INTRODUCTION
Severe gastroenteritis is a major public health problem worldwide and a major contributor to mortality and morbidity in developing countries. Deaths caused by diarrhoeal diseases were estimated to represent 3·2% of the total mortality in World Health Organization (WHO) regions in 2002 [1]. In developed countries the mortality is limited, but the social and economic burdens are high. In the wide range of enteric infections, those caused by Salmonella spp. have been recognized as an important burden. In the United States, about 40 000 cases of human salmonellosis were reported annually in the 1990s, and an estimate of the annual number of non-typhoidal salmonellosis cases by the Centers for Disease Control and Prevention (CDC) was about 1·4 million [Reference Voetsch2]. With around 15 000 hospitalizations and 400 deaths per year, Salmonella spp. were estimated to cause the most annual deaths among foodborne pathogens in the United States and represented a considerable economic impact costing US$ 0·5–2·3 billion annually (human capital vs. labour market approach) [Reference Kennedy3]. Deaths accounted for 66–93% of this cost.
A major change in the epidemiology of salmonellosis was the emergence in the 1980s of one of the numerous Salmonella serotypes, Enteritidis, which has ranked first in isolation for many years in Europe and in North America. This serotype was capable of systemic colonization of poultry and consequently led to a widespread contamination of eggs and poultry meat. The WHO Salmonella surveillance programme showed for the period 1979–1987 that this serotype increased for humans in 24/35 (69%) reporting countries from Europe, North and South America and Africa, with a marked increase which reached 1000% in Argentina [Reference Rodrigue, Tauxe and Rowe4].
Fortunately, during recent years, a decrease in the number of isolates was reported in most countries of the European Union, with the exception of Greece and Portugal where an increasing trend was observed [5, 6]. Spain, Portugal and Italy reported fluctuating incidences over recent years. In 2004, 192 703 cases of human salmonellosis were reported in 24 member states of the European Union, which represents an average of 42·2 cases/100 000 inhabitants. Newly reporting countries such as the Czech Republic, Slovakia and Slovenia had the highest incidence with 300, 235 and 163 cases/100 000 inhabitants, respectively, whereas Hungary, Lithuania and Poland had an incidence near the European mean (from 41 to 75 cases/100 000 inhabitants). S. Enteritidis was the most common serotype reported by the member states (76% of the total Salmonella isolates), ranging from 32% in France (n=6352, Salmonella spp.) to 100% in Cyprus (n=89). Fourteen percent of all serotyped isolates in the European Union was identified as S. Typhimurium.
In an attempt to control S. Enteritidis in Europe and in order to prevent outbreaks of foodborne infections, in 1992 the European Parliament issued a directive (Council Directive 92/117/EEC) establishing measures for protection against specified zoonotic agents in animals and products of animal origin. This Directive proposed that the member states establish monitoring systems and control measures in at least the breeding flocks of poultry. A Salmonella monitoring programme in breeding flocks started in Belgium in 1993. The European directives establishing sanitation measures for poultry flocks were adopted by Belgian royal and ministerial decrees in August, 1998. Enforcement of these measures, as well as enhanced monitoring of breeding flocks of more than 200 chickens, and additional sanitation and monitoring measures for laying flocks and broilers started in January 1999. Despite the implementation of all sanitation and monitoring measures in the breeding sector, the number of S. Enteritidis cases isolated from humans continued to grow (from 3382 cases in 1992 to 10 492 cases in 1999).
A few years later, it became clear that measures aiming at reducing vertical transmission of Salmonella at the breeder-flock level were insufficient to seriously reduce contamination levels of layers and broilers, and thus had only limited impact on human illness caused by Salmonella. To improve the former measures, the European Parliament and the Council of the European Union introduced in 2003 the Regulation (EC) No. 2160/2003 to ensure that proper and effective measures were taken in the member states to detect and control Salmonella at all relevant stages of production, processing and distribution, particularly at the level of primary production, including feed. In Belgium, the application of a new official programme in layers and breeders implementing Regulation (EC) No. 2160/2003 commenced on 1 January 2007. However, the Federal Agency for the Safety of the Food Chain (FASFC) had already strongly recommended S. Enteritidis vaccination of layer hens since 2004.
This paper aims to present the epidemiology (incidence and trends) of human Salmonella infections in Belgium and to review the role of the vaccination programme in layer flocks on the decline of the incidence of human salmonellosis and foodborne outbreaks due to S. Enteritidis.
MATERIALS AND METHODS
Surveillance systems and objectives
Human and food isolates
In Belgium, Salmonella strains isolated from human patients are transferred on a voluntary basis to the National Reference Centre for Salmonella and Shigella (NRCSS) for serotyping by clinical microbiology laboratories (between 171 and 201 laboratories over the last 5 years representing each year more than 90% of the total number of licensed clinical laboratories). Reporting practices in place from the 1970s did not change over the studied period. Surveillance data [patient information, specimen source, symptoms, sporadic case/outbreak, travel history (when supplied), serotype] were recorded.
