INTRODUCTION
Ascension is a small volcanic island situated in the middle of the South Atlantic Ocean. Its nearest land areas are more than 1000 km away (St Helena Island: 1130 km; Liberia, West Africa: 1536 km; Fernando de Noronha archipelago: 2048 km). The approximately triangular island is only 97 km2, being surrounded mostly by rocky shores and small sandy beaches. For a description of general characteristics of the coast of Ascension see Price & John (Reference Price and John1980). As pointed out by these authors, in the beginning of the 20th Century many expeditions used Ascension as a stop-off point during their journeys. However, few observations were made about the marine life of the island. Improvement on the knowledge came later, with publication of annotated lists of species, in particular those by Rosewater (Reference Rosewater1975) for marine molluscs, Manning & Chace Jr (Reference Manning and Chace1990) for decapod and stomatopod crustaceans and Lubbock (Reference Lubbock1980) for shore fish.
Rosewater's (Reference Rosewater1975) list of molluscs included data previously reported by other authors, such as Packer (Reference Packer1968) and mostly Smith (Reference Smith1890a, Reference Smithb), and the information provided by the examination of collections made by Mrs Hutchfield and by R.B. Manning. Rosewater (Reference Rosewater1975) also considered valid the presence of some species on the island based only on verbal communications by Mrs Hutchfield, that is, without the existence of specimens for examination (e.g. Cypraea tigris Linnaeus, 1758 and Tonna galea Linnaeus, 1758). His list covered 89 species, eight of them under the ‘Opisthobranchia’ (Rosewater, Reference Rosewater1975: p. 24), including one pyramidellid. The list also included two species of Siphonariidae. In the past, the family Pyramidellidae was considered part of Opisthobranchia by some authors (e.g. Boettger, Reference Boettger1955) but later included among the group of basal, not well-resolved heterobranchs (Haszprunar, Reference Haszprunar1985). It is now clear that Opisthobranchia per se is not a natural group (Jörger et al., Reference Jörger, Stöger, Kano, Fukuda, Knebelsberger and Schrödl2010; Kocot et al., Reference Kocot, Halanych and Krug2013, among others), and a recent reclassification of traditional groups such as Acteonoidea, Nudibranchia and Sacoglossa has been presented by Wägele et al. (Reference Wägele, Klussmann-Kolb, Verbeek and Schrödl2014).
Among the eight opisthobranch species reported from Ascension by Rosewater (Reference Rosewater1975), most are shelled forms, including two deep water species collected by ‘The Challenger’ (see Table 1). No representatives of other diverse groups, such as Nudibranchia or Sacoglossa, were known from the island until now. Based on material collected in a recent expedition by the Shallow Marine Surveys Group (SMSG) and the South Atlantic Environmental Research Institute (SAERI), we here update the information on the heterobranch sea slugs of Ascension Island, including the description of two new species.
Table 1. Heterobranch sea slugs from Ascension. New records marked* (Eupulmonata not included).

MATERIALS AND METHODS
Material was collected manually from tide pools and through SCUBA diving down to a depth of 15 m, in August–September 2012. Specimens were photographed alive, preserved in 96% ethanol and deposited in the malacological collection of the Zoologische Staatssammlung München (ZSM), Germany. Taxonomic identifications were based mostly on external characters, such as body morphology and colour pattern, in comparison to field guides and checklists (Cervera et al., 2004; Valdés et al., Reference Valdés, Hamann, Behrens and Dupont2006), and original descriptions. At least two species presented an external morphology and colour pattern previously unknown for any described species. Specimens of these species were dissected under a stereomicroscope. The buccal bulb was manually cleaned and immersed in a solution of 10% sodium hydroxide (NaOH) to dissolve soft tissues. Cleaned jaws and radula were transferred to distilled water and mounted for photography in the scanning electronic microscope LEO 1430VP, at the ZSM. For the study of the reproductive system, it was first cleaned and isolated from adjacent systems and then drawn with the aid of a camera lucida.
RESULTS
During the expedition, ten species were collected: Micromelo undatus (Bruguière, 1792); Pleurobranchus areolatus Mörch, Reference Mörch1863; Platydoris angustipes (Mörch, Reference Mörch1863); Diaulula sp.; Felimida atlantica sp. nov.; Phidiana mimica sp. nov.; Umbraculum umbraculum (Lightfoot, 1786); Dolabrifera dolabrifera (Rang, 1828); Aplysia parvula Guilding in Mörch, Reference Mörch1863; and Caliphylla mediterranea A. Costa, 1867. Seven species represent new records for Ascension Island (see species remarks below), among them two new nudibranch species, representing the first record of this group from the island.
