mesomediterranean biogeographic region (Rivas-Martínez et al. Reference Rivas-Martínez, Penas and Díaz2004). Average annual temperatures vary from 9.6°C in winter to 24.1°C in summer and the annual rainfall averages 586 mm (1981–2010 period; IPMA 2015, 2016). As in most areas of southern Portugal, the region of Évora has experienced in the last two decades land-use conversion, with extensive cereal agriculture replaced by irrigated crops, permanent grasslands for livestock rearing and, to a lesser extent, temporary grasslands (i.e. cereal, leguminous or mixed crops for hay production). Nowadays the study area still holds the main habitats of Little Bustard, with a mosaic landscape dominated by permanent/temporary grasslands (70–80%) within which extensive cereals, leguminous crops for grain production (10–20%), and summer crops (5–10%) e.g. maize, sorghum, also occur. The mean field size in the study area is around 70 ha. The study area is partly included in the Évora Special Protection Area for birds. It holds important populations of protected grassland bird species such as Little Bustard and Great Bustard Otis tarda (394 and 39 individuals, respectively; Pinto et al. Reference Pinto, Rocha and Moreira2005, Silva and Pinto Reference Silva and Pinto2006). Little Bustard occurs here at densities (0.4 males/100 ha; Silva and Pinto Reference Silva and Pinto2006) lower than in other areas of southern Portugal (e.g. São Vicente and Vila Fernando plains; Silva et al. Reference Silva, Estanque, Moreira and Palmeirim2014), although locally the species can be found at moderate to high densities (up to three males/100 ha; unpubl. data).
Figure 1. Location of the study area and sampling fields.
Field methodology
We conducted bird counts during five consecutive breeding seasons from 2012 to 2016 using low speed 4x4 car surveys (c.5 km/h). Sampling fields were randomly selected in the plain located at the south of the city of Évora (c.15,000 ha). The main land uses are interspersed over the study area, instead of being concentrated in any particular sector. Sampling was repeated two or more years in 70% of fields (41 unique fields). In total, we performed counts in 33 fields in 2012 (2,518 ha), 37 fields in 2013 (2,605 ha), 34 fields in 2014 (2,299 ha), 24 fields in 2015 (1,512 ha) and 25 in 2016 (1,661 ha). Field size varied between 30 and 180 ha.
To maximise bird detection, surveys were carried out during the first three and a half hours after sunrise or in the last two and a half hours before sunset (one visit per field), coinciding with the species’ activity peaks, from 22 June to 4 July (see Tarjuelo et al. Reference Tarjuelo, Delgado, Bota, Morales, Traba, Ponjoan, Hervás and Mañosa2013). Due to the low densities of this species in the region of Évora, we opted to perform total counts by searching the fields for birds using roads, tracks, livestock paths, firebreaks or driving in the grass. This latter option was permitted by farmers and used when the spatial arrangement of tracks and livestock paths in the field did not allow vehicle access to the core of the field (this procedure has no legal implications and the disturbance of birds is a minor issue since most juveniles were already out of nest and able to fly). With this procedure we ensured an accurate and comparable sampling between fields, accounting for potential differences in the detectability of individual bustards in fields with different vegetation structure (height and cover). Surveys encompassed stops every 200 m to search with binoculars and stops at hilltops to search with a spotting scope. The time spent in each field was proportional to field size. Bird observations comprised (1) solitary males, (2) solitary females, (3) males and females, (4) families (females with juveniles) and (5) mixed groups (families with males). Juveniles, second year males, and females were identified by an experienced observer (NF) and following the criteria provided by Jiguet and Wolff (Reference Jiguet and Wolff2000): size, silhouette and pattern of wing feathers. From the 153 fields surveyed, 141 fields were also sampled during the last fortnights of April and May to obtain numbers of displaying males (one estimate per month), using point counts with a radius of 250 m. One sampling point was randomly located within each field.
