Introduction
The Pronocephaloidea Looss, 1899 is a superfamily of trematodes that mainly infect the intestines of marine and freshwater turtles (Pronocephalidae Looss, 1899), a wide range of birds and mammals (Notocotylidae Lühe, 1909; Nudacotylidae Barker, 1916) and sirenians (dugong and manatees) (Labicolidae Blair, 1979; Opisthotrematidae Poche, 1926; Rhabdiopoeidae Poche, 1926); a few pronocephalids infect marine iguanas and fishes (Blair, Reference Blair, Jones, Bray and Gibson2005). Although there has been only limited molecular exploration of the relationships within the group (Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003), they are convincingly considered the sister taxon to the Paramphistomoidea. Life cycles are known only for notocotylids, one nudacotylid and a handful of pronocephalids. As far as it is known, these always involve the infection of gastropods by ingestion of the egg (Murrills et al., Reference Murrills, Reader and Southgate1985), the development of ‘monostome’ cercariae in rediae, cercariae which emerge from the gastropod and encyst as metacercariae on the surface of potential food of the definitive host and infection of the definitive host when that food is consumed. There are many life cycles reported for the Notocotylidae (e.g. Herber, Reference Herber1942; Stunkard, Reference Stunkard1960; Pike, Reference Pike1969; Kanev et al., Reference Kanev, Vassilev, Dimitrov and Radev1994) involving a wide range of gastropods: caenogastropods (Bithyniidae, Cerithiidae, Hydrobiidae, Littorinidae, Pleuroceridae, Pomatiopsidae, Tateidae, Thiaridae and Viviparidae); neritimorphs (Neritidae); and heterobranchs (Lymnaeidae, Physidae and Planorbidae). In contrast, relatively few pronocephalid life cycles are known. They are known for entirely freshwater species infecting Pleuroceridae or Thiaridae (Horsfall, Reference Horsfall1935; Hsü, Reference Hsü1937) and one species that infects the semi-marine terrapin Malaclemys terrapin (see Hunter, Reference Hunter1967), which has a nassariid (Caenogastropoda) as intermediate host. The single reported nudacotylid life cycle (Ameel, Reference Ameel1944) involves a pomatiopsid (Caenogastropoda). There have so far been no intermediate hosts identified for Labicolidae, Opisthotrematidae, Pronocephalidae or Rhabdiopoeidae that infect marine turtles and sirenians, which, between them, harbour an exceptionally rich fauna of these trematodes.
Here we report five new pronocephaloid cercariae based on combined morphological and molecular data. These species were found in specimens of Rhinoclavis vertagus (Cerithiidae), Nassarius coronatus (Nassariidae) and Thylacodes sp. (Vermetidae).
Materials and methods
Collection and morphological study
Cerithiid and nassariid gastropods were collected by hand from rocks and sand flats surrounding Lizard Island on the northern Great Barrier Reef (14°40′S, 145°27′E). These were identified without difficulty by reference to Wilson (Reference Wilson1993a, Reference Wilsonb). Vermetids were collected by hammer and chisel from intertidal rocks off Dunwich (27°24′S, 153°20′E) and Amity (27°23′S, 153°26′E), North Stradbroke Island, Moreton Bay, Queensland, Australia. The specimens could not be readily identified by reference to any available guide and were characterized by 16S rDNA data. Sequence data were analysed by Dr Timothy Rawlings relative to his unpublished database of vermetid sequences. Two vermetid genotypes, differing by 6 bp and found off Dunwich and Amity respectively, possibly represent two closely related species. These genotypes were nested among species of the genus Thylacodes Guettard, 1770, but did not correspond to any species for which sequence data were available. Sequence data for these specimens are lodged on GenBank (MN574296–97), and morphological vouchers relating to the two genotypes are lodged in the Queensland Museum (MO85861–2).
Cerithiids and nassariids were held in seawater for 24 h and monitored for emerged cercariae; no emerged cercariae were detected, so infections were found only by dissection. Vermetids were held in aerated seawater prior to dissection. Because these gastropods formed large tangled masses encrusting rocks, they could not be monitored individually for cercarial emergence, and all were dissected. Asexual trematode stages were fixed by pipetting into near-boiling saline followed by preservation in 80% ethanol to allow for either morphological or molecular analysis. Specimens for morphology were stained in Mayer's haematoxylin, destained in dilute HCl, neutralized in dilute ammonia solution, dehydrated in a graded ethanol series, cleared in methyl salicylate and mounted on slides in Canada balsam. Mounted specimens were measured using CellSens software (https://www.olympus-lifescience.com/en/software/cellsens) connected to an Olympus SC50 camera mounted onto an Olympus BX53 compound microscope (Olympus, Notting Hill, Australia). Drawings were made with a camera lucida mounted on an Olympus BX53 compound microscope and were digitized in Adobe Illustrator (https://www.adobe.com/au/products/illustrator.html).
