Introduction
The Balleny Islands sit on the edge of the Antarctic Circle in the northern Ross Sea, ~250 km north of the coast of northern Victoria Land (Fig. 1). Along with Scott Island, the Balleny Islands are the only oceanic islands and the most northerly land in the Ross Sea region. There are three main islands in the Balleny group (Sturge, Buckle and Young) and three smaller islands (Row, Borradaile and Sabrina), as well as numerous offshore rock stacks. These volcanic islands are orientated in a north-west to south-east direction and span over a degree of latitude (66°15′S–67°35′S), several degrees of longitude (162°30′E–165°00′E) and ~190 km (Bradford-Grieve & Fenwick Reference Bradford-Grieve and Fenwick2002). The Balleny Islands have never been inhabited and are ice bound throughout the winter. The sea between the Balleny Islands and mainland Antarctica is frequently impenetrable due to heavy ice, and, although the area generally becomes ice free from early to mid-January until April, pack ice may survive through a significant part of the summer (Bradford-Grieve & Fenwick Reference Bradford-Grieve and Fenwick2002).

Fig. 1. a. The Balleny Islands. b. A map of the Ross Sea (bottom left) showing the location of collections from Scott Island, and with detailed insets of collection sites at the Balleny Islands (Inset 1), the Cape Adare and Cape Hallett area (Inset 2) and the inner Ross Sea (Inset 3).
Early accounts documenting macroalgal collections from the Ross Sea and East Antarctica include the work of Barton (Reference Barton and Lankaster1902), Foslie (Reference Foslie1905, Reference Foslie and Nordenskjöld1907), Gepp & Gepp (Reference Gepp and Gepp1905a, Reference Gepp and Gepp1905b, Reference Gepp and Gepp1907, Reference Gepp and Gepp1917), Lucas (Reference Lucas1919), Levring (Reference Levring1945) and Skottsberg (Reference Skottsberg1953). Macroalgal collections from the western Ross Sea were made by J.S. Zaneveld in 1963–1964, 1964–1965 and 1967, including sampling undertaken on the combined New Zealand-United States Expedition to the Ross Sea, Balleny Islands and Macquarie Ridge from the United States Coast Guard icebreaker Glacier (January–March 1965). Data from these collections were subsequently published in a series of reports (Neushul Reference Neushul1965, Zaneveld Reference Zaneveld1966a, Reference Zaneveld1966c, Reference Zaneveld1968, Zaneveld & Sanford Reference Zaneveld and Sanford1980, Wagner & Zaneveld Reference Wagner and Zaneveld1988). The track of the Glacier voyage was provided in Zaneveld & Sanford (Reference Zaneveld and Sanford1980) and the specimens are housed in the Rijksherbarium, Leiden, The Netherlands.
Over the past ~30 years, a range of ecological studies on macroalgae have been undertaken in the Ross Sea (e.g. Miller & Pearse Reference Miller and Pearse1991, Schwarz et al. Reference Schwarz, Hawes, Andrew, Norkko, Cummings and Thrush2003, Reference Schwarz, Hawes, Andrew, Mercer, Cummings and Thrush2005, Norkko et al. Reference Norkko, Thrush, Cummings, Funnell, Schwarz, Andrew and Hawes2004, Reference Norkko, Thrush, Cummings, Gibbs, Andrew, Norkko and Schwarz2007), as well as research on the benthic algal flora in Terra Nova Bay (Cormaci et al. Reference Cormaci, Furnari and Scammacca1992, Reference Cormaci, Furnari, Scammacca, Alongi and Catra1998, 2000). Wiencke & Clayton (Reference Wiencke and Clayton2002) summarized knowledge of Antarctic seaweeds reporting 116 species from the continent, with only 30 of these recorded from the Ross Sea, and specific reference to only 2 species (Chaetomorpha mawsonii, Georgiella confluens) from the Balleny Islands. Cormaci et al. (Reference Cormaci, Furnari, Scammacca, Faranda, Guglielmo and Ianora2000) collated records of macroalgae from the Ross Sea, reporting 37 taxa, many of which have limited distributions or are rarely found. Cormaci et al. (Reference Cormaci, Furnari, Scammacca, Faranda, Guglielmo and Ianora2000) observed ‘a much poorer flora (only 5–10%) is found in latitudes which are higher than 70° S (such as in Terra Nova Bay, Ross Sea) to the minimum of seven taxa recorded by Miller & Pearse (Reference Miller and Pearse1991) from McMurdo Sound’. With 17 taxa recorded from Terra Nova Bay, Cormaci et al. (Reference Cormaci, Furnari, Scammacca, Faranda, Guglielmo and Ianora2000) concluded that this ‘can be considered the richest area in species both in this area and in the stations located to the North’.
