Introduction
Norovirus (NoV) infections are the leading cause of non-bacterial gastroenteritis (GE) worldwide [Reference Kirk1–Reference Koo4]. NoV is spread by fecal–oral route through the ingestion of contaminated food or water, by person-to-person contact and via ingestion of aerosolised particles [Reference Hall5]. The virus is highly contagious, an estimated dose ⩾18 virus particles is sufficient to cause the illness [Reference Glass, Parashar and Estes6]. The incubation period ranges from 12 to 48 h and disease symptoms, include vomiting, abdominal cramps, fever and watery diarrhoea [Reference Patel7]. The virus is environmentally stable and resistant to biodegradation in water (it may remain infectious in the groundwater for at least two months) [Reference Lopman8–Reference Kauppinen, Pitkänen and Miettinen10].
NoVs belong to the family Caliciviridae. Five genogroups (GI–GV) of NoV have been documented, of which GI, GII, and GIV are human pathogens [Reference Zheng11]. GII strains are the most common NoV strains reported, often associated with person-to-person transmission and foodborne outbreaks. GI strains are commonly associated with waterborne outbreaks, presumably due to stability in water [Reference Lysén12, Reference Bitler13].
In July and August 2014, there were exceptionally high temperatures in Finland. The water in the lakes was relatively warm, up to 26°C at public beaches in Tampere. On Monday, 28 July, Tampere Environmental Health received first notifications of acute gastroenteritis (AGE) among persons who had visited public beaches. A large number of AGE notifications were received by phone or e-mail on following days. Majority of persons with AGE visited at least one of six public beaches of four separate lakes (A, B, C, D). An outbreak phone line was opened with a dedicated nurse receiving notifications about AGE associated with swimming in natural waters and giving health advice.
Beaches of above-mentioned lakes were closed on Tuesday 29 July. However, it was unknown if the transmission of AGE was associated with the exposure to lake water, spending time in a crowded beach, eating at the beach, or to other unrecognised factors.
Environmental Health and Infectious Disease department of the City of Tampere in collaboration with the National Institute for Health and Welfare (THL) conducted epidemiological, microbiological and environmental investigations in order to assess the magnitude of the outbreak, identify the source and risk factors of infection and to implement control measures to prevent additional cases.
Methods
Epidemiological investigation
Initial web-based survey among symptomatic beach visitors
We conducted a web-based survey instead of in-depth interviews after the notification of the outbreak. The results of this survey were used to formulate the hypothesis about the outbreak; causative agent, potential role of the beaches and water, and to assess the extent of the secondary spread within households. Persons with any gastrointestinal symptoms with a history of exposure to any public beach of Tampere were invited to participate. The local population was informed about the survey through a public announcement at the website of the city of Tampere and the local newspaper. Questions included the date of swimming; the beach visited, how many persons fell ill in the household; the beginning of AGE symptoms and the first symptom; other symptoms; length of illness; and additional information. The information gathered via outbreak phone line or e-mail was also included in this survey.
A total of 1093 self-reported AGE notifications associated with swimming or spending time by the public beaches in Tampere were received via phone, internet or direct e-mail contact. Of these, 819 (75%) persons were associated with exposure to six public beaches in four separate lakes. The remaining 274 (25%) persons were associated with 26 different public beaches. Of 1093 persons with AGE, 622 (57%) were children (<18 years) and 255 (23%) adults (⩾18 years), 216 had missing information on age (20%).
Retrospective cohort study
Data collection
Subsequently, in order to identify a source of infections and risk factors, we conducted a retrospective cohort study by using web-based questionnaire, during 7–22 August 2014. Similarly to the initial web-based survey, the target group of this questionnaire was people with a history of exposure to any public beaches in Tampere. Information on the study and web-based survey was distributed through local newspapers and website of the City of Tampere. Survey included questions on demographic characteristics of respondents (age, sex, place of residence), date and location of visited beaches, length of swimming time (30 min, 30–60 min or more than 60 min), activities related to water, facilities used at the beaches (toilet, changing room, kiosk, fitness place, play area, carpet wash place), date and time of onset of gastrointestinal symptoms, number of persons who fell ill in the household and general hygienic conditions at the beaches. The results of this study are based on this second survey.