Molecular sequencing and phylogenetic analysis
Sequence fragment amplifications of pronocephaloids were mostly obtained from individual cercariae or rediae through direct polymerase chain reaction (PCR) (see Huston et al., Reference Huston, Cutmore and Cribb2018), but in some cases total genomic DNA was extracted using phenol/chloroform extraction techniques (Sambrook & Russell, Reference Sambrook and Russell2001). Pronocephaloids were genetically characterized by complete ITS2 and partial 28S rDNA, and vermetid hosts by partial 16S rDNA. The ITS2 rDNA region was amplified using the primers 3S (Morgan & Blair, Reference Morgan and Blair1995) and ITS2.2 (Cribb et al., Reference Cribb, Anderson, Adlard and Bray1998), the partial D1-D3 28S rDNA region using LSU5 (Littlewood, Reference Littlewood1994) and 1500R (Snyder & Tkach, Reference Snyder and Tkach2001) and the partial 16S rDNA region using 16SARL-CBU (5′-CGC CTG TWT ADC AAA AAC ATG-3′) and 16SBRH (5′-CCG GTC TGA ACT CAG ATC ACG-3′) (both modified from Palumbi, Reference Palumbi, Hillis, Moritz and Mable1996). Direct PCR for the ITS2 and 28S regions was performed following Huston et al. (Reference Huston, Cutmore and Cribb2018). Genomic DNA-based PCR for all regions was performed following Cutmore et al. (Reference Cutmore, Diggles and Cribb2016). Amplification was carried out on an MJ Research PTC-150 thermocycler (Thermo Fisher Scientific, Brisbane, Australia).. The following profile was used to amplify the 16S region for vermetid gastropods: an initial 94°C denaturation for 3 min, followed by 40 cycles of 94°C denaturation for 30 s, 50°C annealing for 30 s and 72°C extension for 30 s, with a final extension at 72°C for 10 min. Amplified DNA was purified using a Bioline ISOLATE II PCR and Gel Kit (Bioline, Alexandria, Australia) according to the manufacturer's protocol. Cycle sequencing of purified DNA was carried out using ABI Big Dye™ version 3.1 (Thermo Fisher Scientific) chemistry following the manufacturer's recommendations, using the same primers used for PCR amplification as well as the additional 28S primers 300F (Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000) and ECD2 (Littlewood et al., Reference Littlewood, Rohde and Clough1997). Cycle sequencing was carried out at the Australian Genome Research Facility. Geneious® version 10.2.3 (Kearse et al., Reference Kearse, Moir and Wilson2012) was used to assemble and edit contiguous sequences, and the start and end of the ITS2 rDNA region were determined by annotation through the ITS2 Database (Keller et al., Reference Keller, Schleicher, Schultz, Muller, Dandekar and Wolf2009; Ankenbrand et al., Reference Ankenbrand, Keller, Wolf, Schultz and Forster2015) using the ‘Metazoa’ model.
The partial 28S rDNA sequences generated during this study were aligned with sequences of related species from GenBank (table 1) using MUSCLE (Edgar, Reference Edgar2004), with ClustalW sequence weighting and UPGMB clustering for iterations 1 and 2. The resultant alignment was refined by eye using MESQUITE (Maddison & Maddison, Reference Maddison and Maddison2018); no ambiguously aligned sites were identified (those constituting more than three bases and present in two or more of the sequences in the dataset). Bayesian inference analysis of the 28S dataset was performed using MrBayes version 3.2.6 (Ronquist et al., Reference Ronquist, Teslenko and van der Mark2012), run on the CIPRES portal. The best nucleotide substitution model was estimated using jModelTest version 2.1.10 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012). The Akaike Information Criterion and Bayesian Information Criterion predicted the GTR + I + Γ model as the best estimator; Bayesian inference analyses were conducted using the closest approximation to this model and run over 10,000,000 generations (ngen = 10,000,000), with two runs each containing four simultaneous Markov Chain Monte Carlo chains (nchains = 4) and every 1000th tree saved. Analysis used the following parameters: ‘nst = 6’, ‘rates = invgamma’, ‘ngammacat = 4’, and the priors parameters of the combined dataset were set to ‘ratepr = variable’. Samples of substitution model parameters, and tree and branch lengths, were summarized using the parameters ‘sump burnin = 3000’ and ‘sumt burnin = 3000’. Species of Hexangium Goto & Ozaki, 1929 and Neohexangitrema Machida, 1984 were designated as functional outgroup taxa.
Table 1. Collection data and GenBank accession numbers for pronocephaloid species analysed in this study.
Results
Infections of pronocephaloid-type cercariae (oculate, monostome, paired dorsal adhesive pockets and simple tail) were found in three gastropod species, two in R. vertagus (Cerithiidae) from off Lizard Island, one in N. coronatus (Nassariidae) from off Lizard Island and two in the two genotypes of Thylacodes sp. (Vermetidae), one in both genotypes from off Dunwich and Amity, the second only from off Dunwich.
Morphological characterization
Because cercariae could not be assigned to any named species, they are herein assigned a temporary name consisting of an alphabetical (A–E) and geographical identifier: LI for Lizard Island, Great Barrier Reef; NSI for North Strandbroke Island.
Superfamily: Pronocephaloidea Looss, 1899
Pronocephaloid sp. A-LI (fig. 1a, b; measurements in table 2).