Vegetation in fields was classified into four dominant types according to height and haying state: < 15 cm, 15–30 cm, > 30 cm and hayed (hay stubbles). Variation in vegetation height was mostly low within fields and categories were easily assigned visually in the field (in case of doubt, vegetation height was measured). The information on grazing (number of livestock and period of stay in each field) was collected during field work or provided by land managers at least once per month. The effect of livestock type was evaluated by dividing sample fields into two categories: (1) grazed only by cattle and (2) grazed by sheep or mixed livestock. The effect of grazing rotation type was evaluated by classifying fields into five categories: fields under (1) continuous grazing (CON); (2) short-medium term rotation (SMR; livestock returns cyclically to the field after 2–6 weeks since the last grazing event); (3) seasonal grazing (SSG; ungrazed during winter and grazed in spring); (4) seasonal grazing, ungrazed during spring and grazed in winter or totally ungrazed (USWG) and (5) irregular grazing (IRR; the grazing pattern did not fit into any of the previous categories and livestock used the field regularly but with no obvious temporal pattern). Fields under seasonal grazing, ungrazed during spring and grazed in winter and fields totally ungrazed (USWG) were pooled because their vegetation structure was similar in late spring (tall and dense). The effects of stocking rates on Little Bustard population productivity and late breeding habitat selection were evaluated by considering three grazing periods: (1) winter – period of low vegetation growth (January and February - JanFeb), (2) spring – peak of vegetation growth (March to May - MarMay) and (3) late spring – vegetation stops growing and dries out (June). Stocking rates are presented as livestock units (LU) and were calculated according to the following ratio: adult cattle = 1 LU; yearling cattle (6–24 months) = 0.6 LU; cattle aged less than 6 months = 0.4 LU and adult sheep = 0.15 LU (DRE 2016).
Data analyses
First, because the number of males and females and males observed with juveniles was highly variable, we assessed for potential concurring effects of male or group vigilance behaviour (predator vigilance) in the selection of fields by females with juveniles. To do so, we calculated correlations between population productivity (both in juveniles/ha, PFI and juveniles/female, PFE) and displaying male densities in April/May and adult density at late breeding.
Second, we used Generalized Linear Mixed Models (GLMM; Bolker 2009) to assess Little Bustard response to stocking rates at field level in the grazing periods defined previously. GLMMs were restricted to evaluate grazing intensity (stocking rates) because the low number of observations did not allow us to include all habitat variables in the modelling approach (as explained below, the remaining variables were analysed using Kruskal-Wallis and Dunn tests). GLMMs assumed a Poisson distribution of the data and field identity was used as random factor. As said above, juveniles were observed in the company of one or more females and therefore it was not possible to assess at the individual female level for differences between females with and without juveniles. The abundances of males, females and juveniles were used as dependent variables (n = 153 fields) and area of field was used as an offset variable (juvenile results are therefore equivalent to PFI). In order to evaluate potential non-linear responses and thresholds of management utility, quadratic terms of stocking rates were allowed in models, but only for MarMay grazing period which coincides with the peak of vegetation growth (temperature and rainfall strongly limit vegetation growth during JanFeb and June periods therefore, non-linear responses to stocking rates are less likely to occur). The global model used can be described as follows:
LB ∼ JanFeb + (MarMay+MarMay 2) x weatherR + June
where weatherR is a factor variable indicating the weather characteristics of each year (wet or dry), used to identify potential weather-dependent responses to stocking rates (dry years: 2012 and 2015; wet years: 2013, 2014 and 2016; see Table S1 in the online supplementary material for detailed annual weather statistics). Due to the limited number of observations, we restricted interaction terms to the MarMay period and to the male and female dataset. All models were checked for over-dispersion. Model ranking and selection was achieved by calculating Akaike’s Information Criterion corrected for small sample size (AICc). Models with the lowest AICc and within less than four units of ΔAICc were selected as best models and considered similar in performance (Burnham and Anderson Reference Burnham and Anderson2002). The residuals of full and best models were tested for spatial autocorrelation using Moran’s I statistic (Cliff and Ord Reference Cliff and Ord1981).
Lastly, we used non-parametric Kruskal-Wallis tests to assess for relationships between male and female densities, and PFI with (1) the vegetation type, (2) livestock type and (3) grazing rotation type (n = 153 fields). The significance of differences between categories was evaluated using Dunn tests after Bonferroni correction.
All calculations were performed using packages lme4 (Bates et al. Reference Bates, Maechler, Bolker and Walker2015), MuMIn (Bartoń, 2016), spdep (Bivand and Piras Reference Bivand and Piras2015), dunn.test (Dinno Reference Dinno2017) and R for Windows (R Development Core Team 2016).
Results
Bird abundance and population productivity
Field surveys returned a total of 38 records of the species, distributed in 31 out of the 153 sampled fields. The minimum distance between records (observations of the species) was 184 m. Males were detected in 23 fields, females in 25 and juveniles in 14 fields (see Table 1 for detailed statistics). Average PFE was 0.51 ± 0.62 juveniles per female (n = 43 females) and the sex ratio calculated was 1.58 males per female. A strong decrease in bird densities and PFI was detected between 2012 and 2016 (Figure 2).