Strains from meat and meat products, isolated as part of the official monitoring programme of the Belgian Food Agency are also transferred to the NRCSS, but on a mandatory basis. The FASFC, the Public Health Inspections of the Flemish Community, the French Community and the Brussels Common Community Committee (for the Brussels-Capital Region, see Fig. 1), and the sentinel laboratories network for human clinical microbiology provide human and food sampling data to the National Reference Laboratory for Food-borne Outbreaks (NRL FBO). Since 2003 the NRL FBO has acted as a central repository for all human and food data on foodborne outbreaks and gathers laboratory results, food sampling and epidemiological data collected during outbreaks. This allows a coordinated reporting to the Belgian authorities and the European Commission. Before 2003, data were collected by the inspection and surveillance systems but neither compared nor verified for overlapping. The data before 2003 used in this study were those from the FASFC. Moreover, since 2005, all suspected food samples in the case of foodborne outbreaks are analysed by the NRL FBO. In the total number of foodborne outbreaks reported, the outbreaks with unknown aetiology or unknown food vehicle are included. In some Salmonella outbreaks, the serotype was not determined, and as a consequence, the total number of outbreaks due to S. Enteritidis might be underestimated. Due to the close collaboration between NRL FBO and NRCSS, Salmonella isolates from outbreaks are available for further studies, e.g. for comparison by pulse-field gel electrophoresis between human and food isolates. Both laboratories are accredited in accordance with the requirements of the ISO 17025 standard.
Fig. 1. The Federal State of Belgium, the Communities, and the Regions [Source: Belgian Federal Portal: (http://www.belgium.be/eportal/application?languageParameter=en&pageid=contentPage&docId=8011).]
Veterinary isolates
All strains isolated from poultry as part of mandatory Salmonella monitoring programmes, as described in the European Directives 92/117/EEC and 2003/99/EC, are sent to the National Reference Laboratory for Salmonella in Animals for typing. This laboratory also serotypes all strains of the Salmonella spp. baseline study in laying flocks according to Decision 2004/665/EC of the Commission of 22 September 2004. Since 1993, breeder flocks are tested for the presence of Salmonella spp. from the age of about 20 weeks and then every 6 weeks by the regional laboratory of Animal Health Care Flanders (DGZ) for the Flanders region and the Regional Animal Health and Identification Association (ARSIA) for the Walloon Region (see Fig. 1). Also layer and broiler flocks are sampled about 3 weeks before their transport to the slaughterhouse. The bacteriological isolation of Salmonella spp. is carried out by laboratories accredited and recognized by the FASFC. For the isolation of Salmonella from faecal material, semi-solid media [modified semi-solid Rappaport–Vassiladis (MSRV) or Diassalm] are used as selective enrichment agar. Swarmed colonies were plated on Brilliant Green agar (BGA), xylose lysine desoxycholate (XLD) agar or xylose lysine agar tergitol 4 (XLT4) for isolation.
In 1998 and 2005, screenings were carried out by the veterinary services and FASFC in order to determine the prevalence of Salmonella spp. and in particular of S. Enteritidis in layers. In 1998, the screening targeted 61 holdings of layers of all ages. Twenty blood samples per flock were analysed by ELISA for the presence of antibodies against S. Enteritidis and a mixture of 60 faecal samples screened for the presence of Salmonella spp. In 2005, the study targeted 130 flocks at the end of their laying period. Two samples per flock, of dusty material collected beneath the cages or at different places of the house and five mixed faecal samples, or five pairs of boot swabs were screened for the presence of Salmonella spp.
All these national surveillance programmes document the occurrence and trends of serotypes, detect local, regional, national or even international outbreaks, attempt to find and eliminate the sources, and allow the competent authorities to plan and apply preventive strategies to combat salmonellosis. This national Salmonella surveillance is also intended to rapidly interact at the international level via electronic communication (e.g. Enter-net network, RASFF) [Reference Fisher7].
Isolation of Salmonella from suspected food
For the detection of Salmonella in food the official Belgian method SP-VG-M002 was used. [Reference Ghafir8]. After pre-enrichment in buffered peptone water, Diassalm was used as a semi-solid enrichment medium. Strains were isolated on XLD agar and confirmed by biochemical tests (rapid ID 20e, bioMérieux, France).
Serotyping and phage-typing
Serotyping of Salmonella strains is carried out by slide agglutination with commercial antisera following the Kauffmann–White scheme [Reference Popoff9]. Phage-typing is performed on isolates according to the recommendations of the Health Protection Agency (Colindale, UK) [Reference Threlfall and Frost10].
RESULTS
Results of investigations in 2005 and epidemiological history – serotypes
During the period 1970–1987, the total of human Salmonella spp. ranged from 4000 to 8500 laboratory-confirmed cases a year and S. Enteritidis scarcely represented between 1·8% and 5·5% of all Salmonella isolates (Fig. 2). During this period, the serotype Typhimurium was the most prevalent serotype. From 1988 a dramatic rise of S. Enteritidis infections in humans was observed and from 1991, Enteritidis had overtaken Typhimurium. Consequently to the rise of S. Enteritidis, the total number of Salmonella isolates in Belgium increased in the last decade to reach a maximum of 15 774 isolates and an incidence of 160/100 000 inhabitants in 1999 (Table 1).