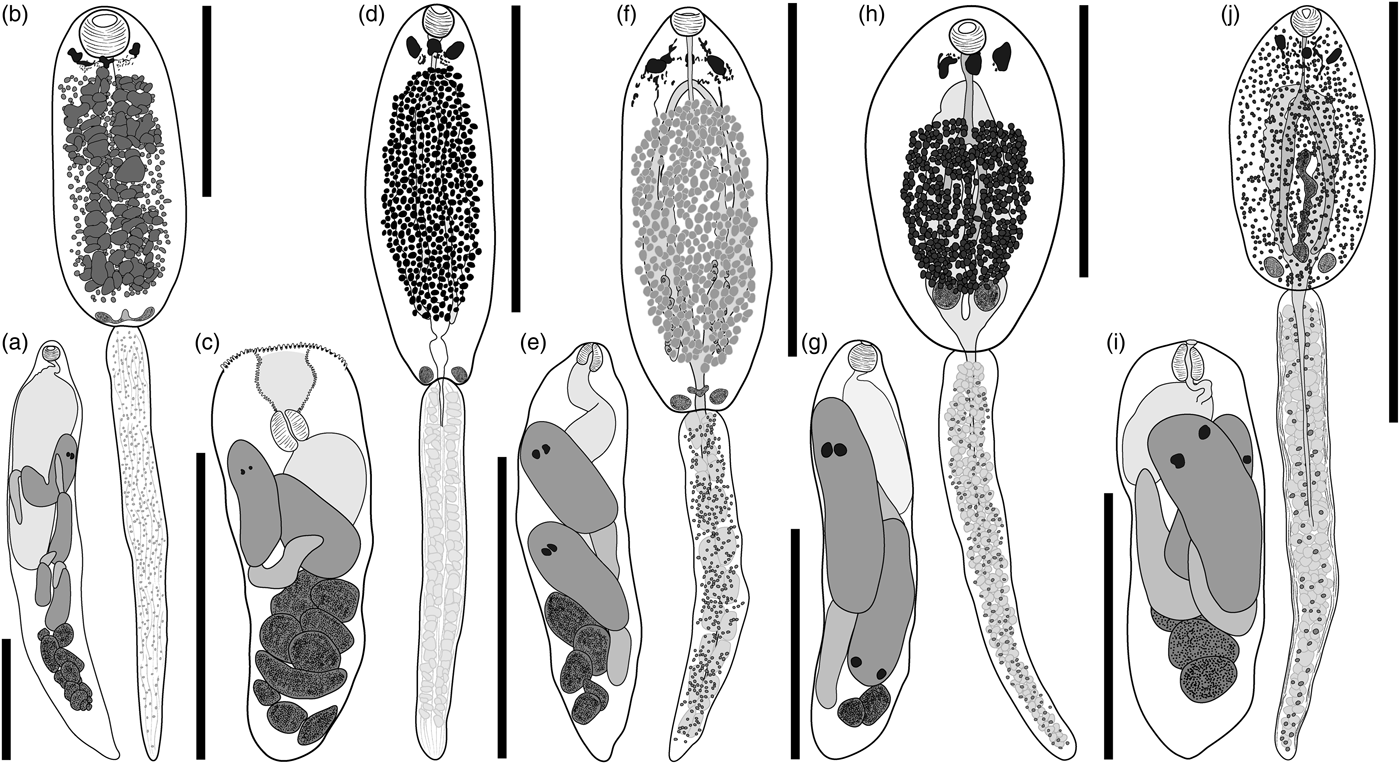
Fig. 1. Rediae and cercariae of five putative pronocephaloids from Queensland marine gastropods. (a, b) Species A-LI, ex Rhinoclavis vertagus, Lizard Island; (c, d) species B-LI, ex R. vertagus, Lizard Island; (e, f) species C-LI, ex Nassarius coronatus, Lizard Island; (g, h) species D-NSI, ex Thylacodes sp., Moreton Bay; (i, j) species E-NSI, ex Thylacodes sp., Moreton Bay. Cephalic collar and adhesive pockets of cercariae not illustrated. Scale bars: 500 µm.
Table 2. Morphometric data for pronocephaloid cercariae expressed in micrometres or as percentages.