SYSTEMATICS
Class GASTROPODA Cuvier, 1795
HETEROBRANCHIA Gray, 1840
ACTEONOIDEA d'Orbigny, 1843
Family APLUSTRIDAE Gray, 1847
Genus Micromelo Pilsbry, 1895
Micromelo undatus (Bruguière, 1792)
(Figure 1A)

Fig. 1. Heterobranch sea slugs from Ascension Island: (A) Micromelo undatus (Bruguière, 1792) (ZSM Mol 20130107); (B) Pleurobranchus areolatus Mörch, Reference Mörch1863 (ZSM Mol 20130104); (C) Platydoris angustipes (Mörch, Reference Mörch1863) (ZSM Mol 20130105); (D) Diaulula sp. (ZSM Mol 20130107, photograph by Simon Morley); (E) Felimida atlantica sp. nov. (holotype, ZSM Mol 20130114); (F) Umbraculum umbraculum (Lightfoot, 1786) (not collected).
MATERIAL EXAMINED
One specimen, 6 mm long (preserved) (crawling on a rock in 5 m depth, English Bay, Ascension Island) (ZSM Mol 20130108), P. Wirtz coll., 10 September 2012.
REMARKS
Micromelo undatus is considered a circumtropical species (Valdés et al., Reference Valdés, Hamann, Behrens and Dupont2006), but this needs to be tested through comparative morphological and molecular studies. It was first recorded from Ascension by Rosewater (Reference Rosewater1975) based on four specimens, one of them collected alive.
EUTHYNEURA Spengel, 1881
NUDIPLEURA Wägele & Willan, 2000
PLEUROBRANCHOIDEA Gray, 1827
Family PLEUROBRANCHIDAE Gray, 1827
Genus Pleurobranchus Cuvier, 1804
Pleurobranchus areolatus Mörch, Reference Mörch1863
(Figure 1B)
MATERIAL EXAMINED
Three specimens, 30 mm long preserved (ZSM Mol 20130103), 32 mm long preserved (ZSM Mol 20130104) and 25 mm long preserved (ZSM Mol 20130113) (found under rocks between 5 and 10 m depth, English Bay and Soudan Bay), coll. P. Wirtz, August–September 2012.
REMARKS
Originally described from St Thomas, in the Caribbean Sea (Mörch, Reference Mörch1863), P. areolatus was later recorded from many localities in the tropical western Atlantic (see Valdés et al., Reference Valdés, Hamann, Behrens and Dupont2006), and also in the eastern Pacific and eastern Atlantic (Cervera et al., Reference Cervera, Calado, Gavaia, Malaquias, Templado, Ballesteros, García-Gomez and Megina2004; Camacho-García et al., Reference Camacho-García, Gosliner and Valdés2005). The first record of this species from Ascension was provided by Rosewater (Reference Rosewater1975) based on a single specimen. It is not clear if the differences in body colour pattern and dorsal papillae reported for P. areolatus may, in fact, be indicative that more than one species is involved (Rudman, Reference Rudman2000). Ascension specimens present the most common reddish pattern known for the species, being very similar to the specimens from Brazil, illustrated by García et al. (Reference García, Troncoso and Domínguez2002: Figure 2H) and Padula et al. (Reference Padula, Bahia, Correia and Sovierzoski2012, Figure 5D).
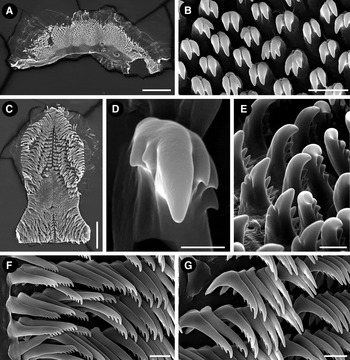
Fig. 2. Felimida atlantica sp. nov. (holotype, ZSM 20130114), SEM micrographs. Labial cuticle: (A) opened labial cuticle; (B) elements of the labial cuticle. Radula: (C) entire radula; (D) detail of the first lateral tooth; (E) first lateral teeth, ventral view; (F) outermost lateral teeth; (G) mid-lateral teeth. Scale bars: A, 200 µm; B, 10 µm; C, 200 µm; D, 5 µm; E, 10 µm; F, G, 20 µm.