More recently, Oliveira et al. (Reference Oliveira, Pellizzari, Medeiros, Yokoya, Gómez and Huovinen2020) compiled records of macroalgae from Antarctica, South Shetland Islands, King George Island and Adelaide Island with 151 species in total (85 Rhodophyta, 34 Ochrophyta and 32 Chlorophyta), incorporating the results of a number of other reports (including Mystikou et al. Reference Mystikou, Peters, Asensi, Fletcher, Brickle and Van West2014, Wiencke et al. Reference Wiencke, Amsler, Clayton, de Broyer, Koubbi, Griffiths, Raymond, Udekem d'Acoz and Van de Putte2014, Pellizzari et al. Reference Pellizzari, Silva, Silva, Medeiros, Oliveira and Yokoya2017). Additional new records of green and red algae have been reported by Dubrasquet et al. (Reference Dubrasquet, Reyes, Sanchez, Valdivia and Guillemin2018, Reference Dubrasquet, Garrido, Bruning, Reyes and Guillemin2021) from the South Shetland Islands and the Antarctic Peninsula. Thus, only ~25–30% of the total Antarctic flora has been reported from East Antarctica.
There are many unresolved taxonomic questions relating to the species recorded from the Antarctic region. Many records are based on drift or dredged specimens, a number of species are known from few collections and names of morphologically similar taxa from outside the region have been applied to Antarctic specimens. Caution therefore is required when comparing lists of taxa recorded from the region. In this account, we describe the macroalgal diversity from the Balleny Islands, Scott Island and the Ross Sea, updating records reported in Nelson et al. (Reference Nelson, Cummings, Halliday, Marriott, D'Archino and Neill2010, Reference Nelson, Cummings, D'Archino, Halliday, Marriott and Neill2017), and compare the diversity of macroalgal and benthic communities and habitats with reports from other parts of the region.
Materials and methods
This study is based primarily on collections lodged in the Herbarium of the Museum of New Zealand Te Papa Tongarewa (WELT; Thiers Reference Thiers2021). Figure 1 shows the localities that specimens were collected from and Table S1 provides full details of collection sites, as well as collection methods and depths, along with reference numbers and GenBank numbers. Samples were collected both opportunistically and as part of targeted research at the Balleny Islands and in the north-western Ross Sea between 2001 and 2006 during expeditions by RV Tangaroa, RV Italica and yacht Tiama. Additional opportunistic collections have been made available to us from the Ross Sea, including material from Franklin Island (76°04′48.0″S, 168°19′12.0″E), Cape Evans (77°38′05.7″S, 166°24′50.6″E) and the McMurdo jetty (77°50′51.5″S, 166°39′27.7″E). A number of the samples examined in this study came from depths well below the euphotic zone and using equipment that was targeting the collection of other organisms.
Macroalgal samples were either pressed in the field or preserved in either 5–10% formalin/seawater or 90% ethanol or were frozen. Some specimens were subsampled for subsequent molecular sequencing, with small portions (~1 × 2 cm) of tissue removed and placed in silica gel. Liquid-preserved specimens were rinsed, examined for epiphytes and then pressed on herbarium paper. Specimens that had been frozen were highly problematic, and on thawing many of the samples disintegrated. Fragmentation was lessened by immersing samples in trays of 70% alcohol as they thawed, followed by rapid preparation of herbarium specimens.
Specimens were examined microscopically by either hand sectioning with a razor blade or by preparing whole mounts. Some slides were stained for several minutes in an acidified aniline blue solution (one part 1% aniline blue solution: nine parts 7% acetic acid solution) and permanently mounted in a 50% aqueous Karo mixture (with phenol crystals added to prevent microbial growth). Permanent slides were examined by light microscopy using a Zeiss AxioCam HRc camera with accompanying AxioVision software mounted on a Zeiss Axiovert microscope.
In addition to the field collections, we examined underwater images taken in 2001 and 2006 from Tangaroa and images taken in 2006 from Tiama to compare with dredge and scuba records.
Molecular sequencing data
Samples of algae were sequenced during this study, including the red algae initially identified as belonging to the genera Ballia, Gainia, Iridaea, Palmaria, Phyllophora and Phycodrys (reported in Lin & Nelson Reference Lin and Nelson2010), four samples of Hapalidiaceae and a sample of the green algal genus Prasiola (reported in Heesch et al. Reference Heesch, Sutherland and Nelson2012). DNA was extracted following a modified cetyl trimethylammonium bromide (CTAB) protocol (Zuccarello & Lokhorst Reference Zuccarello and Lokhorst2005) or, for coralline algae, using the Qiagen DNeasy Blood and Tissue DNA Extraction Kit (Qiagen GmbH, Hilden, Germany), using a modified version of the protocol described by Broom et al. (Reference Broom, Hart, Farr, Nelson, Neill, Harvey and Woelkerling2008). Amplifications were performed in 25 μl polymerase chain reactions (PCRs) containing 1 μl of 1:50 diluted DNA extract, 5 μl of 5 × reaction buffer, 1.5 mmol MgCl2, 10 nmol of each deoxynucleotide triphosphate, 25 pmol of each primer and 0.1 U Kapa 2 G Robust DNA polymerase (Custom Science, Auckland, New Zealand). The plastid-encoded large subunit of the ribulose-1,5-bisphosphate carboxylase/oxygenase (rbcL) gene was amplified from the six red algal genera named above in two parts, using a variety of primer combinations: F145–R898 and F762–R1442 (Kim et al. Reference Kim, Kim and Nelson2010) or F57, F492, F753 (Freshwater & Rueness Reference Freshwater and Rueness1994) or F7 (Gavio & Fredericq Reference Gavio and Fredericq2002) combined with the reverse primers R753 or RrbcS (Freshwater & Rueness Reference Freshwater and Rueness1994), annealing at 45°C. The photosystem II thylakoid membrane protein D1 (psbA) gene was amplified from the Hapalidiaceae specimens and from one specimen initially identified as a Phyllophoraceae sp. specimen using primers psbAF1 paired with psbAR1 (Yoon et al. Reference Yoon, Hackett and Bhattacharya2002). Amplifications were performed in MJ Research PTC-200 (GMI, Ramsey, MN, USA), using an initial denaturation step (95°C, 3 min), followed by a 35 cycles of denaturation (95°C, 15 s), annealing (44–50°C, 20 s) and extension (72°C, 1 min), with a final extension of 2 min at 72°C. The PCR products were visualized on 1% agarose gels, purified by exonuclease I/alkaline phosphatase digestion using standard methods and commercially sequenced in both directions (Macrogen, Inc., Seoul, Korea). Sequences were assembled and aligned using Geneious Prime 2019.0.4 (https://www.geneious.com). Sequences obtained were compared with accessions in GenBank of the National Center for Biotechnology Information (NCBI) using BLAST searches (https://blast.ncbi.nlm.nih.gov/Blast.cgi; Altschul et al. Reference Altschul, Gish, Miller, Myers and Lipman1990), and they also were compared with unpublished sequences from current research projects.