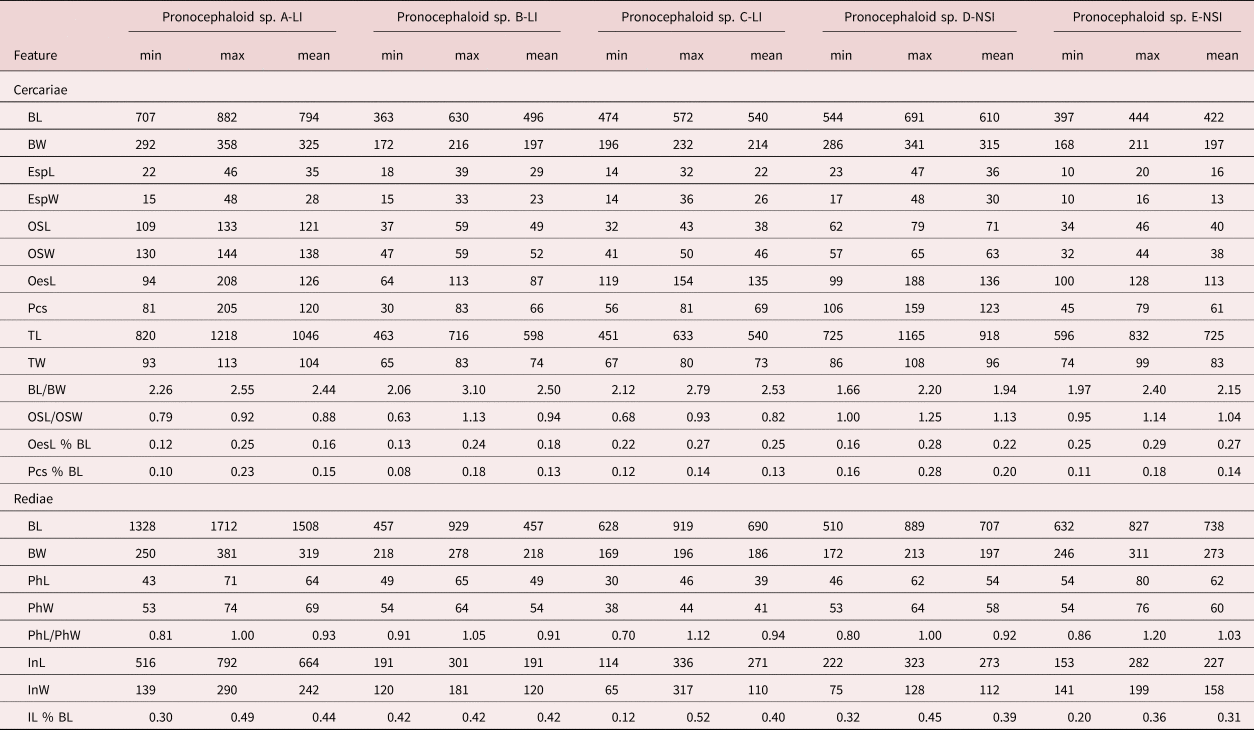
B, body; L, length; W, width; Esp, eyespot (data combined for all three eyespots); OS, oral sucker; Oes, oesophagus, Pcs, post-caecal space; T, tail; Ph, pharynx; I, intestine.
Taxonomic summary
Intermediate host. Rhinoclavis vertagus (Linnaeus, 1767) (Cerithiidae).
Prevalence. Two of 138 (1.45%).
Locality. Off Lizard Island, Great Barrier Reef, Queensland, Australia (14°40′S, 145°27′E).
Voucher material. Seven slides (QM G238198–238204).
Representative DNA sequences. ITS2, four identical replicates (one submitted to GenBank: MN574291); 28S, two identical replicates (one submitted to GenBank: MN574298).
Description
Redia. Based on ten excised specimens. Body elongate, allantoid, attenuating posteriorly, typically containing fewer than five well-developed cercarial bodies in anterior region and fewer than 12 germinal balls in posterior region. Pharynx doliiform to subglobular. Intestine elongate, ellipsoidal, with margins indented, occupying up to one-half of body length. Birth pore distinct, adjacent to pharynx.
Cercaria. Based on ten excised specimens. Trioculate monostome. Body elongate, ellipsoidal. Cephalic collar indistinct. Tegument smooth. Cystogeneous gland-cells extensive in body, filled with opaque, granular substance, extending from pharynx to testicular masses, with distinct gap between gland-field and body margins, obscure most of internal anatomy. Dorsal adhesive pockets inconspicuous, bowl-shaped, on postero-lateral extremities of body. Eyespots three (two in immature cercariae), in anterior portion of pre-equatorial region of body, closely associated with ganglionic mass, often irregular and diffused, conical, with proximal portion near dorsal surface, expanding ventrally; central eyespot slightly posterior to parallel lateral eyespots. Additional pigment scattered in pre-equatorial body region. Oral sucker terminal, subglobular. Oesophagus bifurcates near posterior margin of cephalic collar; caeca terminate blindly in posterior third of body. Reproductive primordia indistinct. Testicular masses two, parallel, joined by U-shaped group of cells, near posterior extremity, at level of adhesive pockets. Ovarian complex indistinct. Excretory bladder variable in shape; consists of two extensively diverticulated lateral arms, uniting anteriorly near central eyespot and posteriorly near testicular primordia. Tail simple, longer than body, attenuating posteriorly.
Pronocephaloid sp. B-LI (fig. 1c, d; measurements in table 2).
Taxonomic summary
Intermediate host. Rhinoclavis vertagus (Linnaeus, 1767) (Cerithiidae).
Prevalence. One of 138 (0.72%).
Locality. Off Lizard Island, Great Barrier Reef, Queensland, Australia (14°40′S, 145°27′E).
Voucher material. Seven slides (QM G238205–238211).
Representative DNA sequences. ITS2, three identical replicates (one submitted to GenBank: MN574292); 28S, two identical replicates (one submitted to GenBank: MN574299).
Description
Redia. Based on ten excised specimens. Body elongate, blunted anteriorly, tapering posteriorly, typically containing fewer than five well-developed cercarial bodies in anterior region and fewer than 12 germinal balls in posterior region. Mouth cavity large, at anterior extremity, lined with noticeable annulations. Pharynx at base of mouth cavity, ellipsoidal. Intestine sac-like, occupying up to one-half of body length. Birth pore inconspicuous, adjacent to pharynx.