Population at risk and case definition
The population at risk were people visiting any of the public beaches in Tampere during 21 July and 6 August 2014. We defined a case as a person who visited any public beach in Tampere from 21 July to 6 August 2014 and developed diarrhoea or vomiting or two other symptoms (nausea, stomach ache or fever). Persons within the same household who developed AGE symptoms after contact with a sick individual with compatible symptoms were excluded from the analysis (secondary cases). From the study were excluded also people who travelled abroad 1 week prior to the onset of AGE symptoms.
Statistical analysis
We conducted two analyses. Firstly, we performed a lake-specific analysis restricted to persons who visited only one lake during the study period. We calculated the risk ratio (RR) of becoming a case using the chi-square test (‘cstable’ command in STATA). Secondly, we conducted pooled analysis (all lakes combined). Identified risk factors for illness and protective factors with a P value ⩽0.2 in the univariate analysis were included in a multivariable logistic regression model to assess independent association with an illness (expressed as odds ratio, OR). To avoid multicollinearity of risk factors associated with the contact to water (getting water in the mouth while swimming, submerging head under water, swallowing water while swimming) we included in the model only one variable (swallowing water while swimming). In a backward procedure, we excluded exposures with P > 0.1 from the model. Data were analysed by R software (http://cran.r-project.org) and STATA 13.
Ethical considerations
The participation in the study was voluntary. Return of fulfilled questionnaire was tantamount to informed consent. The personal information was removed after checking for possible double entry of data.
Laboratory investigation
Based on the phase of the illness (symptomatic or lately cured) and lake visiting history, persons with AGE symptoms were asked to submit stool samples for diagnostics. The stool samples were analysed for rotavirus and adenovirus (EIA detecting the viral antigens), and for bacterial pathogens (Salmonella, Campylobacter, Yersinia and Shigella) by routine methods in Fimlab (local diagnostic laboratory) in Tampere. Subsequently, all the stool samples were analysed for NoVs by a molecular-based reverse-transcriptase polymerase chain reaction (RT–PCR) (to the genogroup level) and electron microscopy (EM) for other viruses in Huslab Laboratories in Helsinki. Sixteen samples were subjected to typing and sequencing in Viral Infections Unit of the National Institute for Health and Welfare (THL).
Environmental investigation
In Finland, water samples are collected routinely three or four times during the swimming season for monitoring of water quality. The quality of bathing water is assessed by monitoring the levels of fecal indicator bacteria: Escherichia coli and intestinal enterococci. The European Union norm of 1000 and 400 colony-forming units (CFU) or most probable number (MPN) per 100 ml for E. coli and intestinal enterococci, respectively, is used as a threshold for safe bathing in inland water. If these values are exceeded, the municipal health protection authority is obliged to assess the impact of water quality on human health.
After the first signs of the outbreak, additional water samples were obtained from all beaches for microbiologic analysis. The laboratory analysis for E. coli, intestinal enterococci, cyanobacteria, and algae were performed in Water Protection Association of the River Kokemaenjoki, and for NoV and adenovirus were performed in Expert Microbiology Unit of THL. In addition, environmental health officers inspected the beach areas at implicated lakes for general conditions, available infrastructure and hygienic indicators of trash or fecal traces.
Results
Epidemiological investigation
Descriptive epidemiology
Altogether 1656 persons responded to the second survey. We excluded 155 self-reported secondary cases within households, 28 persons who did not visit public beaches and 20 persons who had travelled abroad 1 week prior onset of AGE symptoms.