NUDIBRANCHIA Cuvier, 1817
EUCTENIDIACEA Tardy, 1970
Family DISCODORIDIDAE Bergh, 1891
Genus Platydoris Bergh, 1877
Platydoris angustipes (Mörch, Reference Mörch1863)
(Figure 1C)
MATERIAL EXAMINED
Two specimens, 35 and 42 mm long preserved (under a rock in 10 m depth, English Bay) (ZSM Mol 20130105), coll. P. Wirtz, 6 September 2012.
REMARKS
Widespread in the tropical western Atlantic, with many records along the Caribbean to south-eastern Brazil (Valdés et al., Reference Valdés, Hamann, Behrens and Dupont2006; Padula et al., Reference Padula, Bahia, Correia and Sovierzoski2012), P. angustipes is herein for the first time recorded from Ascension Island, representing the easternmost known record for the species.
Genus Diaulula Bergh, 1878
Diaulula sp.
(Figure 1D)
MATERIAL EXAMINED
One specimen, 22 mm long preserved (under a rock in 10 m depth, North East Bay) (ZSM Mol 20130107), coll. S. Morley, 31 August 2012.
REMARKS
Due to the high number of similar morphotypes or species, the family Discodorididae represents one of the most puzzling groups in the Atlantic Ocean (Camacho-García et al., Reference Camacho-García, Pola, Carmona, Padula, Villani and Cervera2014). This morphotype from Ascension resembles Diaulula hummelincki (Ev. Marcus & Er. Marcus, 1963) in the colour of the rhinophores and gill, and young specimens of Discodoris branneri MacFarland, 1909 in general colour and external morphology (Alvim & Pimenta, Reference Alvim and Pimenta2013). The specific identity of this material can only be clarified after further comparative morphological and molecular studies.
Family CHROMODORIDIDAE Bergh, 1891
Genus Felimida Ev. Marcus, 1971
Felimida atlantica sp. nov.
(Figures 1E, 2, 6A)
TYPE MATERIAL
Holotype: 12 mm long, preserved. Dissected, radula and jaws mounted on stub for SEM, reproductive system studied (English Bay, water depth: 15 m, under a large rock) (ZSM Mol 20130114), coll. P. Wirtz, 9 September 2012.
EXTERNAL MORPHOLOGY
Body long, oval in shape, flattened. Mantle smooth, with a series of densely arranged, small, irregular granular glands (mantle dermal formations, ‘MDFs’) along its border, except on the anterior margin. Foot relatively straight, posterior region projected and pointed. Oral tentacles short and conical. Rhinophoral sheath low, base of rhinophores smooth, distal portion with 15 adjacent lamellae. Gill relatively short, with five unipinnate leaves.
BODY COLOUR
Body predominantly white or translucent white. Dorsal mantle with three longitudinal series of small orange dots, running from each rhinophore and from the midpoint between them in the direction of the gill. Mantle edge bordered by a thin yellowish orange band. Rhinophores and gill whitish, pale cream (Figure 1E). Ventral mantle and foot white.
LABIAL CUTICLE AND RADULA
Oral tube initially narrow, widening posteriorly in the junction with the labial cuticle; when open, labial cuticle is irregular, semi-oval in shape (Figure 2A). Labial cuticle covered by many small, mostly bicuspid elements. In some elements, each cusp may be subdivided, presenting a total of three or four cusps (Figure 2B). Radula wider in its posterior portion; posterior end straight (Figure 2C). Radular formula 42 × 26.0.26 in the 12 mm fixed holotype (ZSM Mol 20130114). First lateral tooth with a large base and a prominent central cusp; up to three external short and triangular cusps disposed in series; one or two short internal cusps near central one. Lateral teeth thin and elongated, with a series of apical, small, rounded cusps; four in the first laterals and six in the most external ones. Outermost lateral teeth straighter than lateral ones, with a shorter base and up to seven apical, small, rounded cusps (Figures 2D–G).