Cercaria. Based on ten excised specimens. Trioculate monostome. Body elongate, ellipsoidal. Cephalic collar indistinct. Tegument smooth. Cystogeneous gland-cells extensive in body, filled with opaque, granular substance, extending from pharynx to testicular masses, with distinct gap between gland-field and body margins. Dorsal adhesive pockets conspicuous, bowl-shaped, each on distinct projection on postero-lateral extremities of body. Eyespots three (two in immature cercariae), in anterior portion of pre-equatorial region of body, closely associated with ganglionic mass, laterally parallel, often irregular and diffused, conical, with proximal portion near dorsal surface, expanding ventrally. Additional pigment scattered in pre-equatorial body region. Oral sucker terminal, spherical. Oesophagus bifurcates near posterior margin of cephalic collar; caeca terminate blindly in posterior third of body. Reproductive primordia distinct. Testicular masses two, well-differentiated, parallel, near posterior extremity. Ovarian complex a conspicuous longitudinal mass of cells, passing from testes to mid-body. Excretory bladder variable in shape, consists of two extensively diverticulated lateral arms, uniting anteriorly near central eyespot and posteriorly near testicular primordia. Numerous large, lightly stained gland-cells associated with excretory bladder. Tail simple, longer than body, attenuating posteriorly.
Pronocephaloid sp. C-LI (fig. 1e, f; measurements in table 2).
Taxonomic summary
Intermediate host. Nassarius coronatus (Brugvière, 1789) (Nassariidae).
Prevalence. One of 43 (2.33%).
Locality. Off Lizard Island, Great Barrier Reef, Queensland, Australia (14°40′S, 145°27′E).
Voucher material. Seven slides (QM G238212–238218).
Representative DNA sequences. ITS2, five identical replicates (one submitted to GenBank: MN574293); 28S, two identical replicates (one submitted to GenBank: MN574300).
Description
Redia. Based on ten excised specimens. Body elongate, allantoid, typically containing fewer than five well-developed cercarial bodies in anterior region and fewer than 12 germinal balls in posterior region. Pharynx subglobular. Intestine elongate, ellipsoidal, occupying up to one-half of body length. Birth pore distinct, posterior to pharynx.
Cercaria. Based on ten excised specimens. Trioculate monostome. Body elongate, ellipsoidal. Cephalic collar inconspicuous. Tegument smooth. Cystogeneous gland-cells dense and extensive, extending from region just posterior to eyespots to bifurcation of excretory vesicle, with distinct gap between gland-field and body margins, containing opaque, granular substance. Dorsal adhesive pockets, inconspicuous, conical, each on postero-lateral extremities of body. Eyespots three (two in immature cercariae), in anterior portion of pre-equatorial region of body, closely associated with ganglionic mass, often irregular and diffused, conical, with proximal portion near dorsal surface, expanding ventrally; central eyespot indistinct, smaller than and situated anterior to parallel, lateral eyespots. Additional pigment extensive in pre-equatorial body region, forming web-like pattern around eyespots. Oral sucker terminal, cup-shaped. Oesophagus bifurcates near posterior margin of cephalic collar; caeca terminate blindly in posterior third of body. Reproductive primordia distinct. Testicular masses two, well-differentiated, parallel, near posterior extremity. Ovarian complex a conspicuous longitudinal mass of cells, passing from testes to mid-body. Excretory bladder elongate, balloon-like; consists of two extensively diverticulated lateral arms, uniting anteriorly near central eyespot and posteriorly near testicular primordia. Numerous large, lightly stained gland-cells associated with excretory bladder. Tail simple, longer than body, attenuating posteriorly.
Pronocephaloid sp. D-NSI (fig. 1g, h; measurements in table 2).
Taxonomic summary
Intermediate host. Thylacodes sp. Genotypes 1 (Dunwich) and 2 (Amity) (Vermetidae).
Prevalence. Dunwich: three of 400 (0.75%); Amity: one of 355 (0.28%).
Locality. Off Dunwich (27°24′S, 153°20′E) and Amity (27°23′S, 153°26′E), North Stradbroke Island, Queensland, Australia.
Voucher material. Four slides (QM G238219–238222).
Representative DNA sequences. ITS2, seven identical replicates (one submitted to GenBank: MN574294); 28S, three identical replicates (one submitted to GenBank: MN574301).
Description
Redia. Based on ten excised specimens. Body elongate, allantoid, typically containing fewer than five well-developed cercarial bodies in anterior region and fewer than 12 germinal balls in posterior region. Pharynx subglobular. Intestine elongate, ellipsoidal, occupying up to one-half of body length. Birth pore distinct, adjacent to pharynx.