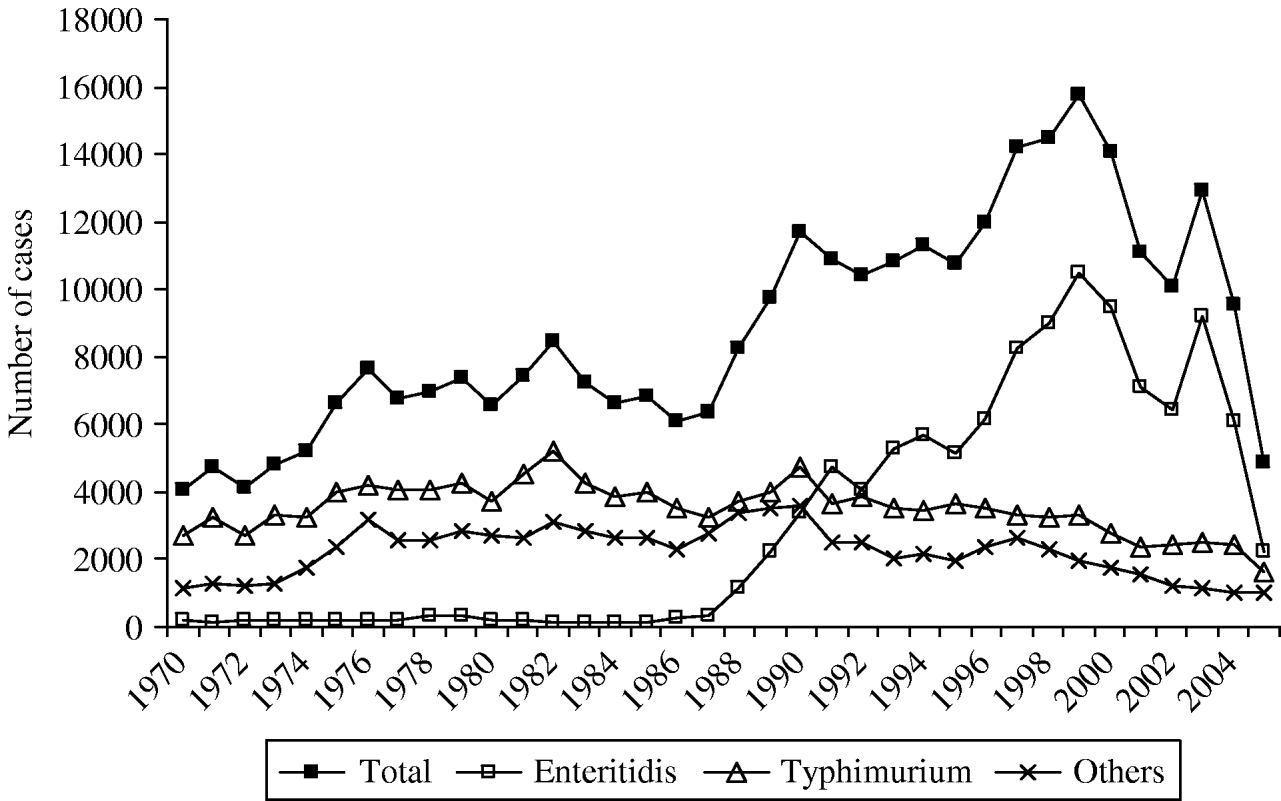
Fig. 2. Trend of the human Salmonella isolates, and of the two major serotypes Enteritidis and Typhimurium over the last 34 years in Belgium; number of laboratory-confirmed cases.
Table 1. Human Salmonella: number of laboratory-confirmed cases. Occurrence of S. Enteritids and S. Typhimurium over the last 20 years in Belgium

Salmonella strains isolated by clinical microbiology laboratories from human patients are transferred on a voluntary basis to the NRCSS for serotyping.
Since then the total number of laboratory-confirmed cases declined to 14 088, 10 783 and 10 075 cases in 2000, 2001 and 2002, respectively (Table 1). In 2003, an increase in the total number of human salmonellosis was again recorded (28% more than in 2002). This resulted from the increase (42%) of the serotype Enteritidis in 2003 which represented for the first time more than 70% of the total number of cases. Due to the absence of epidemiological investigation, this peak could not be attributed to one or several large outbreaks or an increase of sporadic cases. In 2005, the total number of cases caused by Salmonella spp. and by S. Enteritidis fell to 4872 and 2208 cases, respectively. This represented a decrease of 58% for Salmonella spp. and of 71% for S. Enteritidis compared with the average annual number of cases in the period 2000–2004. Compared to 2004, the decrease in 2005 was not specific to a particular season (Fig. 3), although a higher reduction was observed from September to November (around 75% decline vs. 45–65% for the other months).