Alignments were produced using newly generated data, previous data (Nelson et al. Reference Nelson, Cummings, Halliday, Marriott, D'Archino and Neill2010) and available sequences from GenBank. The rbcL and psbA datasets were aligned using MAFFT in Geneious. Maximum-likelihood analyses were implemented using IQ-TREE (Trifinopoulos et al. Reference Trifinopoulos, Nguyen, von Haesele and Minh2016). IQ-TREE was used to select the molecular evolution models (ModelFinder; Kalyaanamoorthy et al. Reference Kalyaanamoorthy, Minh, Wong, von Haesler and Jermiin2017) under the Bayesian information criterion. Genes were partitioned by codon as appropriate. The datasets were subjected to nonparametric bootstrap analysis (500 replicates; Felsenstein Reference Felsenstein1985) in IQ-TREE. Trees were annotated in FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/) and Inkscape 1.1 (http://inkscape.org).
Results
Field observations
Underwater photography at the Balleny Islands revealed macroalgae occupying a range of habitats, with dense stands of Himantothallus grandifolius and Desmarestia menziesii forming forests in the nearshore. Macroalgae were not restricted to hard substrate and shallow water (<30 m). There were also macroalgae growing on cobbles and on small patches of harder substrate amongst soft sediment environments at depths of up to ~100+ metres.
The red algae Palmaria decipiens and Ballia sp. were the most commonly seen species on underwater video from sites at Borradaile Island, while the green algae Monostroma hariotii and Lambia antarctica were also identified along video transects. Encrusting non-geniculate coralline algae were noted on what appeared to be stable cobble beds. The large brown algae H. grandifolius and D. menziesii, the red alga Georgiella confluens and bladed red algae (potentially Notophycus fimbriatus, Halymeniaceae and Iridaea sp.) were also noted.
In the Ross Sea, one dive at Cape Hallett (72°18′37.4″S, 170°15′22.1″E) found large stands of the kelp H. grandifolius (Cummings et al. Reference Cummings, Thrush, Schwarz, Funnell and Budd2006). To date, this appears to be the southern limit of its distribution (Zaneveld Reference Zaneveld, Young and McLachlan1966b, Wiencke & Clayton Reference Wiencke and Clayton2002). At the shallow-water Terra Nova Bay locations (74°48′00.0″S, 164°38′56.4″E), two foliose algal species (Phyllophora antarctica, Iridaea sp.) and non-geniculate corallines were present, as well as an encrusting red alga Gainia mollis (Cummings et al. Reference Cummings, Thrush, Marriott, Funnell, Norkko and Budd2008). Drift Desmarestia thalli were collected from Franklin Island (76°04′48.0″S, 168°19′12.0″E), as well as Iridaea sp. and Plocamium sp. Only red algae were observed at Cape Evans (77°38′21.5″S, 166°24′11.9″E) and McMurdo (77°50′51.5″S, 166°39′27.7″E) (Phyllophora, Iridaea sp. and Tethysphytum antarcticum).
Species list
We report 30 taxa, 27 recorded from the Balleny Islands and 18 taxa recorded from the western Ross Sea. Fifteen of the western Ross Sea taxa are also recorded from the Balleny Islands, with three taxa recorded solely from the Ross Sea.
CHLOROPHYTA
ULVOPHYCEAE
BRYOPSIDALES
Bryopsidaceae
Lambia antarctica (Skottsb.) Delépine
Type locality: Melchior Islands.
Habitat: Recorded from subtidal zone to 50 m depth; at the Balleny Islands collected at 12–15 m depth.
Distribution: East Antarctica: Balleny Islands, Wilkes Land, Mac.Robertson Land.
West Antarctica: South Orkney Islands, Antarctic Peninsula, South Shetland Islands.
Representative specimen: Balleny: WELT A023615 - Scott Cove, Buckle Island, Shears, 18.ii.2006.
