To the Editor—In the 3SITES trial,Reference Parienti, Mongardon and Mégarbane 1 the incidences of central venous catheter (CVC)-related bloodstream infections (CRBSIs) after 1:1:1 randomized catheterization of the internal jugular, subclavian, or femoral vein, respectively, were compared among adult intensive care unit (ICU) patients. The low total CRBSI incidence in this trial was stated to be consistent with data from other ICUs. 2 Unfortunately, however, the total CRBSI incidence was not clearly indicated in the 3SITES trial. Using the data provided,Reference Parienti, Mongardon and Mégarbane 1 we calculated a CRBSI rate of 1.0% (26 per 2,532 CVCs); for all 3 CVC insertion sites, the mean CRBSI incidence was 1.7 per 1,000 CVC days.
We believe that the reported incidence was rather low and was biased by the diagnostic criteria used.Reference Schalk, Hanus, Färber, Fischer and Heidel 3 For CRBSI diagnosis in the 3SITES trial, only CVC-tip colonization combined with detection of the same pathogen in peripheral blood was taken into account. Differential time to positivity of central and peripheral blood cultures was not used in the 3SITES trial.Reference Parienti, Mongardon and Mégarbane 1 However, this is a well-established parameter for CRBSI diagnosisReference Mermel, Allon and Bouza 4 in addition to the aforementioned criterion; it is also a criterion of definite CRBSI.Reference Schalk, Hanus, Färber, Fischer and Heidel 3 , Reference Hentrich, Schalk and Schmidt-Hieber 5 Furthermore, the cutoff of colony-forming units (CFU) for CVC-tip colonization was 1,000 in quantitative culture,Reference Parienti, Mongardon and Mégarbane 1 which is higher than is usually recommended.Reference Mermel, Allon and Bouza 4 , Reference Hentrich, Schalk and Schmidt-Hieber 5 Instead of quantitative cultures, semiquantitative cultures with a CFU cutoff of 15 should be used for diagnosis of CVC-tip colonization.Reference Mermel, Allon and Bouza 4 However, a CFU cutoff of 15 in semiquantitative culture is the recommended equivalent of a cutoff of 100 in quantitative culture.Reference Mermel, Allon and Bouza 4 , Reference Hentrich, Schalk and Schmidt-Hieber 5 In addition, the median duration of CVC catheterization in the 3SITES trial was only 5 days;Reference Parienti, Mongardon and Mégarbane 1 however, CRBSIs are known to be associated with the length of CVC use in situ.Reference Schalk, Hanus, Färber, Fischer and Heidel 3 , Reference Pepin, Thom and Sorkin 6
Due to the diagnostic criteria used and the low number of CVC catheterization days, we believe the CRBSI rate and incidence, respectively, in the 3SITES trial were underestimated and do not represent a real-life setting. Therefore, we compared CRBSI data used in the 3SITE trial with CRBSI data from our SECRECY registry. SECRECY (German Clinical Trial Register No. DRKS00006551) is a real-life registry of CRBSIs in patients with hematological and oncological malignancies;Reference Schalk, Hanus, Färber, Fischer and Heidel 3 , Reference Schalk, Färber, Fischer and Heidel 7 this is a high-risk population for CRBSI, comparable to the ICU patients in the 3SITES trial.
Using SECRECY data up to January 25, 2016, we report on 447 CVCs (internal jugular vein, 419 CVCs [93.7%]), which accounts for a total of 6,700 CVC days. A comparison of diagnostic criteria and characteristics of CRBSI in the 3SITES trial vs the SECRECY registry is provided in Table 1. In this real-life setting, CVCs were in situ longer and the CRBSI rate and incidence were higher than in the 3SITES trial. In the SECRECY registry, median CRBSI onset occurred on day 15.5 after CVC insertion, which is much longer than the median time the CVCs were used in situ in the 3SITES trial (ie, 5 days). Thus, the points discussed here may explain the lower CRBSI incidence in the 3SITES trial, and they should be considered when interpreting data on CRBSI in clinical trials.
TABLE 1 Comparison of Diagnostic Criteria and Characteristics of CRBSI
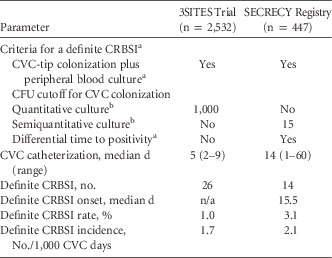
NOTE. CRBSI, central venous catheter-related bloodstream infection; CVC, central venous catheter; CFU, colony-forming units; n/a, not available.
a See Mermel et alReference Mermel, Allon and Bouza 4 and Hentrich et al.Reference Hentrich, Schalk and Schmidt-Hieber 5
b A CFU cutoff of 15 in semiquantitative culture is the recommended equivalent of a cutoff of 100 in quantitative culture (see Mermel et alReference Mermel, Allon and Bouza 4 and Hentrich et alReference Hentrich, Schalk and Schmidt-Hieber 5 ).
ACKNOWLEDGMENT
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: Both authors report no conflicts of interest relevant to this article.