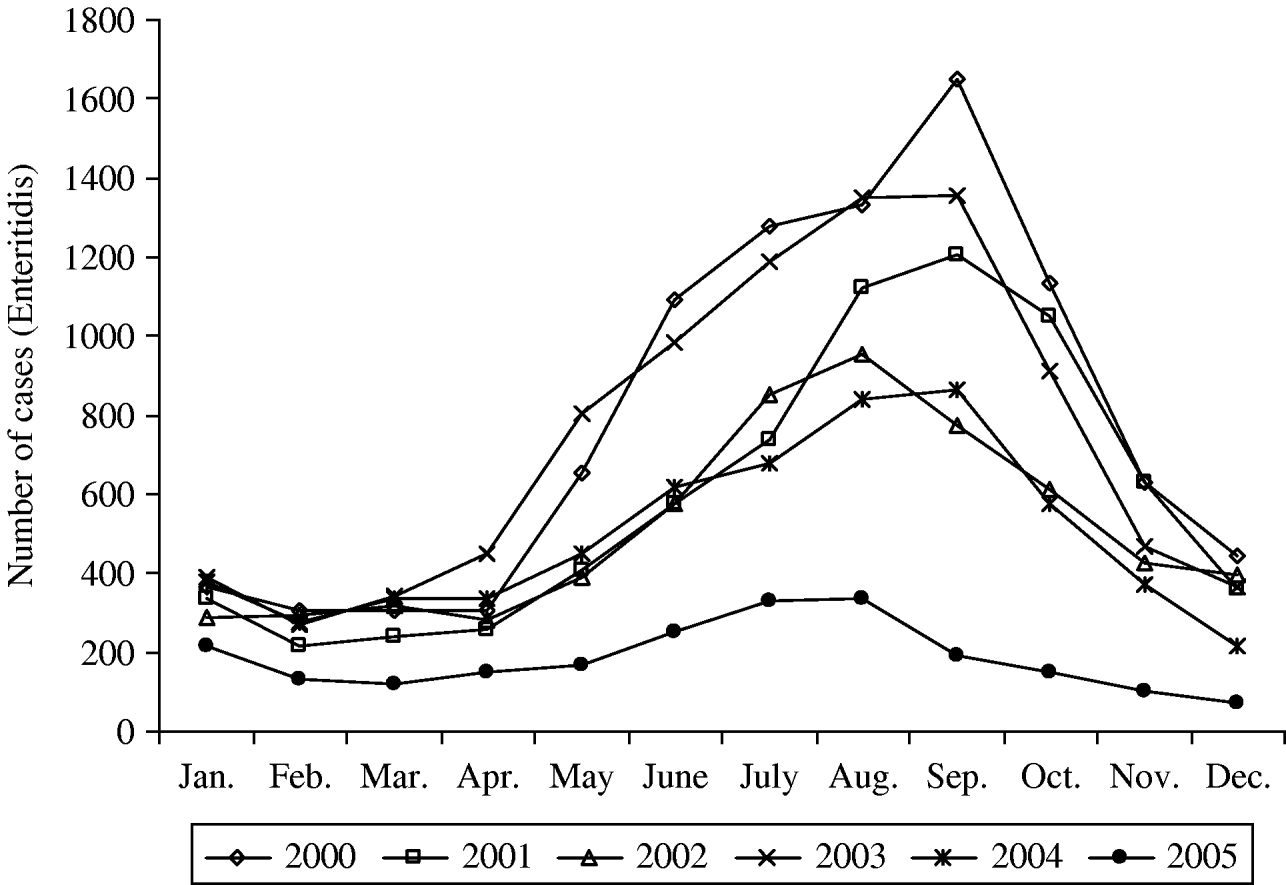
Fig. 3. Seasonal distribution of S. Enteritidis for the period 2000–2005.
Investigation results of foodborne outbreaks
Investigation results are presented as percentages of the total number of reported foodborne outbreaks for each year because the way of reporting the foodborne outbreaks changed in 2003 and has improved considerably since then. This explains the increase in the total number of reported outbreaks over recent years (the numbers are mentioned as indicators of the reporting). From the late 1990s, a decrease was observed in the percentage of foodborne outbreaks caused by Salmonella (all serotypes): from 53% in 1999 (n=21) in 40 reported outbreaks to 17% in 2002 (n=14) in 83 reported outbreaks (Fig. 4). In 2003 and 2004 the percentage of outbreaks caused by Salmonella spp. increased again towards 62% (n=63) and 53% (n=30), respectively, in 101 and 57 outbreaks. In 2005, a decrease was again recorded and Salmonella spp. was identified as the causative agent in 19% of the 105 foodborne outbreaks. In 2000, S. Enteritidis represented 26% (n=9) of the total number of foodborne outbreaks, decreased towards 8% in 2002 (n=7), increased in 2003 and 2004, respectively, to 22% and 21% (n=22 and n=12) and again decreased to 8% (n=8) in 2005.