Cercaria. Based on ten excised specimens. Trioculate monostome. Body elongate, ellipsoidal. Cephalic collar inconspicuous; lobes extend about one-third of body length. Tegument smooth. Cystogeneous gland-cells extending from oesophageal bifurcation to testicular masses, with distinct gap between gland-field and body margins, filled with an opaque, granular substance. Dorsal adhesive pockets inconspicuous, bowl-shaped, each on postero-lateral extremities of body. Eyespots three (two in immature cercariae), in anterior portion of pre-equatorial region of body, closely associated with ganglionic mass, laterally parallel, often irregular and diffused, conical, with proximal portion near dorsal surface, expanding ventrally. Additional pigment scattered in pre-equatorial body region. Oral sucker subterminal, spherical. Oesophagus bifurcates near posterior margin of cephalic collar; caeca terminate blindly in posterior third of body. Reproductive primordia distinct. Testicular masses two, well-differentiated, parallel, near posterior extremity. Ovarian complex a conspicuous longitudinal mass of cells, passing from testes to mid-body. Excretory bladder variable in shape; consists of two extensively diverticulated lateral arms, uniting anteriorly near central eyespot and posteriorly near testicular primordia. Numerous large, lightly staining gland-cells associated with excretory bladder. Tail simple, longer than body, attenuating posteriorly.
Pronocephaloid sp. E-NSI (fig. 1i, j; measurements in table 2).
Taxonomic summary
Intermediate host. Thylacodes sp. Genotype 1 (Vermetidae).
Prevalence. One of 400 (0.25%).
Locality. Off Dunwich, North Stradbroke Island, Queensland, Australia (27°24′S, 153°20′E).
Voucher material. Five slides (QM G238223–238227).
Representative DNA sequences. ITS2, four identical replicates (one submitted to GenBank: MN574295); 28S, two identical replicates (one submitted to GenBank: MN574302).
Description
Redia. Based on ten excised specimens. Body elongate, allantoid, blunted anteriorly, typically containing fewer than five well-developed cercarial bodies in anterior region and fewer than 12 germinal balls in posterior region. Mouth cavity large, at anterior extremity, lined with cilia-like projections. Pharynx ellipsoidal, at base of mouth cavity. Intestine elongate, ellipsoidal, occupying up to one-half of body length. Birth pore distinct, adjacent to pharynx.
Cercaria. Based on ten excised specimens. Trioculate monostome. Body elongate, ellipsoidal. Cephalic collar inconspicuous; lobes extend about one-third of body length. Tegument smooth. Cystogeneous gland-cells sparse, extending throughout body, filled with opaque, granular substance. Dorsal adhesive pockets conspicuous, cup-shaped, each on postero-lateral extremities of body. Eyespots three (two in immature cercariae), in anterior portion of pre-equatorial region of body, closely associated with ganglionic mass, laterally parallel, often irregular and diffused, conical, with proximal portion near dorsal surface, expanding ventrally. Additional pigment scattered in pre-equatorial body region. Oral sucker terminal, infundibuliform. Oesophagus bifurcates near posterior margin of cephalic collar; caeca terminate blindly in posterior third of body. Reproductive primordia distinct. Testicular masses two, well-differentiated, parallel, near posterior extremity. Ovarian complex a conspicuous longitudinal mass of cells, passing from testes to mid-body. Excretory bladder variable in shape; consists of two extensively diverticulated lateral arms, uniting anteriorly near central eyespot and posteriorly near testicular primordia. Tail simple, thick-walled, longer than body, attenuating posteriorly.
Molecular data
28S rDNA sequences were generated for all five morphotypes. BLAST analysis for all five returned closest matches with pronocephaloids. Alignment of the new sequence data with those of related taxa (table 1) yielded 1266 characters for analysis. Sequences representing all six pronocephaloid families formed individual clades, but nodal support was poor for many major nodes (fig. 2). The best-represented family (Notocotylidae) formed a well-supported clade, as did the two species of Opisthotrematidae and the two species of Rhabdiopoeidae. The five cercarial infections are scattered in the phylogeny, none forming a well-supported clade with any other taxon. Species A, B, C and E formed a clade with the sole pronocephalid in the analysis, but this relationship was poorly supported. ITS2 rDNA sequences are provided principally to assist future matching of lifecycle stages. The ten combinations of comparison between the five sequences differed by 64–91 bp.