General comments: Although Wiencke et al. (Reference Wiencke, Amsler, Clayton, de Broyer, Koubbi, Griffiths, Raymond, Udekem d'Acoz and Van de Putte2014) reported this species to be restricted to West Antarctica, it has also been reported from Wilkes Land and Mac.Robertson Land (Guiry & Guiry Reference Guiry and Guiry2021).
ULOTRICHALES
Monostromataceae
Monostroma hariotii Gain
Type locality: Deception Island.
Habitat: Epiphytic or epilithic annual, growing sub-tidally to ~20 m; dredge records from 26 to 30 m and from 120 m.
Distribution: Sub-Antarctic Islands: Macquarie Island, Falkland Islands, Kerguelen Islands.
East Antarctica: Ross Sea, Balleny Islands, Adélie Coast, Wilkes Land.
West Antarctica: South Orkney Islands, Antarctic Peninsula, South Shetland Islands.
Representative specimens: Balleny: WELT A023660 - Borradaile Island Landing, Shears, 15.ii.2006. Ross Sea: WELT A026028, A026030 - Possession Island, RV Tangaroa, 11.ii.2001.
TREBOUXIOPHYCEAE
PRASIOLALES
Prasiolaceae
Prasiola crispa (Lightf.) Kütz. (Fig. 2a,b)

Fig. 2. a,b. Prasiola crispa in the penguin rookery at Sabrina Island, just south of Buckle Island.
Type locality: Scotland.
Habitat: Found near guano deposits/seabird colonies; at the Balleny Islands found on cliffs 100+ m above shore in a penguin nesting area.
Distribution: Widespread in colder regions of the Northern and Southern hemispheres.
East Antarctica: Ross Sea, Balleny Islands, Adélie Coast, Wilkes Land, Ingrid-Christensen Coast.
West Antarctica: Antarctic Peninsula, South Shetland Islands.
Representative specimens: Balleny: WELT A027861A-C - Cape McNab, Buckle Island, Smith & Shears, 18.ii.2006.
General comments: Sequence data place the sample from the Balleny Islands in a clade with samples from Cork, Eire and British Columbia, Canada (Heesch et al. Reference Heesch, Sutherland and Nelson2012).
OCHROPHYTA
CHRYSOMERIDOPHYCEAE
CHRYSOMERIDALES
Chrysomeridiaceae
Antarctosaccion applanatum (Gain) Delépine
Type locality: Deception Island.
Habitat: Epiphytic on Plocamium sp. growing at 15–20 m.
Distribution: East Antarctica: Balleny Islands.
West Antarctica: Antarctic Peninsula, South Shetland Islands.
Representative specimens: Balleny: WELT A023612 - Cape Smythe, Sturge Island, Shears, 19.ii.2006.
PHAEOPHYCEAE
ECTOCARPALES
Acinetosporaceae
Geminocarpus geminatus (Hook.f. & Harv.) Skottsb.
Type locality: San Martin's Cove, Cape Horn.
Habitat: Epiphytic, epizoic or epilithic; at the Balleny Islands found as an epiphyte on Georgiella confluens at 15–20 m depth, in the Ross Sea dredged at 24–38 m depth.
Distribution: Recorded from throughout the sub-Antarctic islands, New Zealand and Chile.
East Antarctica: Ross Sea, Balleny Islands.
West Antarctica: South Georgia, Antarctic Peninsula, South Shetland Islands.
Representative specimens: Balleny: WELT A023614 - Cape Smythe, Sturge Island, Shears, 19.ii.2006. Ross Sea: WELT A026035 - Possession Island, RV Tangaroa, ii.2001.
General Comments: This name has been applied to collections from a wide geographical region. There may be greater diversity within this taxon than is currently recognized. Ricker (Reference Ricker1987) discussed differences between sub-Antarctic and Antarctic collections.
Chordariaceae
Elachista antarctica Skottsb.
Type locality: Melchior Island, Antarctic Peninsula, on Leptosarca simplex, coll. I.M. Lamb, 3.ii.1945 (Ricker Reference Ricker1987).
Habitat: Apparently obligately epiphytic on Palmaria decipiens; at the Balleny Islands found growing subtidally at 14 m depth, at Possession Island growing on Palmaria dredged from 26–30 m.
Distribution: Macquarie Island.
East Antarctica: Balleny Islands.
West Antarctica: Antarctic Peninsula, South Shetland Islands.
Representative specimens: Balleny: WELT A023577 - Borradaile Island, Shears, 15.ii.2006. Ross Sea: WELT A026035 - Possession Island, RV Tangaroa, ii.2001.