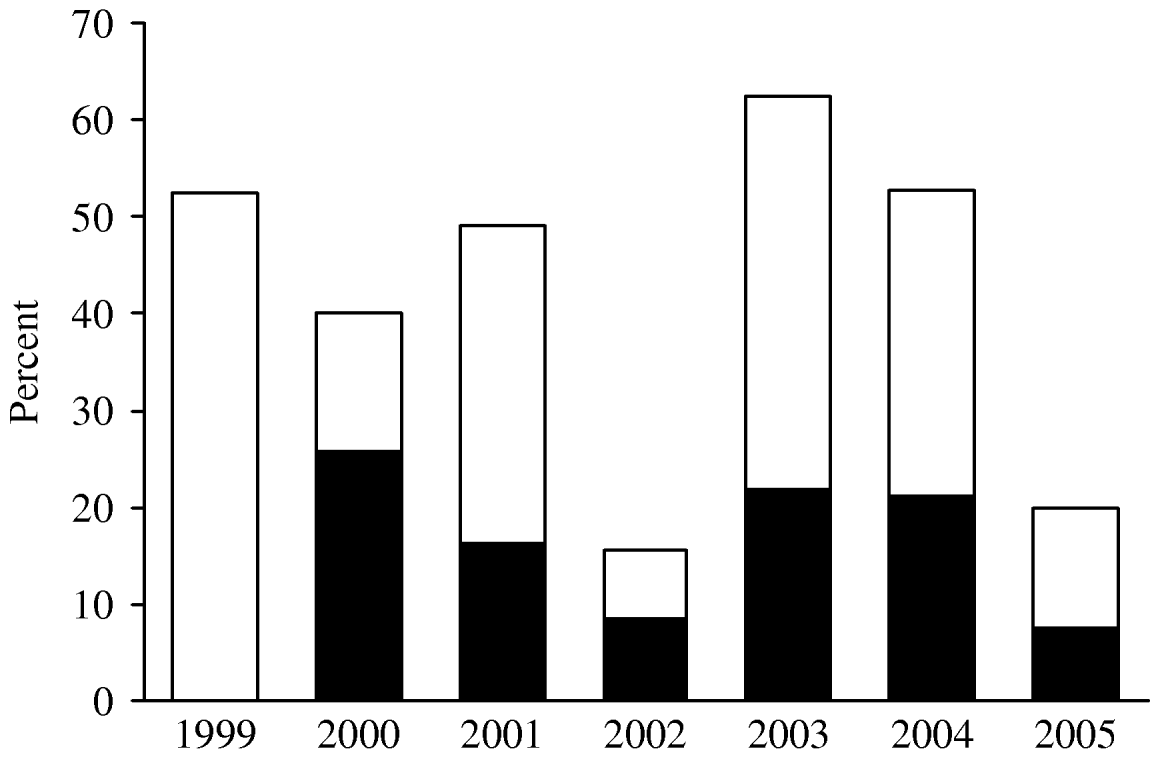
Fig. 4. Percentages of the reported foodborne outbreaks due to Samonella spp. and S. Enteritidis in Belgium over the period 1999–2005. □, Percentage of FBO with Salmonella spp. as aetiological agent; ■, Percentage of FBO with Salmonella Enteritidis as aetiological agent (no data available for the FBO with S. Enteritidis as aetiological agent in 1999).
It is worth noting that the nature of the predominant food vehicle in foodborne outbreaks also changed from eggs and food prepared with raw eggs to meat and meat preparations (poultry, pork, beef, veal, lamb and meat mixtures). Eggs and preparations with raw eggs were implicated as a vehicle in 23% of outbreaks in 1999, with a peak of 34% in 2003 but, in 2005, this food vehicle was responsible for only 8% of the total outbreaks. On the other hand, the percentage of outbreaks where meat and meat preparations were the vehicle increased in this period from 21% to 35%.
Results of monitoring programmes in primary poultry production
From 1993, all Belgian breeder flocks were regularly screened for the presence of Salmonella spp. (Table 2). In Flanders where most breeder flocks are located (about 90% in 2005), the percentage of flocks contaminated with S. Enteritidis increased from 3% to 7·7% over the period 1993–1998. However, since 1999, a marked decrease was observed with <1% of flocks infected. In Wallonia, the data available from 2000 also indicated a decrease in the number of infected breeder flocks ranging from 7·4% in 2000 to 0% in 2005. The same tendency was observed for S. Typhimurium infections in breeders.
Table 2. Isolation of Salmonella spp., S. Enteritidis and S. Typhimurium from breeder flocks in Belgium from 1993 to 2005 (percentages of positive flocks)
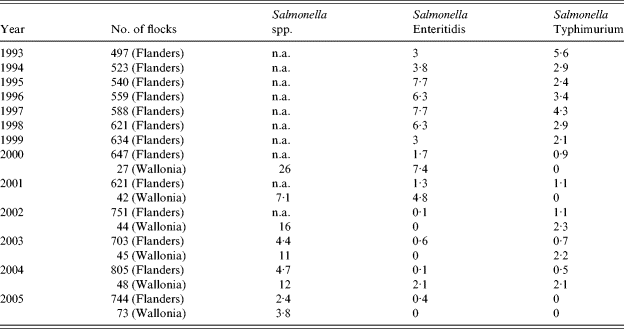
n.a., Not available.