Table 1. Summary statistics by type of record (number of fields) and age/sex (number of birds) for the Little Bustard population in the region of Évora during the late breeding seasons of 2012 to 2016.

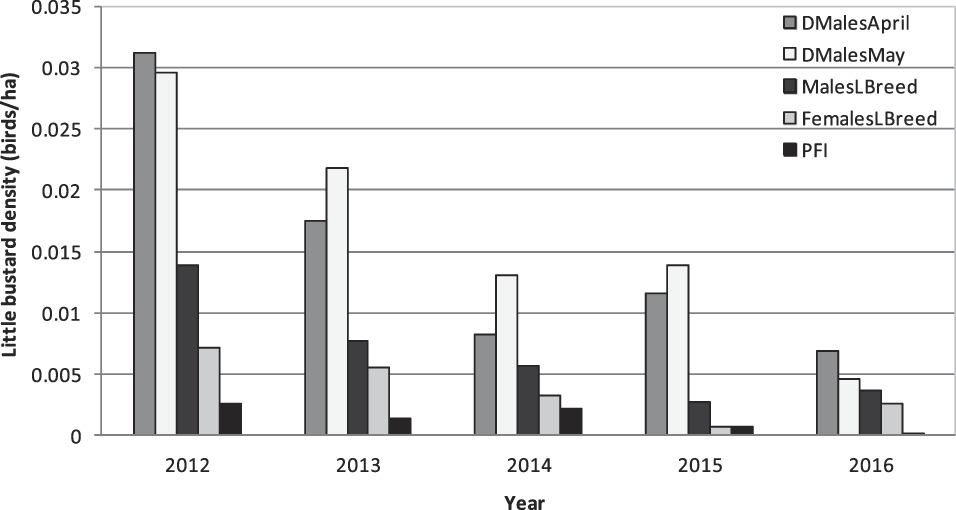
Figure 2. Variation of Little Bustard density from 2012 to 2016 in the region of Évora. Data refers to the average density of displaying males in April and May (DMalesApril and DMalesMay) and to male, female (MalesLBreed and FemalesLBreed) and PFI (population productivity in juveniles/ha) during late breeding.
PFI was positively correlated with the density of displaying males in April and particularly with that found in May (April: r = 0.18, p = 0.038; May: r = 0.35, P < 0.001; n = 141). However, no significant correlations were obtained between PFE and the density of displaying males (April: r = 0.22, P = 0.36; May: r = -0.05, P= 0.84; n = 20). We found no significant correlations between the density of adults (adult male and female) or males in fields at late breeding and PFI (Adults: r = 0.12, P = 0.50; Males: r = 0.01, P = 0.95; n = 30) or PFE (Adults: r = -0.22, P = 0.25; Males: r = -0.24, P = 0.19; n = 30).
Stocking rates
The results of GLMM modelling indicate that higher abundance of Little Bustard males was found in fields (1) with high stocking rates between March and May in wetter years, and in fields with stocking rates around one LU/ha in dry years, and (2) ungrazed or under low stocking rates during the months of January and February (Table 2 and Figure 3). The intensity of grazing during June seems to be less important in determining the abundance of males (Table 2). The results for female abundance were mostly similar to those of males (Table 2), but in this case optimum stocking rates in dry years were found around 0.8 LU/ha (Figure 3).
Table 2. Summary results of GLMM modelling approach on the relationship between the abundance of Little Bustard males, females and juveniles (PFI, see methods), and stocking rates in three grazing periods from January to June (JanFeb – January to February, MarMay – March to May and June – June; quadratic terms are presented). The AICc, ΔAICc and Akaike weights of each model are presented. Only models with ΔAICc < 4 and the null model are presented.


Figure 3. Simulations of predicted abundance of males (top-left), females (top-right) and juveniles (bottom) during March-May grazing period, obtained from GLMM models. Bootstrap confidence intervals for predictions (95%) are presented. Simulations for males and females are shown by weather regime (grey lines – wet years, black lines – dry years).
Higher PFI was found in fields under moderate stocking rates (c.0.9 LU/ha; Figure 3). Just as for males and females, PFI was higher in fields ungrazed or under low stocking rates during winter (Table 4). Again, the intensity of grazing during June seems to be of lesser importance in models (Table 4). Moran’s I tests revealed no significant autocorrelation in the residuals of the GLMMs of males, females and juveniles (Table S2).