DESMARESTIALES
Desmarestiaceae
Desmarestia menziesii J.Agardh (Fig. 3a)
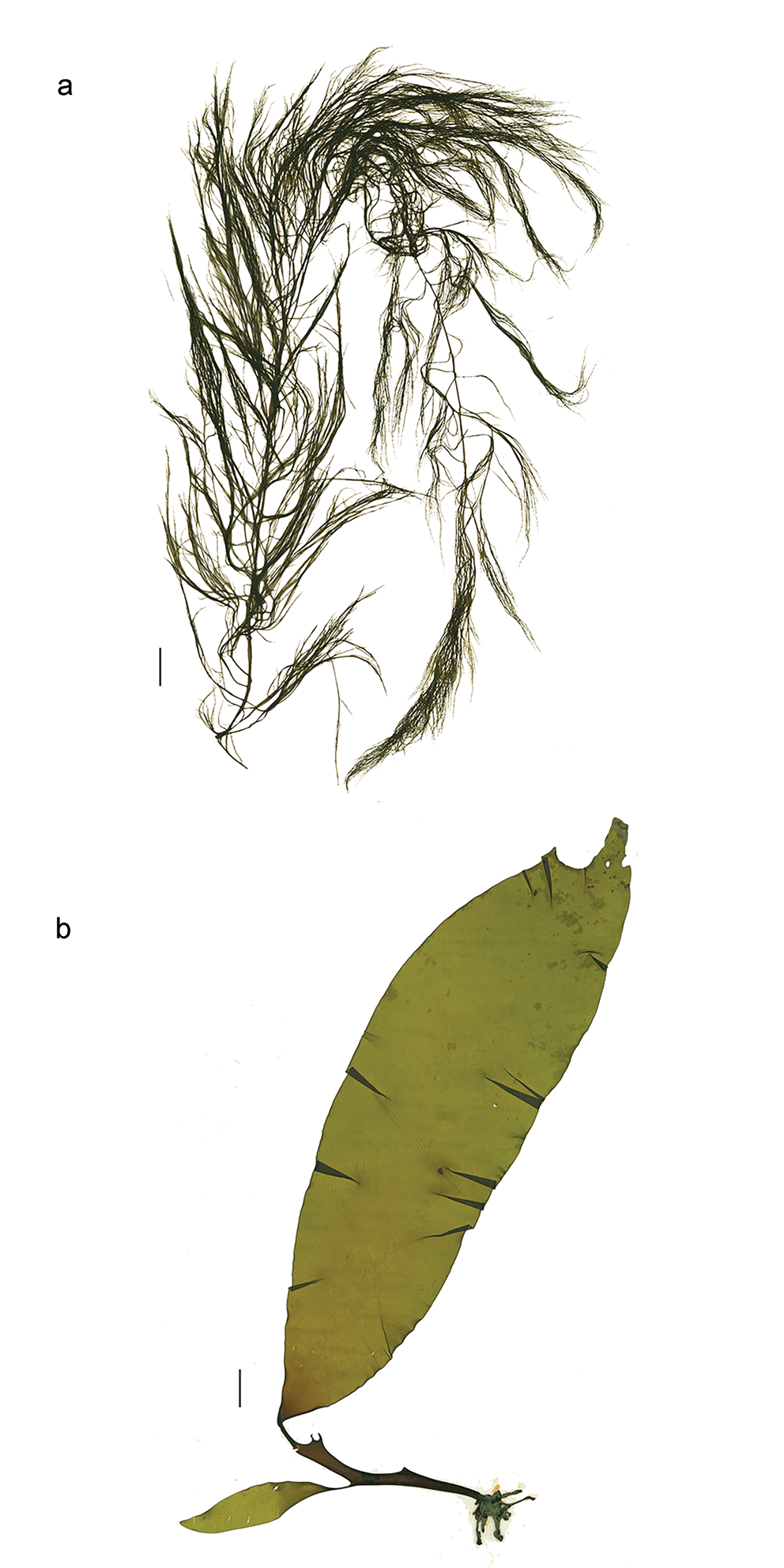
Fig. 3. a. Desmarestia menziesii, herbarium specimen WELT A023600 from Borradaile Landing. b. Himantothallus grandifolius, herbarium juvenile specimen WELT A023601 from Borradaile Landing. All scale bars = 2 cm.
Type locality: Ad oras novae Shetlandiae (Menzies).
Habitat: Reported to grow epilithically to 35 m; strongly shade and cold adapted; Balleny Island scuba collections from 10–15 m depth; Balleny Island and Ross Sea dredge samples from 25–1570 m depth.
Distribution: Endemic and widespread in Antarctica from 76°S in the Ross Sea to South Georgia.
East Antarctica: Ross Sea, Balleny Islands, Wilkes Land.
West Antarctica: South Georgia, Antarctic Peninsula, South Shetland Islands, Anvers Island.
Representative specimens: Balleny: WELT A023594 - Borradaile Island, Shears, 22.ii.2006. WELT A025994 - Sturge Island, RV Tangaroa, ii.2001. WELT A025987, A025995-A025997 - Young Island, RV Tangaroa, ii.2001. Ross Sea: WELT A034422, Wilcox, 13.ii.2012; WELT A026024-A026027, A026032-A026033 - Possession Island, RV Tangaroa, ii.2001.
General comments: Although several species of Desmarestia have been recorded from the Ross Sea (Cormaci et al. Reference Cormaci, Furnari, Scammacca, Faranda, Guglielmo and Ianora2000, Wiencke & Clayton Reference Wiencke and Clayton2002), we have seen only one species amongst the collections. We consider that it is unlikely Desmarestia confervoides (type locality: Concepción Chile) is present in the Ross Sea.
Himantothallus grandifolius (A.Gepp & E.S.Gepp) Zinova (Fig. 3b)
Type locality: Scotia Bay, South Orkney Islands, April 1904.