REPRODUCTIVE SYSTEM
Hermaphroditic, triaulic, anterior portion occupying a relatively small space between the buccal mass and digestive gland. Hermaphrodite duct wide, flattened and short; ampulla moderately long, thin, situated above the female gland. Prostate elongated, with many folds; distal deferent duct wide and folded, disposed laterally and ventrally to seminal receptacle; transition between deferent duct and penis well demarcated; male and female atrium in a common space. Vagina very thin and long, projected below to the seminal receptacle; seminal receptacle cylindrical, 1/2 of bursa size, inserting ventrally in the vagina region through a small and curved region; bursa copulatrix rounded. Uterine duct long, thin, resembling vagina, projecting ventrally from bursa copulatrix in direction to the gonopore, inserting female gland mass near to oviduct. Oviduct short. Female glands well developed, nidamental region with a rounded portion, ventrally to vagina (Figure 6A).
ETYMOLOGY
The specific name refers to the Atlantic Ocean.
GEOGRAPHIC DISTRIBUTION
Only known from its type locality: English Bay, Ascension Island, South Atlantic Ocean.
REMARKS
Due to the body form, smooth mantle, the arrangement of MDFs, pectinate radular teeth and the arrangement of the reproductive system, the single specimen studied is allocated in the genus Felimida. According to Johnson & Gosliner's hypothesis (Reference Johnson and Gosliner2012) Felimida comprises Atlantic species previously attributed to the genera Chromodoris and Glossodoris. Felimida atlantica sp. nov. resembles four other Atlantic and Mediterranean Felimida species: Felimida grahami (Thompson, 1980) and Felimida paulomarcioi (Domínguez, García & Troncoso, Reference Domínguez, García and Troncoso2006) from the Caribbean Sea and Brazil, respectively, Felimida kpone (Edmunds, Reference Edmunds1981) from Ghana, and Felimida purpurea (Risso in Guérin, 1831), from the Mediterranean and the eastern Atlantic. All these species share the general whitish dorsal mantle, with or without orange/pink spots or lines, with a marginal yellowish orange line, and purple/reddish pigment in the rhinophores and branchial leaves (Edmunds, Reference Edmunds1981; Debelius & Kuiter, 2008). Also, F. purpurea and F. paulomarcioi share a very similar reproductive system (García-Gomez, Reference García-Gómez2002; Domínguez et al., Reference Domínguez, García and Troncoso2006), while the reproductive system of F. grahami and F. kpone were not described up to date. These species are differentiated by details in coloration and radular morphology. Felimida atlantica sp. nov differs from all by having white rhinophores and gill. This is an important feature because the colour of these structures does not present wide variation in Felimida species. Differences in the radular morphology are more difficult to state because the general pattern in Felimida is very similar. Few specimens of each Felimida species were anatomically studied and potential intraspecific variation, including ontogenetic, is not well known. However, the innermost lateral teeth seem to carry some specific information. Innermost lateral teeth of Felimida atlantica sp. nov. have one, mostly two, internal and only three external cusps (Figure 2D, E). This agrees with the variation reported for F. purpurea, two internal, three–four external cusps (García-Gomez, Reference García-Gómez2002), but these species differ in the colour of the rhinophores and the gill and also in the dorsal colouration, F. purpurea not presenting the small orange dots found in F. atlantica sp. nov. The dorsal pattern of F. atlantica sp. nov. agrees with the description of F. grahami, F. paulomarcioi and F. kpone, with orange/reddish spots disposed in longitudinal lines. In fact, it is not clear if F. paulomarcioi does not simply represent a variation of F. grahami, as commented by Padula et al. (Reference Padula, Bahia, Vargas and Lindner2011). Felimida atlantica sp. nov. differs from these and other known Felimida species by the diagnostic white rhinophores and gill, combined with the reduced number of cusps in the innermost lateral teeth.
DEXIARCHIA Schrödl, Wägele & Willan, 2001
Family FACELINIDAE Bergh, 1889
Genus Phidiana Ev. Marcus, 1971
Phidiana mimica sp. nov.
(Figures 3A, B, 4, 5, 6B)

Fig. 3. Heterobranch sea slugs from Ascension Island: (A) Phidiana mimica sp. nov., dorsal view (holotype, ZSM Mol 20130109); (B) Phidiana mimica sp. nov., lateral view (holotype, ZSM Mol 20130109); (C) Dolabrifera dolabrifera (Rang, 1828) (ZSM Mol 20130112, photograph by Simon Morley); (D) Caliphylla mediterranea A. Costa, 1867 (ZSM 20130111).