Vegetation structure, grazing timing and livestock type
The results of Kruskal-Wallis tests revealed significant differences in the density of the species between types of vegetation considered (males: ![]() $\chi _3^2$ = 22.00, P < 0.001; females:
$\chi _3^2$ = 22.00, P < 0.001; females: ![]() $\chi _3^2$ = 13.02, P < 0.001; PFI:
$\chi _3^2$ = 13.02, P < 0.001; PFI: ![]() $\chi _3^2$ = 8.70, P = 0.03). Dunn tests indicate that the density of males and females was significantly higher in fields with short vegetation (<15 cm) compared to hay stubbles, tall vegetation and medium-sized vegetation (Table 3). PFI tended to be higher in fields with short vegetation (<15 cm) compared to hay stubbles and tall vegetation but results were only marginally significant.
$\chi _3^2$ = 8.70, P = 0.03). Dunn tests indicate that the density of males and females was significantly higher in fields with short vegetation (<15 cm) compared to hay stubbles, tall vegetation and medium-sized vegetation (Table 3). PFI tended to be higher in fields with short vegetation (<15 cm) compared to hay stubbles and tall vegetation but results were only marginally significant.
Table 3. Summary statistics of Dunn tests on the relationships between male, female and juvenile densities (PFI) with vegetation type (significant p-values are presented in bold characters). Vegetation categories are presented as follows: less than 15 cm - < 15; between 15 and 30 cm – 15-30; more than 30 cm - >30, and; stubbles (hayed vegetation) – hayed.

The timing and rotation of grazing in the fields was found to be an important parameter for the species (males: ![]() $\chi _4^2$ = 28.42, P < 0.001; females:
$\chi _4^2$ = 28.42, P < 0.001; females: ![]() $\chi _4^2$ = 21.58, P ≤ 0.001; PFI:
$\chi _4^2$ = 21.58, P ≤ 0.001; PFI: ![]() $\chi _4^2$ = 13.21, P = 0.01). Dunn tests revealed that fields ungrazed or grazed only during winter and ungrazed in spring (USWG) have significantly lower densities of males, females and lower PFI than those under seasonal grazing, ungrazed during winter and grazed in spring (SSG; Table 4). Male and female densities were significantly lower in fields under a short-medium rotation term (SMR) than those of SSG fields. Also, male densities were significantly lower in USWG fields compared to fields under continuous grazing and in fields under irregular grazing compared to SSG fields. No differences were found in respect to the type of livestock (males:
$\chi _4^2$ = 13.21, P = 0.01). Dunn tests revealed that fields ungrazed or grazed only during winter and ungrazed in spring (USWG) have significantly lower densities of males, females and lower PFI than those under seasonal grazing, ungrazed during winter and grazed in spring (SSG; Table 4). Male and female densities were significantly lower in fields under a short-medium rotation term (SMR) than those of SSG fields. Also, male densities were significantly lower in USWG fields compared to fields under continuous grazing and in fields under irregular grazing compared to SSG fields. No differences were found in respect to the type of livestock (males: ![]() $\chi _1^2$ = 1.30, P = 0.25; females:
$\chi _1^2$ = 1.30, P = 0.25; females: ![]() $\chi _1^2$ = 0.50, P = 0.48; PFI:
$\chi _1^2$ = 0.50, P = 0.48; PFI: ![]() $\chi _1^2$ = 1.00, P = 0.32).
$\chi _1^2$ = 1.00, P = 0.32).
Table 4. Summary statistics of Dunn tests on the relationships between male and female densities, and PFI with grazing rotation type (significant p-values are presented in bold characters). Grazing rotation categories are presented as follows: ungrazed or seasonal, ungrazed during spring – USWG; continuous grazing – CON; short-medium term rotational grazing - SMR; irregular grazing – IRR; seasonal, ungrazed during winter – SSG.

Discussion
In the grasslands of Évora the Little Bustard shows a clearly negative population trend (Figure 2, own unpubl. data). This negative trend could be due to low population recruitment owing to various different causes (food shortage or limited access to invertebrate prey, high predation rates). Like in other areas of Iberia and France, this decrease in recruitment is likely to be linked with agricultural intensification (Bretagnolle Reference Bretagnolle2001, Morales et al. Reference Morales, Traba and Arroyo2015). This intensification includes (1) the increase of irrigated crops around grassland fields, (2) the replacement of extensive grasslands by intensive/irrigated forage crops and (3) haying intensification (Faria et al. Reference Faria, Morales and Rabaça2016). According to our results, low population recruitment can be associated with habitat management factors acting in grasslands (e.g. increase in the area of tall/dense swards for haying and mown swards), and reflected in the strong decrease in the number of breeding males and females that settled each spring in our study area. Also, observations at late breeding indicate a considerable deviation in sex ratio in favour of males which may in the short-term further decrease the recruitment rate of the population, thus threatening its persistence (see Morales et al. Reference Morales, Bretagnolle and Arroyo2005).