On the other hand, Salmonella spp. infected numerous Belgian layer flocks. A study completed by the veterinary services at the end of 1997 and the beginning of 1998 revealed that infection levels of layer flocks (n=61) were 30% for Salmonella spp., 21% for S. Enteritidis, and 1·6% for S. Typhimurium. In 2005, the reference study on the prevalence of Salmonella in layer flocks in the European Union revealed for Belgium an infection level of 35·4% for Salmonella spp., 26·2% for S. Enteritidis and 0% for S. Typhimurium. This study involved 130 Belgian farms and confirmed the predominance of S. Enteritidis infections (66·3% of the total isolates). Ninety of these 130 Belgian farms were vaccinated against S. Enteritidis.
Distribution of the different phage types in 2005; comparison with the period 2000–2005
From 2000 to 2005, 7% (n=2804) of S. Enteritidis isolates were phage-typed (randomly selected). From 2000 to 2004, PT4 was the predominant phage type accounting for 28–55% of the isolates (Fig. 5). PT21 was the second most common phage type in the same time period accounting for 24·6–35% of the isolates each year. In 2005, however, PT21 became the most common phage type accounting for 37·56% of the isolates, followed by PT4, which accounted for 23·6%. Only PT1 (6·5%), PT6 (5·7%), PT8 (7·6%), and the newly emerging PT34 (5·9%) were found in >5% of the isolates. PT14b which also emerged during 2001–2004 almost completely disappeared in 2005.
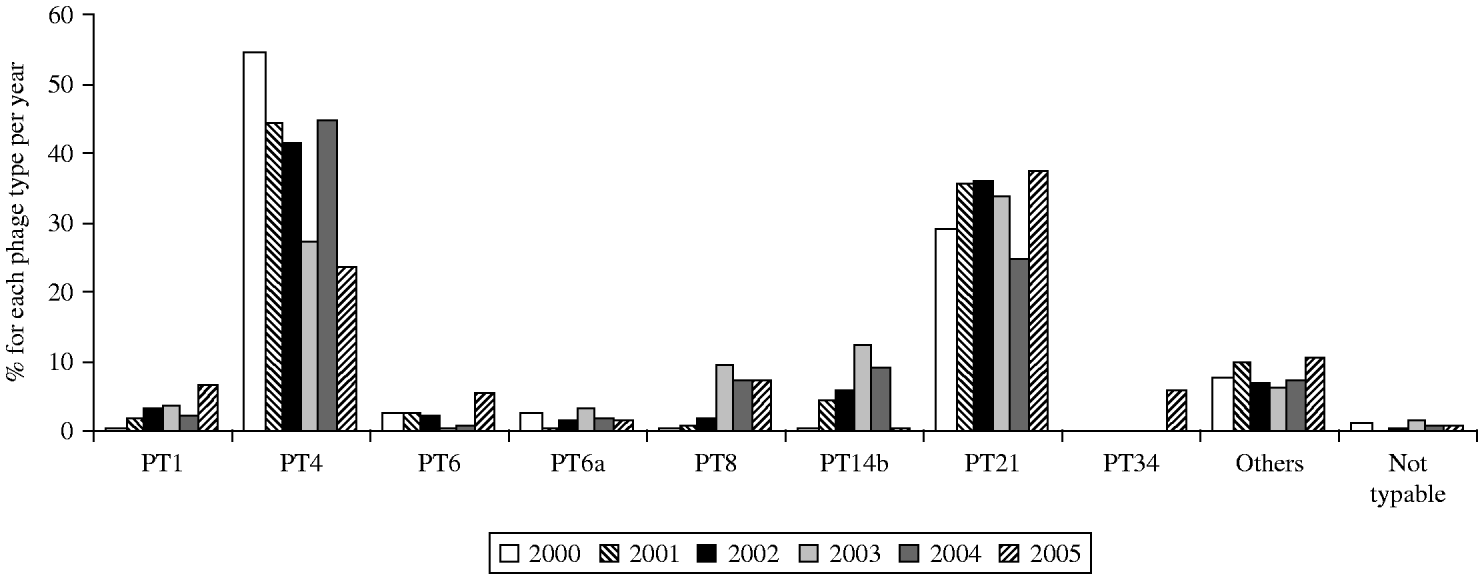
Fig. 5. S. Enteritidis: distribution of the most important phage types in 2000 (n=376), 2001 (n=488), 2002 (n=495), 2003 (n=492), 2004 (n=479), and 2005 (n=474).
Regarding layer flocks, in preliminary data on 11/25 flocks tested, S. Enteritidis PT4 was found, and in an additional 10 flocks PT21 was detected; in both occasions alone or in combination with other phage types. Other phage types were also found, but to a far smaller extent.
DISCUSSION
Human illness due to non-typhoidal Salmonella is mainly caused by consumption of contaminated food. Incidents affecting only a few individual are common but outbreaks involving large numbers of cases are observed on some occasions [Reference Bell and Kyriakides11]. While potentially all livestock animals can be infected with Salmonella spp., contaminated poultry meat and eggs are the main sources of S. Enteritidis for humans [Reference Telzak12–Reference Henzler14].