Fig. 4. Phidiana mimica sp. nov.: (A) left jaw (photograph on stereo microscope) (paratype, ZSM 20130109). SEM micrographs: (B) border of the jaw (paratype, ZSM 20130109); (C) entire radula (holotype, ZSM 20130110); (D) radular teeth (holotype, ZSM 20130110); (E) detail of radular teeth (holotype, ZSM 20130110). Scale bars: A, 250 µm; B, C 100 µm; D, E, 20 µm.

Fig. 5. Phidiana mimica sp. nov., teratological radula of the paratype (ZSM 20130109): (A) radular teeth, dorsal view; (B) lateral view of radular teeth showing teratology. Scale bars: A, 25 µm; B, 20 µm.

Fig. 6. Reproductive system, dorsal view: (A) Felimida atlantica sp. nov. (holotype, ZSM 20130114); (B) Phidiana mimica sp. nov. (holotype, ZSM 20130109). Scale bars: A, 1 mm; B, 0.5 mm. am, ampulla; bc, bursa copulatrix; dd, deferent duct; fg, female gland; hd, hermaphrodite duct; pe, penis; pr, prostate; rs, receptaculum seminis; ud, uterine duct; va, vagina.
TYPE MATERIAL
Holotype: 4 mm long preserved. Dissected, radula and jaws mounted on stub for SEM, reproductive system not studied (English Bay, water depth: 10–15 m, under a rock) (ZSM Mol 20130110), coll. P. Wirtz, 9 September 2012.
Paratype: 7 mm long preserved. Dissected, radula and jaws mounted on stub for SEM, reproductive system studied (English Bay, water depth: 10–15 m, under a rock) (ZSM Mol 20130109), coll. P. Wirtz, 9 September 2012.
EXTERNAL MORPHOLOGY
Body long and narrow, distinct and elongated head with long and pointed oral tentacles; rhinophores comparatively short and smooth. Foot with the same width as the body, except the anterior portion that is wider. Anterior region of the foot curved with thin lateral projections; posterior end short and pointed. Cylindrical and elongated cerata distributed in groups; anterior group forming a short arch, posterior groups in lines; each group with 8–15 cerata. Anus situated at the base of the anterior cerata of the second ceratal cluster, on the right side of the body. Genital aperture situated laterally on the right side of the body below the first group of cerata. The position of the renal pore could not be determined.
BODY COLOUR
Body orange; dorsally, from the base of each oral tentacle a thin dorsal white line runs in direction to the region between the rhinopores. Dorsal region above the pericardium with a triangular white spot. Dorsal region posterior to the pericardium orange. Foot corners translucent white. Oral tentacles with orange-reddish bases, central region fade yellowish and distal portion white. Rhinophores reddish with white tips. Cerata deep red in their lower half, with a short bluish zone in transition to the white distal portion (Figure 3A, B). It seems that the deep red colour of the cerata is derived from the content of the digestive gland, but it could not be confirmed through the photographs or examining the preserved material. The bluish zone and the distal white portion are pigments on the surface of the cerata.
JAWS AND RADULA
Uniseriate radula with 17 (holotype ZSM Mol 20130110) and 19 teeth (paratype ZSM Mol 20130109); radular teeth with a prominent and smooth central cusp (Figure 4D) and five or six lateral smooth denticles (Figure 4E). The paratype is a teratological specimen with asymmetrical teeth. Some teeth present one side with few (3–6) large denticles and the other with up to 20 small denticles or even smooth (Figure 5B). Holotype and paratype with thin and relatively high jaws (Figure 4A). Masticatory border of the jaws long, projected and denticulate, with a single row of large and spaced, spoon-like teeth (Figure 4A, B).
REPRODUCTIVE SYSTEM
Hermaphroditic, androdiaulic. Proximal gonoduct thin and long. Ampulla elongated, wide, with a turn on its proximal region. Distal portion of ampulla narrowing, postampullary gonoduct short, dividing into deferent duct and oviduct. A differentiate prostate not present. Deferent duct very short, thin, connected to a curved, muscular, penis. A small projection is present in the superior portion of the penis, appearing as a small protuberance above the gonopore (Figure 6B). Vagina elongated, moderately wide, connecting to the female gland mass, oviduct and the stalk of receptaculum seminis; the latter with irregular shape and surface, having a distinct yellowish colour.