As supported by our results, the management of grazing is a key issue for the species. Low stocking rates between January and February are important to ensure the growth of vegetation biomass to levels that guarantee sustainable use of vegetation by livestock during spring. However, grazing intensity requirements during spring can differ substantially between males and females (with or without juveniles). Also, grazing intensity requirements seem to depend on each year’s weather features, at least those of males and females. Females losing their brood join males in fields intensively grazed during spring, inside the breeding range. Indeed, this behaviour was observed from early June in five radio-tracked females (own unpubl. data). This behaviour is less likely to occur in females with juveniles due to the limited ability of juveniles to move from one field to another.
Females with juveniles seem to prefer short or medium swards, which to some extent differs from the findings of Silva et al. (Reference Silva, Estanque, Moreira and Palmeirim2014), who did not find any relationship with vegetation height. The occurrence in short swards may in some cases be related to livestock management under high stocking rates (large herds using small pastures for short periods), which are tricky to model since their effect seems to depend on the interaction with other grazing management and weather variables. The effect of this variable was not evaluated in our study, and should be addressed in future research. Under this type of management, the continuous removal of vegetation in fields that formerly presented taller vegetation, together with the low vegetation regeneration from late May onwards, results in most cases in short vegetation (or even depleted fields). Therefore, the use or abandonment of these fields by Little Bustard families may depend on field size and on the availability of habitats with medium-height vegetation in rearing ranges (the main habitats for females during nesting season; Faria et al. Reference Faria, Rabaça and Morales2012), since as stressed above, juveniles may not at this time be fully prepared to abandon nesting fields.
Hay stubbles were found to be unattractive for the species. This contrasts with the selection of cereal stubbles for grain production reported in other Iberian locations in the same phenological period (Tarjuelo et al. Reference Tarjuelo, Delgado, Bota, Morales, Traba, Ponjoan, Hervás and Mañosa2013). Hayed fields often present shorter stubbles than those of cereal for grain and are very homogeneous in terms of vegetation structure, offering juveniles little concealment from predators compared to stubbles of cereal for grain or short grazed swards. Also, grazed areas with short and sparse vegetation allow an easier chase and capture of arthropods (see Lapiedra et al. Reference Lapiedra, Ponjoan, Gamero, Bota and Mañosa2011 for similar observations). According to our field observations (but see also Jiguet Reference Jiguet2002), arthropods are the main food resource during this time of the year, being captured in rapid chases and these seem to be much more abundant and accessible in grazed areas than in hay stubbles or tall vegetation. Therefore, the avoidance of hay stubbles may reflect habitat requirements similar to those of other grassland bird species that abandon fields after mowing (Grüebler et al. Reference Grüebler, Schuler, Spaar and Naef-Daenzer2015, Faria et al. Reference Faria, Morales and Rabaça2016).
Some limitations of this study should be acknowledged. Particularly those due the small number of bird observations, which limited our quantitative models to stocking rates, hampering an integrated analysis with other potentially relevant habitat variables (e.g. vegetation height or grazing rotation pattern). Also, our field methodology only allowed a preliminary evaluation of the potential effects of predator vigilance in the distribution of birds, since no specific behavioural observations were made. Finally, extrapolation of our results to areas with different climate, soil conditions and landscape structure (e.g. more fragmented or heterogeneous) should be made with caution.
We conclude that the loss of suitable habitat for Little Bustard families (i.e. fields under low to moderate stocking rates during winter and spring) due to an unsuitable grazing management (undergrazing and overgrazing) and to an increase in the area used for hay production, has a strong impact on habitat use and on the species’ productivity, threatening population persistence and the conservation of declining Little Bustards.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270917000387
Acknowledgements
We thank all land managers who kindly supplied information on grassland management and allowed us to work on their land. To João Paulo Silva for his valuable comments on a previous version of this manuscript. Nuno Faria was funded by grant SFRH/BD/74840/2010 from the Portuguese Science and Technology Foundation (FCT- Fundação para a Ciência e a Tecnologia), supported by funding POPH/FSE, and by the REMEDINAL3-CM network (S-2013/MAE-2719) of the Autonomous Community of Madrid.