Before 1980, S. Enteritidis was isolated at a medium-to-low frequency from humans and animals in most European countries. During the period 1956–1959, the isolation rates in Western Germany and West Berlin of human S. Enteritidis ranged from 4·4% to 5·9% whereas those of S. Typhimurium were between 34% and 41% [Reference Poppe and Saeed15]. Similarly, in Belgium, the isolation rates of the serotype Enteritidis ranged from 1·8% to 5·5% between 1970 and 1987, whereas the rates for the serotype Typhimurium were 34–71%.
Between 1979 and 1987, S. Enteritidis isolated from humans increased in 24/35 (69%) countries reporting to the WHO. In 1979, only 2/21 (10%) countries with reported data had S. Enteritidis as their most common serotype. However in 1987, 9/21 (43%) countries reported S. Enteritidis as their most common serotype; 8/9 (89%) were European countries [Reference Rodrigue, Tauxe and Rowe4]. In the United States, S. Enteritidis steadily increased in frequency from being the sixth most common serotype in 1960 to the most frequently isolated serotype from humans in 1990 [Reference Aserkoff, Schroeder and Brachman16, Reference Mishu17].
From the late 1980s until recently, shell eggs were recognized as the most common vehicle of infection in outbreaks [Reference St Louis18, Reference Ejidokun19]. From 1985 to 1996, a total of 660 outbreaks of S. Enteritidis were reported to the CDC. Eggs alone or egg-containing foods were implicated in 233/293 (79%) outbreaks with known vehicles [Reference Angulo, Swerdlow, Saeed, Gast, Potter and Wall20]. In England, Wales and The Netherlands, the consumption of undercooked hen eggs and to a lesser extent chicken meat were also identified as main risk factors for salmonellosis [Reference Cowden21–Reference Delarocque-Astagneau24]. We showed that the percentage of reported outbreaks in Belgium caused by Salmonella spp. was high until 2004 (between 40% and 63%), except in 2002 (16%), and decreased in 2005 to 20%. The percentage caused by S. Enteritidis roughly followed the same evolution as Salmonella spp. However, as the serotype was not always reported or determined for each foodborne Salmonella spp. outbreak, the number of outbreaks due to S. Enteritidis might have been underestimated.
Eggs and preparations with raw eggs were responsible of 23% of registered foodborne outbreaks in 1999, with a peak of 34% in 2003. In response to the high number of egg-associated outbreaks, the FASFC conducted a prevention campaign that included public announcements on television during the summer of 2003. This campaign stressed the importance of adequate refrigeration of food containing fresh eggs.
However, in recent years a decrease in the number of human Salmonella isolates has been reported in European countries, with few exceptions [6]. In Belgium, after the rise of S. Enteritidis infections observed in humans from 1988 to 1999, the number of S. Enteritidis cases diminished except in 2003 when this serotype exceeded for the first time 70% of the total Salmonella cases. That year, a significant excess isolation of S. Enteritidis was also reported by The Netherlands, a neighbouring country. Van Pelt et al. [Reference Van Pelt25] explained that strong evidence was found to suggest that the increase in importation of contaminated eggs, as the result of the avian influenza outbreak in spring 2003 which also affected the North of Belgium, was the most probable reason for this excess. The change in the phage types of S. Enteritidis (Fig. 3) in 2003 in Belgium also indicated a possible change in the trade of eggs or egg products. In 2004, the total number of human Salmonella isolates and particularly the serotype Enteritidis was gradually decreasing but during 2005 a substantial decrease (71%) of S. Enteritidis infections compared with the average annual number of cases in the period 2000–2004 was recorded.
First reported in Europe in the 1980s, S. Enteritidis phage type (PT) 4 quickly became established in European poultry flocks and consequently emerged in human isolates. In countries where S. Enteritidis PT4 has emerged, it rapidly replaced other phage types and resulted in at least a fivefold increase in the rate of S. Enteritidis isolates recovered from humans [Reference Rampling26]. The isolation rates of S. Enteritidis from human sources increased gradually during the period 1990–1994, and PT4 became the most prevalent S. Enteritidis phage type in 1992 [Reference Poppe and Saeed15]. From 1998 the distribution of phage types in S. Enteritidis has changed substantially. In 1998, fewer than 62% of the 34998 isolates that have been phage-typed in 12 European countries were PT4 whereas data for 2003 from 14 countries (27 431 cases) showed that PT4 comprised only 32·1% of the total [27]. Phage types such as PT1, PT8, PT14b and PT21 showed over the 6-year period an increase in prevalence. PT6 and PT6a remained around 3–5% in prevalence. These seven phage types (PT4 included) accounted for about 90% of all subtyped strains of S. Enteritidis. In Belgium a shift in the distribution of phage types was also observed. However, PT4 and PT21, the two main phage types, represented altogether about 80% in 2000–2002 and 65% in 2003–2005 of all the S. Enteritidis phage types. PT8 and PT14b markedly increased over recent years (especially in 2003) but almost disappeared in 2005. It is noteworthy that conversion of PT8 to PT14b during passage in in vivo chicken infection experiments has been reported previously [Reference Fadl and Khan28]. While PT8 is the dominant phage type in Czech Republic, Denmark and Slovakia, PT14b dominates in Greece but has also been found in eggs used in the UK catering trade [Reference De Jong and Ekdahl29, 30].