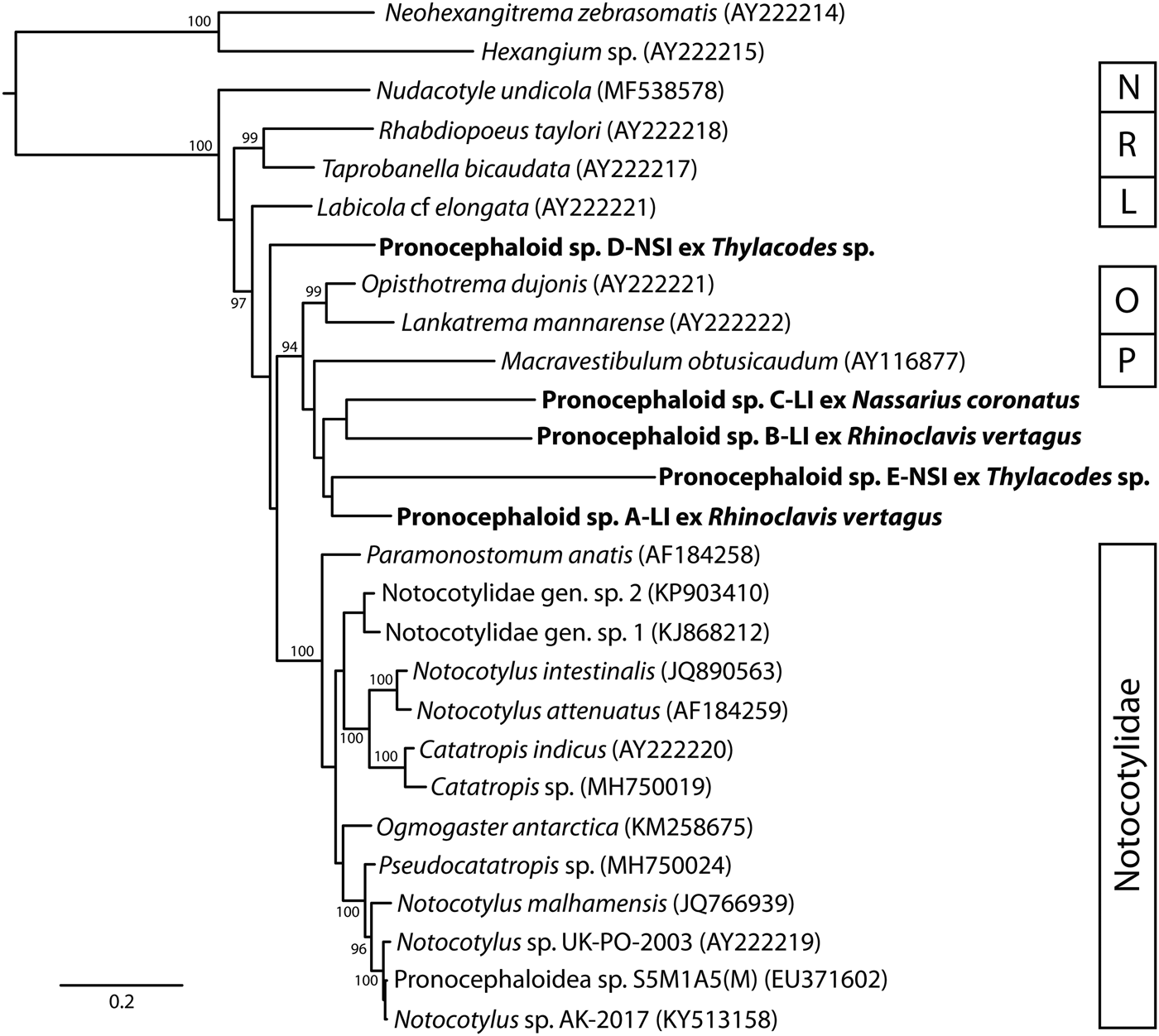
Fig. 2. Phylogenetic position of five pronocephaloid cercariae (in bold) relative to known pronocephaloid taxa based on Bayesian inference analyses of the partial 28S rDNA dataset. Posterior probabilities are shown above the nodes; values <85 are not shown. The scale bar indicates the expected number of substitutions per site. Abbreviations: L, Labicolidae; N, Nudacotylidae; O, Opisthotrematidae; P, Pronocephalidae; R, Rhabdiopoeidae.
Discussion
The combined morphology and molecular phylogenetic analyses of the five species reported here demonstrate that they belong to the superfamily Pronocephaloidea. As currently conceived, the Pronocephaloidea comprises six families: Labicolidae Blair, 1979 has one species parasitic in the dugong; Notocotylidae Lühe, 1909 has approximately 20 species parasitic in marine birds and mammals, and many more transmitted in freshwater habitats; Nudacotylidae Barker, 1916 has two genera with species parasitic in both marine and terrestrial mammals; Opisthotrematidae Poche, 1926 has 11 species in six genera, all parasites of sirenians; Pronocephalidae Looss, 1899 is a large family mainly parasitizing aquatic turtles (marine and fresh water; species of about ten genera in marine turtles), but with a few species in fishes and marine iguanas; Rhabdiopoeidae Poche, 1926 comprises four monotypic genera, all parasites of sirenians. As far as we can determine, life cycles are known only for species of Notocotylidae, Nudacotylidae and Pronocephalidae. There are many life cycles known for Notocotylidae (e.g. Martin, Reference Martin1956; Rohde & Onn, Reference Rohde and Onn1967; Pike, Reference Pike1969; Kanev et al., Reference Kanev, Vassilev, Dimitrov and Radev1994), for one nudacotylid (Ameel, Reference Ameel1944) and a handful for Pronocephalidae (e.g. Hsü, Reference Hsü1937; Hunter, Reference Hunter1967). The cercariae described in these studies are (essentially universally) characterized as monostomes (possessing only a single anterior muscular structure, debatably either a pharynx or an oral sucker), possessing eyespots, a simple tail and, diagnostically, paired dorsal adhesive pockets. These pockets have been reported repeatedly, and under many names, but we consider the designation of ‘dorsal adhesive pockets’ proposed by Krupenko & Gonchar (Reference Krupenko and Gonchar2017) to be appropriate for them. These structures were present in all five of the cercariae reported here.
Although the identity of the five cercariae reported here as pronocephaloids does not appear to be in doubt, it is not clear to which family they belong. Moreton Bay has a rich fauna of shorebirds and passing baleen whales, which may act as hosts to notocotylids, a major population of green turtles (Chelonia mydas), and smaller numbers of some other turtle species known to harbour a rich (though unreported) fauna of pronocephalids, and a major population of dugongs (Dugong dugon), which can be expected to harbour Labicolidae, Opisthotrematidae and Rhabdiopoeidae. Lizard Island has fewer shorebirds, but turtles (especially C. mydas) and dugongs both occur there regularly. Probably the only pronocephaloid family absent from the two collecting sites is the Nudacotylidae, which are known only from North and South America.