ETYMOLOGY
From the Latin mimicus (and the Greek mimikos) due to the similarity in external appearance of the new species to Phidiana lynceus Bergh, Reference Bergh1867.
GEOGRAPHICAL DISTRIBUTION
Only known from its type locality: English Bay, Ascension Island, South Atlantic Ocean.
REMARKS
At first glance, based on general body morphology and colour, the studied specimens could erroneously be identified as belonging to Phidiana lynceus, a common tropical western Atlantic species, recorded also from the Canary Islands and Ghana (Edmunds, Reference Edmunds1975; Cervera et al., Reference Cervera, Calado, Gavaia, Malaquias, Templado, Ballesteros, García-Gomez and Megina2004). However, there are differences in the shape of the rhinophores, being lamellated in P. lynceus and smooth in P. mimica sp. nov., and in the colour of some regions of the body, such as the rhinophores and dorsal region, posterior to the head. The rhinophores of P. lynceus have transparent bases, the central region orange or red and a yellowish distal portion (Valdés et al., Reference Valdés, Hamann, Behrens and Dupont2006: p. 257), while they are reddish with white tips in P. mimica sp. nov. A central longitudinal white line runs along the entire dorsal region in P. lynceus, while in P. mimica sp. nov. it is absent. The jaws and the radula of the two species are also different: the jaws of P. lynceus are proportionally longer than the jaws of P. mimica sp. nov.; P. lynceus has 6–9 denticles on each side of the prominent central cusp of the radular teeth (Bergh, Reference Bergh1867; Padula, Reference Padula2007), while the central cusp is smooth in the teeth of P. mimica sp. nov.
Another species that resembles P. mimica sp. nov. is Phidiana indica (Bergh, 1896) from the tropical Indo-Pacific but also recorded in the Mediterranean as an exotic species (Zenetos et al., Reference Zenetos, Gofas, Russo and Templado2003). However, the general body colour pattern is different, P. indica presenting blue and yellow areas on the oral tentacles and cerata which are absent in P. mimica sp. nov.
As pointed recently in a broad molecular phylogenetic study in aeolids (Carmona et al., Reference Carmona, Pola, Gosliner and Cervera2013: figure 1), the traditional generic placements in the family Facelinidae seem to not reflect the natural history of the group and apparently need a general revision. We tentatively allocated the new species in the genus Phidiana based on characteristics of external morphology, jaw, radula and reproductive system (Rudman, Reference Rudman1980, Reference Rudman1999). An exception is the smooth rhinophores, as most Phidiana species have perfoliated rhinopores, but P. indica, for example, does not. This species formerly was placed into the genera Learchis, Caloria, Hervia and Facelina by different authors (see Rudman, Reference Rudman1999).
One unusual feature of the paratype of P. mimica sp. nov. is the asymmetrical morphology of the radular teeth, with a single tooth presenting different shapes on each side of the central cusp, and adjacent teeth presenting different morphology (Figure 5B). It probably represents an abnormality, indeed illustrates how the systematics of aeolid nudibranchs can be even more complicated. Only new comprehensive studies may clarify the boundaries within the Facelinidae subgroups.
TECTIPLEURA Schrödl et al., 2011
EUOPISTHOBRANCHIA Jörger et al., Reference Jörger, Stöger, Kano, Fukuda, Knebelsberger and Schrödl2010
UMBRACULOIDEA Dall, 1889 (1827)
Family UMBRACULIDAE Dall, 1889
Genus Umbraculum Schumacher, 1817
Umbraculum umbraculum (Lightfoot, 1786)
(Figure 1F)
MATERIAL EXAMINED
One specimen photographed alive, material not collected.
REMARKS
Another species considered to have a wide geographical distribution in the Atlantic, Indo-Pacific and also eastern Pacific waters (Uribe et al., Reference Uribe, Nakamura, Indacochea, Pacheco, Hooker and Schrödl2013). Many names were synonymized to Umbraculum umbraculum, and at least some may represent valid names of more restricted distributed, cryptic, species. This species was first recorded from Ascension under the name Umbraculum mediterraneum (Lamarck, 1819) based on a single shell (Rosewater, Reference Rosewater1975: p. 26). During the recent survey, a living specimen was photographed (Figure 1F) and several more were observed by the second author, confirming the occurrence of the species in the island.