Altogether 1453 persons were enrolled in the cohort study. Of these 68% (978/1431) were women and the median age was 33 years (interquartile range (IQR) 13–43 years), 100 (7%) were children <5 years of age (Table 1). The highest number of people visited lake A (342 persons), followed by D (289 persons), C (211 persons) and B (163 persons).
Table 1. The attack rate of gastroenteritis symptoms by age and gender
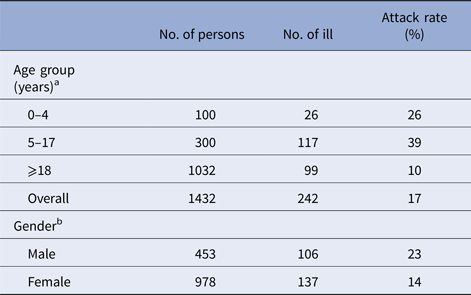
Waterborne outbreak, Tampere, 2014.
a Data on age are missing for 21 persons.
b Data on gender are missing for 22 persons.
We identified 244 persons who met the case definition (overall attack rate: 17%). The attack rate among those who visited: lake A was 23% (78/342); lake B, 33% (54/163); lake C, 13% (27/211) and lake D, 16% (46/289). The highest attack rate was among 5–17 years old (39%), followed by 0–5 years old (26%) (Table 1). The median age of cases (12 years) was significantly lower than non-cases (32 years) (P-value <0.05). The most commonly reported symptoms were nausea (212, 87%), vomiting (193, 79%), stomach ache (192, 79%), diarrhoea (137, 56%) and fever (78, 32%). The median duration of symptoms was 24 h. Twenty cases (8%) sought medical attention and two cases were hospitalised (aged 3 and 66 years). No deaths were reported. The number of cases increased from 23 July onwards and peaked on 28 and 29 July (46 and 42 cases, respectively), subsequently declining rapidly after the closure of the beaches on 29 July (Fig. 1).
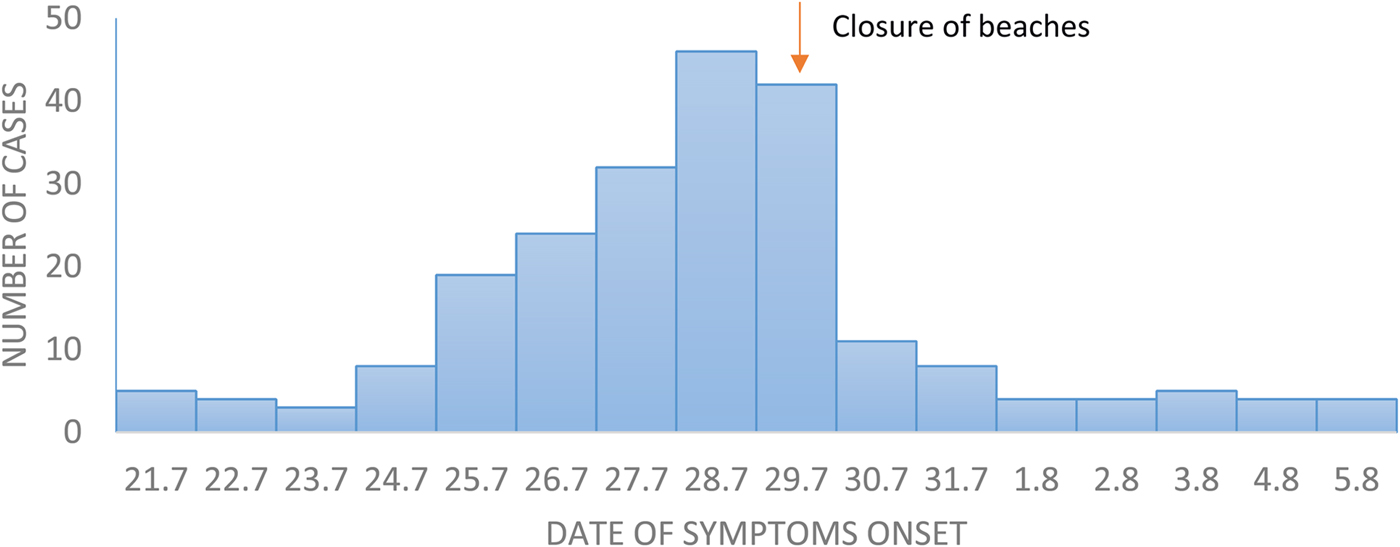
Fig. 1. Distribution of cases of gastroenteritis (n=223*) among visitors of lakes in Tampere according to date of symptoms onset. Waterborne outbreak Tampere, 2014.