Our phylogenetic analysis included representatives of all six pronocephaloid families. Although support for many of the clades is weak, several observations and inferences can be made. The single sequence of a nudacotylid was sister to all other pronocephaloids. The two species of Rhabdiopoeidae form a well-supported clade that is the next most-basal clade in the Pronocephaloidea. The single sequenced labicolid species is the next most-basal taxon and is isolated from all other taxa. The two opisthotrematid species form a moderately supported clade. The multiple notocotylid taxa formed a strongly supported clade to the exclusion of all other taxa including the five cercarial species reported here. There is no strong evidence of a relationship between any of these families and the cercariae reported here. We predict that most, if not all, will prove to be pronocephalids on the basis that the phylogenetic analysis appears least consistent with relationship to Nudacotylidae, Rhabdiopoeidae, Labicolidae or Notocotylidae. The Pronocephalidae is almost certainly the richest pronocephaloid taxon in the areas of study given the abundance of turtles near the collection sites. The pronocephalid fauna of marine turtles in Australia has not yet been characterized, but Santoro et al. (Reference Santoro, Greiner, Morales and Rodriguez-Ortiz2006) reported 15 pronocephalid species in C. mydas from Costa Rica. As such, it is perhaps inherently most likely that most, if not all, the present species will ultimately match with species of that family. We also suspect that this diverse assemblage of species will ultimately prove to be paraphyletic relative to the specialized families parasitic in sirenians. It is striking that the available molecular data for the Pronocephalidae is so poor; only one 28S gene sequence is available – that of Macravestibulum obtusicaudum from a freshwater turtle (Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003).
Cerithiids have been reported as hosts for pronocephaloids twice previously. Cable (Reference Cable1956) reported an unidentified cercaria, which he predicted would prove to be that of a pronocephalid. Martin (Reference Martin1956) reported a cerithiid as the intermediate host of the notocotylid Catatropis johnstoni Martin, Reference Martin1956. For the Nassariidae, there is just one report of a pronocephaloid infection, that of Pleurogonius malacaclemys Hunter, 1961 by Hunter (Reference Hunter1967). Intriguingly, this species infects the semi-marine terrapin M. terrapin, rather than one of the seven fully marine turtle species.
The Vermetidae (order Littorinimorpha: Vermetoidea) is a new host family and superfamily for the Pronocephaloidea. However, other families of the order Littorinimorpha (Bithyniidae, Hydrobiidae, Littorinidae, Pomatiopsidae and Tateidae) have been reported extensively as hosts for notocotylids (e.g. Stunkard, Reference Stunkard1960; Dollfus, Reference Dollfus1966; Rohde & Onn, Reference Rohde and Onn1967; Graefe, Reference Graefe1968; Smith & Hickman, Reference Smith and Hickman1983). Vermetids have the form of tube-worms, forming asymmetrical calcareous tubes encrusting on rocks or among other substrates. They may occur in large numbers but are easily overlooked as part of the gastropod community because their morphology is so unusual. Indeed, several species were initially described as polychaetes given the close resemblance of their tubes to those of Serpulidae. Vermetids have been reported as hosts to several other lineages of trematodes – an echinostomatid, a hemiurid, a mesometrid, a microphallid and a possible opecoelid (Prévôt, Reference Prévôt1969, Reference Prévôt1971a, Reference Prévôtb; Jousson et al., Reference Jousson, Bartoli and Pawlowski1999). Most recently, and of special relevance to the present findings, Cribb et al. (Reference Cribb, Crespo-Picazo, Cutmore, Stacy, Chapman and García-Párraga2017) reported an infection of a turtle-infecting spirorchiid from a vermetid in Spain. Indeed, the genesis of the current study of Moreton Bay vermetids was to seek further infections of spirorchiids, which are known to be abundant in marine turtles there (Chapman et al., Reference Chapman, Cribb, Blair, Traub, Kyaw-Tanner, Flint and Mills2015, Reference Chapman, Owen, Flint, Magalhaes, Traub, Cribb, Kyaw-Tanner and Mills2017). If one or both of the present species from vermetids should prove to be a pronocephalid, it will be intriguing that species of two distantly related trematode families that infect turtles should both infect vermetid gastropods. Whether such a shared host group might be coincidental or related to an aspect of transmission ecology remains to be explored. Cribb et al. (Reference Cribb, Crespo-Picazo, Cutmore, Stacy, Chapman and García-Párraga2017) discussed the possibility that vermetids might be carried on the carapaces of turtles, creating a situation where the definitive host carries the intermediate hosts of their parasites with them. We think that vermetids are worthy of further study for parasites, especially in areas where turtles are abundant.
Acknowledgement
We thank Drs John Healy and Timothy Rawlings for their advice on the collection and identification of vermetids.
Financial support
This work was generously supported by a research grant from the Sea World Research and Rescue Foundation Inc.
Conflicts of interest
None.
Ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.