ANASPIDEA Fischer, 1883
Family APLYSIIDAE Lamarck, 1809
Genus Dolabrifera Gray, 1847
Dolabrifera dolabrifera (Rang, 1828)
(Figure 3C)
MATERIAL EXAMINED
Two specimens, 6 and 10 mm long, preserved (under rocks in 12 m depth in Northeast Bay) (ZSM Mol 20130112), collector not recorded, 6 September 2012. One specimen, 13 mm long, preserved (under rocks on 10 m depth, English Bay) (ZSM Mol 20130106), collector not recorded, 6 September 2012.
REMARKS
This species is distributed circumglobally in tropical and sub-tropical waters (Rudman, Reference Rudman2003). However, as commented for Micromelo undatus and Umbraculum umbraculum, this wide distribution should be investigated with more detailed, comparative morphological and molecular studies. This is the first record of D. dolabrifera from Ascension Island.
Genus Aplysia Linnaeus, 1767
Aplysia parvula Guilding in Mörch, Reference Mörch1863
MATERIAL EXAMINED
Two specimens, 8 and 10 mm long, preserved (under rocks) (ZSM Mol 20130115), collector not recorded, September 2012.
REMARKS
This is the first record of Aplysia parvula from Ascension and the second record of an Aplysia species, after the record of A. dactylomela Rang, 1828 from the island by Rosewater (Reference Rosewater1975). Unfortunately, no photographs of living specimens of A. parvula were taken. Aplysia parvula is distributed in tropical to warm temperate waters worldwide, but preliminary molecular data point to the existence of a complex of species (V. Padula, unpublished data); this may represent a similar case to A. dactylomela, for which cryptic species were detected through molecular analysis (Alexander & Valdés, Reference Alexander and Valdés2013).
PANPULMONATA Jörger et al., Reference Jörger, Stöger, Kano, Fukuda, Knebelsberger and Schrödl2010
SACOGLOSSA Ihering, 1876
Family CALIPHYLLIDAE Tiberi, 1881
Genus Caliphylla A. Costa, 1867
Caliphylla mediterranea A. Costa, 1867
(Figure 3D)
MATERIAL EXAMINED
Two specimens, 4 and 5 mm long, preserved (on the alga Bryopsis plumosa , 8 m depth in English Bay, at night) (ZSM Mol 20130111), coll. P. Wirtz, 8 September 2012.
REMARKS
Originally described from the Mediterranean Sea, Caliphylla mediterranea was later recorded from different localities in the Caribbean Sea, Brazil and Senegal (Gascoine, Reference Gascoigne1979; Padula et al., Reference Padula, Bahia, Correia and Sovierzoski2012). It is here recorded from Ascension Island for the first time. It is unclear if the material of all these localities really belongs to the same species or represents a complex of cryptic species. Amphiatlantic distribution was supported for some, and rejected for other sacoglossan species in a recent study by Carmona et al. (Reference Carmona, Malaquias, Gosliner, Pola and Cervera2011).
DISCUSSION
The addition of seven new records, including two new species, almost doubles the number of ‘opisthobranch’ species known from Ascension Island (Rosewater, Reference Rosewater1975). A list of all heterobranch sea slugs recorded from Ascension is given in Table 1. Due to Ascension's geographical position and isolation, a recurrent question is the origin of its shallow water fauna and flora and the degree of endemism (Price & John, Reference Price and John1980). Among the 10 heterobranch species studied herein, four are considered circumglobal (M. undatus, D. dolabrifera, U. umbraculum and A. parvula) and one is recorded from the eastern Pacific and both sides of the Atlantic (P. areolatus). Such a range of distribution would not be naturally expected, as the maintenance of genetic structure between different oceans is highly incompatible to the biology of opisthobranch species with mostly benthic life (Goddard, Reference Goddard2004). Recent studies on sea slug species with similar wide geographical distribution revealed the existence of cryptic species. ‘Navanax aenigmaticus’, for example, is not distributed in the eastern Pacific, and on both sides of the Atlantic as previously thought, but indeed is a complex of three cryptic species, each restricted to one of these geographical regions (Ornelas-Gatdula et al., Reference Ornelas-Gatdula, Camacho-García, Schrödl, Padula, Hooker, Gosliner and Valdés2012). Hawaian specimens of ‘Aplysia dactylomela’ are not conspecific to Atlantic and Mediterranean ones (Alexander & Valdés, Reference Alexander and Valdés2013; Valdés et al., Reference Váldes, Alexander, Crocetta, Yokes, Giacobbe, Poursanidis, Zenetos, Cervera, Caballer and Galil2013). The existence of many more such cases is likely (Uribe et al., Reference Uribe, Nakamura, Indacochea, Pacheco, Hooker and Schrödl2013).