Cohort study
In the univariable analysis performed for each lake separately, water activities were associated with the illness (Table 2). In addition, using fitness playground at lake C and play area at lake D, were associated with the illness. However, those risk factors explained only a small number of cases. In the pooled analysis, getting water in the mouth while swimming (Risk ratio (RR) 3.32; 95% CI: 2.36–4.68), submerging head underwater (RR 3.24; 95% CI: 2.27–4.62), swallowing water while swimming (RR 2.85; 95% CI: 2.18–3.72) and playing on the wet sand in the beach (RR 1.90; 95% CI: 1.50–2.41) were significantly associated with AGE symptoms (Table 3). Those swimming 30–60 min had almost twice the risk of falling ill (RR 1.95; 95% CI: 1.38–2.74) and those swimming >60 min had four times the risk (RR 4.01; 95% CI: 2.76–5.90) when compared to those swimming less than 30 min. Other risk factors such as using a toilet on the beach or changing room were not associated with the illness.
Table 2. Attack rates (AR) and relative risk of acute gastroenteritis associated with specific risk factors, stratified by the lake

Waterborne outbreak, Tampere, 2014.
Table 3. Attack rates (AR) and relative risk of acute gastroenteritis associated with specific risk factors stratified by age (years), pooled data from four lakes

Waterborne outbreak, Tampere, 2014.
In the multivariable analysis significant risk factors after adjusting for age were swallowing water while swimming (OR 1.74; 95% CI: 1.09–2.78), swimming in lake B (OR 2.35; 95% CI: 1.49–3.72) and lake A (OR 1.66; 95% CI: 1.15–2.39). Swimming in lakes C and D were not associated with higher risk of disease.
Laboratory investigation
A total of 23 stool samples were obtained from AGE cases. NoV was found in 19 (83%), rotavirus in three (8%), and Campylobacter jejuni in one case (9%). Sixteen NoV samples were sequenced. Of 16 sequenced isolates, 11 belonged to GI (GI.2, GI.4, GI.7), one to GII (GII.2) and in four cases the sequencing could not be done because of the poor quality of samples. The median time difference between the onset of symptoms and sampling was 3 days (between 0 and 11 days), for the 19 cases where information was available.
Environmental investigation
Lakes A and B were located in the western part of the city with approximately 1 km of distance. Lakes were not directly connected by any watercourse. Lakes C and D were located in the eastern part of Tampere, approximately 5 km apart. The characteristics and available infrastructure at particular lakes are summarised in Table 4.
Table 4. Infrastructure available at each lake
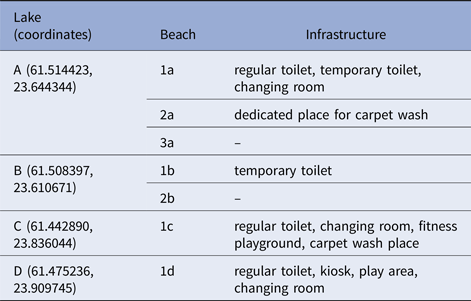
Waterborne outbreak, Tampere 2014.