On the veterinary side, the principal preventive measures taken in breeder flocks since 1993 focused on a wide vaccination (near 100% in 2004) against S. Enteritidis, an antibiotic treatment if contaminated, and sanitation measures and monitoring programmes every 6 weeks in all flocks in order to detect the bacterium. Consequently, a clear reduction in prevalence of S. Enteritidis was observed since 1999 (Table 2) in breeder flocks suggesting the beneficial impact of the programme. However, a similar improvement was not observed in layer hens (only sanitation measures, a restricted monitoring programme until 2005 and a weak percentage of vaccination coverage until 2004). The comparison between the results of the 1998 and the 2005 studies has to be viewed carefully because the sampling methods differed somewhat on parameters such as: age of layers, number of samples, and analyses per flock. These differences should not significantly affect the results as representative sampling was carried out for both years. Production of Salmonella-negative 1-day-old layer chickens did not seem to be a sufficient factor to reduce the infection level in adult hens, indicating the importance of horizontal transmission. Moreover, the antibiotic treatment of breeders infected with S. Enteritidis until 2004 was probably insufficient to prevent the transmission of the bacterium towards the chicks and therefore may have had a negative effect on the infection rate in layers because these chicks were integrated in the same manner as those resulting from unscathed flocks.
A European screening [31] of layer hens performed in 2005 (Decision 2004/665/EC and SANCO/34/2004 Rev 3) confirmed that S. Enteritidis infection was frequent in Belgium, with an infection rate of 26·2% of the layer flocks. In addition, the most prevalent phage types found in layer flocks are the same as those found among humans, i.e. PT4 and PT21, suggesting that table eggs from Belgian farms could be an important source for contamination of local egg products and humans. Nevertheless, the same screening indicated a weaker contamination by this bacterium in the flocks of vaccinated layers (22% of the 90 vaccinated flocks) than in the flocks of unvaccinated layers (35% of the 40 unvaccinated flocks) suggesting a beneficial impact of vaccination in the control of S. Enteritidis in layers. The European study [31] reveals similar results. S. Enteritidis prevalence in layer flocks is dependent on the vaccination status with a lower prevalence in vaccinated flocks (4%) than in unvaccinated flocks (12·9%).
It was estimated on the basis of vaccine sales by the FASFC that, in 2005, about 90% of the new layer flocks were vaccinated against S. Enteritidis in comparison with 30% in 2004. Vaccines were administered without legal obligation but vaccination was strongly advised by the competent authority/veterinary services (FASFC) and supported by the grocery store industry.
Moreover, several studies show that vaccination with vaccines, currently used in Belgium, can reduce faecal shedding and systemic spread of S. Enteritidis in laying hens, and thus reduce external and internal egg contamination [Reference Van Immerseel32]. A live vaccine registered for prophylactic use against S. Enteritidis, the rough strain S. Gallinarum 9R, commonly used in Belgian layer flocks, has been shown to reduce the flock-level incidence of S. Enteritidis infections in a large-scale study in The Netherlands [Reference Feberwee33]. While the flock-level incidence was 2·5% (2/80 flocks) in vaccinated flocks, the flock-level incidence in unvaccinated flocks was 11·5% (214/1854 flocks). More recently, Gantois et al. [Reference Gantois34] showed that oral vaccination with the live vaccines TAD Salmonella vac® E (commonly used in Belgian layer flocks), TAD Salmonella vac® T and a combination of both live vaccine strains, at day 1, week 4 and week 16 decreased internal organ colonization, including reproductive tract colonization, and egg contamination.
Although no cause-and-effect relationship between the decline in human S. Enteritidis infections and vaccination of laying hens in Belgium has been statistically proven, the above-mentioned data indicate that increased vaccination status of flocks has most probably contributed to decreasing egg contamination. The new monitoring programme of Salmonella spp. in layers adopted from 2007 will allow evaluation, in the medium term, of the real effect of vaccination on S. Enteritidis carriage in laying hens. On the other hand, the rise of infection in a population also induces an immunity of the population to this bacterium. This herd immunity is the probable cause of the fluctuations seen in infections of animals with different Salmonella serotypes [Reference Cogan and Humphrey35]. Therefore the ubiquity of S. Enteritidis, in poultry as well as in humans, during these two last decades could also have led to its decline, concomitantly to the vaccinations, sanitary and prevention campaigns. However, we have to remain watchful since the decline of S. Enteritidis might open a niche for new Salmonella serotypes.
ACKNOWLEDGEMENTS
The NRC Salmonella and Shigella is very grateful to L. Willems, D. Baeyens, H. Steenhout, F. De Cooman and D. Delbecq for their technical help.
DECLARATION OF INTEREST
None.