It is becoming clear that the traditional sea slug taxonomy, based on a reduced number of characters, does not allow secure delimitation of similar or even morphologically identical species (e.g. Krug et al., Reference Krug, Vendetti, Rodriguez, Retana, Hirano and Trowbridge2013), but additionally requires sound molecular approaches (see Jörger et al., Reference Jörger, Norenburg, Wilson and Schrödl2012, Jörger & Schrödl, Reference Jörger and Schrödl2013). For the circumglobal, widespread species considered in the present work, the identification provided here is tentative until comprehensive and integrative studies elucidate the real number of species involved and the correct names to be applied.
Briggs (Reference Briggs1974, Reference Briggs1995) proposed that Ascension and St Helena together constitute a separate biogeographical province in the Atlantic. Data available on more studied groups, such as reef fish, which due to their life habits and biology can be compared to some benthic invertebrates, corroborate this idea (Bullock, 1980; Floeter et al., Reference Floeter, Rocha, Robertson, Joyeux, Smith-Vaniz, Wirtz, Edwards, Barreiros, Ferreira, Gasparini, Brito, Falcón, Bowen and Bernardi2008). At the same time, it is known that Ascension and St Helena shallow water marine fauna receives strong influence from both the western and eastern Atlantic, including the occurrence of amphiatlantic species (Floeter et al., Reference Floeter, Rocha, Robertson, Joyeux, Smith-Vaniz, Wirtz, Edwards, Barreiros, Ferreira, Gasparini, Brito, Falcón, Bowen and Bernardi2008). In general, the influence of the western Atlantic seems to be stronger (Manning & Chace Jr, Reference Manning and Chace1990), but see Floeter et al. (Reference Floeter, Rocha, Robertson, Joyeux, Smith-Vaniz, Wirtz, Edwards, Barreiros, Ferreira, Gasparini, Brito, Falcón, Bowen and Bernardi2008: p. 38). Briggs & Bowen (Reference Briggs and Bowen2012) compiled biogeographical data and reported the open-water expanse of the mid-Atlantic, that is, the mass of water separating the western Atlantic and the eastern Atlantic, as a soft barrier for dispersion. The almost absent data on heterobranch sea slugs from the east coast of Africa limits the discussion in our case. From one side, the discovery of two new species (F. atlantica and P. mimica), unknown from elsewhere, reinforces the biogeographical province status of Ascension (Briggs, Reference Briggs1974, Reference Briggs1995, Floeter et al., Reference Floeter, Rocha, Robertson, Joyeux, Smith-Vaniz, Wirtz, Edwards, Barreiros, Ferreira, Gasparini, Brito, Falcón, Bowen and Bernardi2008). On the other hand, the occurrence of the nudibranch Platydoris angustipes, currently only known from the tropical western Atlantic and from Ascension Island, corroborates the marine faunal affinity between these two regions.
ACKNOWLEDGEMENTS
P.W. is grateful to Paul Brickle and the Shallow Marine Surveys Group for the invitation to take part in the August–September 2012 expedition to Ascension Island, and to Kostas Tsiamis for drawing attention to the Caliphylla specimens for the identification of Bryopsis. Thanks to Stedson Stroud and Jolene Sim of the Ascension Conservation Department for their help during the expedition. We are grateful to the Shallow Marine Surveys Group and the South Atlantic Environmental Research Institute for organizing the expedition. We are very grateful to Ascension Island Government, the members of staff at the Conservation Centre and Ascension Island Dive Club for their cooperation, accommodation and hospitality. We are also grateful to British Forces South Atlantic Islands for their logistic support. We thank Simon Morley for the photograph of Diaulula sp. and Dolabrifera dolabrifera and Enrico Schwabe (ZSM) for assistance with the SEM and the management of specimens in the ZSM collection.
FINANCIAL SUPPORT
The funding for this work came from a grant to the Shallow Marine Surveys Group from the Darwin Initiative (EIDCF012). V.P. has a PhD grant from the CNPq-Brazil and DAAD-Germany. Laboratory work is supported by DFG grants SCHR667/9,13 to M.S.