Water samples from four implicated lakes (six beaches) were obtained at least four times during summer swimming period. All tested samples had acceptable values of intestinal enterococci (range from 0 to 5 CFU), E. coli (range from 1 to 27 CFU) and cyanobacteria. Water samples were taken on 29 July and 6 August from lake A were negative for NoV and adenovirus.
Environmental health officers indicated overcrowding and poor hygienic conditions at public beaches. In addition, 40% of responders (202 persons) indicated that on the beach were visible trashes and diapers. Also, some noticed poor hygiene of toilets, overfilled trash bins, children urinating or defecating on the beach and washing babies’ bottoms in the lake.
Discussion
Results from the initial web-based survey and retrospective cohort study indicated that over a thousand people had AGE after exposure to lakes in Tampere. In univariate analysis activities related to water in the four lakes were significantly associated with AGE. Results of the multivariable analysis suggested that mainly two lakes (A and B), located in the western part of the city, were associated with the illness. Clinical symptoms compatible with NoV infection reported among 244 cases and positive stool samples suggest that the possible causative agent of this widespread outbreak was NoV. Taking into account distance between two lakes and detection of several types of NoV in the stools of affected persons, the source of the outbreak was presumably multiple infected persons coupled with secondary transmission between persons and water contamination. The outbreak could spread further between lakes through infected beach visitors. However, the exact primary source of the outbreak could not be determined and there were no obvious sources of infections close to the beach areas.
Several factors may have contributed to the size of the outbreak. The weather was unexceptionally warm for an extended period during the major holiday season and therefore many people spent time on the overcrowded beaches during subsequent days. In addition, inadequate emptying of the trash bins and cleaning of the area, risky behaviors on the beaches such as inappropriate disposal of diapers, washing babies’ bottoms in the lake and defecating in the public areas, could have contributed to the extent of the outbreak. Taking into account the considerable genetic diversity of NoV, low infectious dose, large-scale shedding (also by asymptomatic persons) and resistance to biodegradation in water and easy person-to-person transmission, only a few primary cases could cause an epidemic.
In July and August 2014, an increased number of suspected outbreaks of gastroenteritis related to bathing water was reported to the Finnish food- and waterborne outbreak registry [Reference Kauppinen14]. Epidemiological and microbiological data indicated NoV as the main causative agent. The investigations suggested that the likely source of contamination was infected beach users. In one outbreak, an external source of contamination was identified.
NoV has been identified as the etiologic agent in numerous outbreaks at lake swimming beaches, swimming pools and treated water settings worldwide [Reference Dziuban15–Reference Hoebe24]. In 1997–2006, in the USA, NoV was associated with 20.6% and 6.7% of -untreated and -treated recreational water outbreaks, respectively [Reference Yoder16]. In Sweden, at least 163 people were affected after swimming in a lake [Reference Sartorius22]. Warm weather and overcrowding of swimming areas were highlighted as risk factors. In the USA, a study indicated that swimming in a lake was significantly associated with illness amongst 70 people [Reference Zlot19]. Similar factors contributing to the current outbreak were indicated: high temperatures, the potentially poor water circulation within the beach area and presence of young children at the shallow beach. Also, other studies indicated that swimming in recreational water poses a risk of AGE [Reference Sanborn and Takaro25, Reference Wiedenmann26]. In a randomised controlled trial assessing infectious disease risks from bathing in fresh recreational waters, swimmers swallowing contaminated water were at higher risk of getting AGE than non-exposed non-swimmers (8.6 % vs. 2.8 %, P value <0.001) [Reference Wiedenmann26]. In addition, activities with limited contact with water (for example boating or fishing) increased the risk of AGE by 40%–50% compared to non-water recreational activities. However, due to lack of data, difficulties in defining the source of infection and complex process of analysis of viruses in environmental water samples, waterborne outbreaks are probably under-reported [Reference Hamza27].