Habitat: A canopy-forming species occurring in dense subtidal stands, this species is considered to be strongly shade and cold adapted. It has been observed to grow at depths of 7–70 m on vertical rocks and boulders in conditions of moderate to low turbulence and also on less stable substrata such as pebbles and gravel (Wiencke & Clayton Reference Wiencke and Clayton2002); scuba collections from the Balleny Islands were made at 20 m depth; dredge collections from the Ross Sea and Balleny Islands are from 18 to 1500 m depth.
Distribution: Widespread in East and West Antarctica.
East Antarctica: Ross Sea (Victoria Land), Balleny Islands, Wilkes Land.
West Antarctica: South Georgia, Antarctic Peninsula, South Shetland Islands, Trinity Island, Anvers Island.
Representative specimens: Balleny: WELT A025999A/B - Young Island, RV Tangaroa, ii.2001. Ross Sea: WELT A026031A/B, A026039-A026041 - Possession Island, RV Tangaroa, ii.2001.
RHODOPHYTA
FLORIDEOPHYCEAE
CORALLINOPHYCIDAE
Non-geniculate coralline algae
Representative specimens: Balleny: ASJ433 - west of Sturge Island, RV Tangaroa, 4.iii.2004. Ross Sea: WELT A028363 - Cape Evans, Marriott & Mercer, 12.xi.2002. WELT A028361 - Dunlop Island, Marriott, Norkko & Mercer, 25.x.2002. WELT A028364 - Spike Cape, Marriott, 30.x.2002.
General comments: The identity of a number of non-geniculate coralline samples remains uncertain and further work is required. Recent research has established that molecular sequence data are required to confirm the identity of most non-geniculate coralline algae (Twist et al. Reference Twist, Cornwall, McCoy, Gabrielson, Martone and Nelson2020).
HAPALIDIALES
Hapalidiaceae
Tethysphytum antarcticum K.Sciuto, E.Moschin & I.Moro
Type locality: Tethys Bay (Ross Sea, Antarctica; 74°41S, 164°07E).
Habitat: Subtidal, epilithic - on cobbles and rock surfaces.
Distribution: East Antarctica: Ross Sea, Balleny Islands.
Representative specimens: Balleny: WELT A023680 - Borradaile Island, Shears, 15.ii.2006 (GQ323776). Ross Sea: WELT A034416-18 - Cape Evans., Anderson, 8.xi.2004.
General comments: Sequences from three samples from Cape Evans (77°38′21.5″S, 166°24′11.9″E) were identical to sequences LR812099 and LR861817 from Tethys Bay, Ross Sea (Sciuto et al. Reference Sciuto, Moschin, Alongi, Cecchetto, Schiaparelli and Caragnano2021), extending the known distribution of this species 3° further south (Fig. S1). The psbA sequence (GQ323776) of a sample from the Balleny Island (previously identified as belonging to the ‘Mesophyllum/Synarthrophyton’ complex) had a 5 bp difference from the Ross Sea sequences.
NEMALIOPHYCIDAE
BALLIALES
Balliaceae
Ballia sp. previously reported as Ballia callitricha (C.Agardh) Kütz.
Habitat: On rock, cobbles and epiphytically.
Distribution: East Antarctica: Balleny Islands, Ross Sea.
Representative specimens: Balleny: WELT A023663 - Borradaile Island Landing, Shears, 15.ii.2006 (GenBank GQ323777). Ross Sea: WELT A026037 - Possession Island, RV Tangaroa, ii.2001.
General comments: Ballia callitricha (C.Agardh) Kütz. (type locality: Falkland Islands; holotype: Gaudichaud; Herb. Agardh, LD 19357) is reported to occur throughout cold temperate regions of the Southern Hemisphere in the Pacific (Australia, New Zealand, Chile) as well as the Atlantic (South Africa, Falkland Islands), both East and West Antarctica and from a number of sub-Antarctic islands. In New Zealand it is reported from the Three Kings Islands through to the sub-Antarctic, including the Chatham Islands (Adams Reference Adams1994). The results of sequencing Ballia sp. (GC323777) suggest that the use of species names and concepts in this genus needs to be more closely examined. The psbA sequence of a Balleny Island sample that was identified morphologically and anatomically as B. callitricha is only 91% homologous with a B. callitricha isolate (DQ168014) from Wellington (North Island, New Zealand; Fig. S2).
PALMARIALES
Palmariaceae
Palmaria decipiens (Reinsch) R.W.Ricker
Type locality: South Georgia, lectotype: M 67.83/66 (Ricker Reference Ricker1987).
Habitat: Usually epilithic although sometimes epiphytic; lower eulittoral to upper sublittoral to 30 m; scuba samples from the Balleny Islands and Ross Sea were collected at 1 m (intertidal) to 20 m depth, and dredge samples were collected from 63 to 1570 m.
Distribution: Common throughout Antarctica and sub-Antarctic islands; southernmost location is 77°S in Ross Sea.
Sub-Antarctic Islands: Heard Island, Macquarie Island, Kerguelen, Auckland Islands, Campbell Island, Antipodes Islands.
East Antarctica: Ross Sea, Balleny Islands, Wilkes Land.
West Antarctica: South Georgia, South Orkney Islands, Antarctic Peninsula, South Shetland Islands, Deception Island, Graham Land.
Representative specimens: Balleny: WELT A023642 - Borradaile Island Landing, Shears, 15.ii.2006. Ross Sea: WELT AA026005-A026007 - Possession Island, RV Tangaroa, 20.ii.2001. WELT A026036, A026029 - Possession Island, RV Tangaroa, 20.ii.2001.
General comments: We consider that the genus Palmaria in the Antarctic region requires taxonomic investigation, as the sequences from the Balleny Islands and East and West Antarctica form a clade that is distinct from that of Palmaria palmata, the type of the genus (Fig. S3). Guillemin et al. (Reference Guillemin, Dubrasquet, Reyes and Valero2018) found very low genetic diversity in samples from the Antarctic Peninsula, reflecting the impacts of historical perturbations on macroalgae in the region.
RHODYMENIOPHYCIDAE
CERAMIALES
Callithamniaceae
Callithamnion sp.
Habitat: Dredged from 90 to 95 m depth.
Representative specimens: Ross Sea: WELT A026001 - North Possession Island, RV Tangaroa, ii.2001.
General comments: Known from a single collection of three small, incomplete, sterile specimens (finely filamentous, 2.5–3.0 cm in height; rhizoids descending on lower main axis and alternate branching throughout).
Ceramiaceae
Pterothamnion sp.
Habitat: Epiphytic.
Representative specimens: Balleny: WELT A023674 - Young Island, RV Tangaroa, 11.iii.2006.
General comments: Known from a single collection dredged from 65 to 83 m depth. Moe & Silva (Reference Moe and Silva1980) reported on Pterothamnion antarcticum from West Antarctica. The specimens from the Balleny Islands (delicate filamentous thallus up to 2 cm in height, with sessile gland cells and sessile tetrasporangia) appear to differ in their branching from P. antarcticum.
Wrangeliaceae
Antarcticothamnion polysporum R.L.Moe & P.C.Silva
Type locality: Elephant Island, South Shetland Islands. Holotype: UC 144016, Moe (130), 15.i.1974.
Habitat: Epiphytic, epizoic or epilithic and often densely epiphytized; at the Balleny Islands, scuba collections have been made at 12–20 m depth; dredge samples came from depths of 26–120 m.
Distribution: East Antarctica: Ross Sea, Balleny Islands, Vestfold Hills.
West Antarctica: Antarctic Peninsula, South Shetland Islands.
Representative specimens: Balleny: WELT A23620 - Buckle Island, RV Tangaroa, ii.2006; A023622 - Scott Cove, Buckle Island, Shears, 18.ii.2006. Ross Sea: WELT A026038 - Possession Island, RV Tangaroa, ii.2001.
General comments: The material from the Ross Sea and Balleny Islands is tentatively placed in this species pending the availability of further material and more detailed study. Although Gepp & Gepp (Reference Gepp and Gepp1907) described Spongoclonium orthocladum from Cape Adare, this species has not been subsequently collected and remains poorly known. Moe & Silva (Reference Moe and Silva1979) examined the type specimen of S. orthocladum and concluded that it is not a species of Spongoclonium but rather ‘seems closely related to Antarcticothamnion’. As also noted by the Gepps, the heavy settlement of diatoms obscured the branching pattern.
Georgiella confluens (Reinsch) Kylin (Fig. 4a)

Fig. 4. a. Georgiella confluens, herbarium specimen WELT A023630 from east of Sturge Island. b. Notophycus fimbriatus, herbarium specimen WELT A023637 from Cape Smythe, Sturge Island. All scale bars = 2 cm.
Lectotype locality: ‘Nordstrand de Landzunge, Pinguin Bay’ (Hommersand et al. Reference Hommersand, Freshwater, Lopez-Bautista and Fredericq2005). Lectotype: (tetrasporic), 3 July 1883; Botanische Staatssammlung, Munich (M) M-0100496.
Habitat: Epilithic, epiphytic, subtidal; Moe & Silva (Reference Moe and Silva1983) report it growing between 5 and 15 m, although they also report it growing in clear offshore waters to 30 m; strongly shade and cold adapted; scuba collections from Balleny Islands at 12–20 m depth and dredge samples from depths to 292 m.
Distribution: East Antarctica: Balleny Islands.
West Antarctica: South Georgia, South Orkney Islands, Antarctic Peninsula, South Shetland Islands, Graham Land, South Sandwich Islands.
Representative specimens: Balleny: WELT A025991 - Sturge Island, RV Tangaroa, 26.ii.2001. WELT A025988-A025990, A025998 - Young Island, RV Tangaroa, 3.iii.2001.
General comments: Moe & Silva (Reference Moe and Silva1983) examined a specimen from Sabrina Island; this constituted the first record of this species outside West Antarctica.
Delesseriaceae
Phycodrys antarctica (Skottsb.) Skottsb.
Type locality: Graham's Land.
Habitat: Subtidal on rock; Balleny Islands scuba collections were made at depths of 15 m and dredge samples from the Ross Sea and Balleny Islands were made from depths of 30–420 m.