In our study, children were more frequently affected. This may be related to higher exposure to water since children playing often in the shallow water and sand, swallowing more water while swimming, staying in the water longer and having extensive hand-to-mouth exposure. Other studies also showed higher susceptibility of children to swimming-associated gastroenteritis illness [Reference Wade28].
NoV was not detected in water samples. Levels of bacterial fecal indicators in the lake-water were not exceeded during the epidemic period. However, standard methods for assessing water quality involving monitoring of the level of Enterococcus spp. or Escherichia coli are sensitive to temporal and spatial variation and do not relate well to viruses [Reference Prüss23]. Virus levels are often low and identification from water sample requires concentration from large volumes of water, which was not performed during the outbreak. However, failure to detect pathogen cannot exclude the virus as a cause of an outbreak. According to Tillet et al. [Reference Tillett, de Louvois and Wall29], this outbreak met the criteria for possibly associated with water (descriptive epidemiology suggest that the outbreak is water related and there is no obvious alternative explanations and supporting microbiological evidence is missing).
Although the probable source of contamination were beach users, we could not exclude a possibility of other sources of the outbreak, such as flashing of sewage from mobile toilets directly to the lake. During investigation also other possible sources of infection were considered: the presence of summer camps near lakes, various animals farms, the presence of sports team in the town or attendance in the amusement park. However, results from the initial survey did not show enough evidence for these alternative explanations.
Our study had several limitations. The case definition had high sensitivity but low specificity, which could lead to the inclusion of false positive and overestimation of the number of cases. The recruitment of study subjects was based on press releases and participation in the study was voluntary, which may introduce volunteer bias. Some people could respond to both, initial web-based survey and cohort study. People with more severe symptoms or parents of young children may tend to participate more often. It was not possible to estimate the exact number of lakes visitors and number of infected persons due to contaminated lake water. Minor bias may be related to self-reporting of symptoms, which were not validated by medical examination. Furthermore, non-swimmers may also be exposed to infection, since NoV may be transferred from water to the air through fomites. Thus, comparing swimming-associated illness estimation to non-swimmers on the beach as an unexposed group could underestimate the effect of contaminated water.
Closure of swimming beaches in Tampere, advice on hygienic precautions and rapid outbreak alerts were efficient in controlling swimming in areas affected by NoV outbreak. Since there were no-evidence based guidelines, the decision on reopening of lakes was based on expert evaluation of epidemiological professionals. To prevent future outbreaks, the hygienic precautions in the beach areas need further improvements, especially during warm seasons with many visitors to recreational areas. This involves a requirement for both the users to follow hygienic rules, as well as for the municipal services to clean the beaches.
Recreational waters contaminated with the human fecal material are a public health concern. Traditional fecal indicators currently used to monitor the quality of recreational water are not sufficient to prevent viral outbreaks [Reference Turcios30, Reference Updyke31]. Usually, a large number of cases is needed to alarm public health authorities and identify a water as a possible source of infection. In addition, expensive and time-consuming laboratory methods for virus identification in water samples limit the ability to detect causative pathogens [Reference Bosh32]. Some studies indicate F-RNA coliphage, as a modest positive predictor of NoV and rotavirus [Reference Tineke33, Reference Lin and Ganesh34]. However, there have not been consistent results of coliphage-virus association. These results suggest a need for new indicators of water quality. As there is no method to disinfect a lake water and NoVs can survive in water for extended periods, there is also a need for the development of evidence-based recommendation regarding the timing of safe reopen of recreational water venues associated with outbreaks.
Acknowledgements
The authors acknowledge Tuula Sillanpää, Paula Saxholm, Minna Seppälä, Marjaana Leinonen, Maarit Miskala, Marika Haapala, Anne Kauhanen, Tuula Ala-Honkola, Satu Aalto and other nurses and environmental and hospital staff that participated in the outbreak investigation. We acknowledge Dr Paweł Stefanoff for a critical review of the manuscript.
Declaration of interests
None.