Distribution: East Antarctica: Ross Sea, Balleny Islands, Adélie Coast, Mac.Robertson coast, Enderby Land.
West Antarctica: South Orkney Islands, Antarctic Peninsula, South Shetland Islands.
Representative specimens: Balleny: WELT A025993 - Sturge Island, RV Tangaroa, ii.2001. GQ479931 - Wendy's rock, Borradaile Island, Shears, 22.ii.2006. Ross Sea: WELT A026008 - Cape Hallett, RV Tangaroa, ii.2001. WELT A026003-A026004 - Possession Island, RV Tangaroa, ii.2001.
General comments: Sequence data from a specimen from the Balleny Islands was found to be 99.93% similar to sequences from a specimen collected from Avers Island, West Antarctica (Lin & Nelson Reference Lin and Nelson2010).
Delesseriaceae
Description: Small epiphytic blades.
Representative specimens: Balleny: WELT A023695 - Wendy's Rock, Borradaile Island, Shears, 22.ii.2006.
General comments: Insufficient material to place in a genus, but this taxon clearly differs from Phycodrys, the only other member of the Delesseriaceae reported from the Balleny Islands.
GIGARTINALES
Areschougiaceae
Notophycus fimbriatus R.L.Moe (Fig. 4b)
Type locality: Argentine Islands, Antarctic Peninsula (65°15′S, 64°16′W). Holotype: UC 1494459 (female plant), Moe, 19.ii.1975; 22 m. Isotype: UC 1475018.
Habitat: Epilithic, in low eulittoral pools through to depths of 15–22 m subtidally; scuba samples from the Balleny Islands were collected at 15–16 m depth; samples from Possession Island were dredged at 30 m depth and from the Balleny Islands at 230–292 m depth.
Distribution: East Antarctica: Ross Sea, Balleny Islands.
West Antarctica: South Orkney Islands, Antarctic Peninsula, South Shetland Islands.
Representative specimens: Balleny: WELT A023638 - Wendy's Rock, Borradaile Island, Shears, 22.ii.2006. WELT A026000A-D - Young Island, RV Tangaroa, ii.2001. Ross Sea: WELT A026034, A026023 - Possession Island, RV Tangaroa, ii.2001.
General comments: Page et al. (Reference Page, Alcock, Gordon, Kelly-Shanks, Nelson, Neill and Watson2002) reported this species for the first time from East Antarctica from both the Balleny Islands and the Ross Sea.
Gainiaceae
Gainia mollis R.L.Moe
Type locality: Henkes Island, northern Marguerite Bay, Antarctic Peninsula (67°48′S, 68°56′W). Holotype: UC 1475017, 15.ii.1975; 15 m.
Habitat: Reported as growing on encrusting coralline red algae and rocks at 6–25 m depth (Moe Reference Moe1985).
Distribution: East Antarctica: Ross Sea (Terra Nova Bay, Victoria Land), Vestfold Hills.
West Antarctica: Antarctic Peninsula, South Shetland Islands.
Representative specimens Ross Sea: WELT A026045 - Possession Island, RV Tangaroa, ii. 2001.
General comments: The sample reported here was from a single dredge collection from 26 to 30 m depth and is sterile, and hence this placement must be regarded as tentative until further mature material can be examined.
Dumontiaceae
Description: Dark red circular crusts on subtidal rock (sterile).
Representative specimens: Balleny: WELT A023664-65 - Borradaile Island Landing, Shears, 15.ii.2006
General comments: The Balleny Islands collection had previously been identified as Hildenbrandia sp. Sequence data (ON211589) display no close homology to any other data currently in GenBank, with the closest relationship being to members of the Dumontiaceae (Fig. S4).
Gigartinaceae
Iridaea sp. (Fig. 5a)
Previously referred to as Iridaea cordata (Turner) Bory de Saint-Vincent.

Fig. 5. a. Iridaea sp., herbarium specimen WELT A023635 from Faraglione in the inner Ross Sea. b. Phyllophorella sp., herbarium specimen WELT A023659 from Wendy's Rock, Borradaile Island. All scale bars = 2 cm.
Habitat: Scuba samples came from 3 to 20 m depth and from Cape Evans in the south (77°38′S) through to Borradaile Island (66°33′S); only one dredge sample included Iridaea sp., from the Balleny Islands at 70–120 m depth.
Representative specimens: Balleny: WELT A23632 - Borradaile Landing, Shears, 15.ii.2006. Ross Sea: WELT A023634 - Cape Evans, Schwarz, 30.xi.2001; WELT A034420, Wilcox, 13.ii.2012.
General comments: The name ‘Iridaea cordata’ (type locality: Isla de los Estados, Argentina; Hughey et al. Reference Hughey, Silva and Hommersand2001) has been applied to a wide range of specimens found commonly in the shallow subtidal waters of West and East Antarctica. Antarctic specimens (from the South Shetland Islands) that were morphologically similar to plants from South America (Ancud, Chile) were found to differ by > 3% rbcL base pair distance (Hommersand & Fredericq Reference Hommersand, Fredericq, Chapman, Anderson, Vreeland and Davison2003), suggesting that they belonged in separate species. Recent research has shown that I. cordata from southern South America differs from the species present on the Antarctic Peninsula, which is a single genetic entity present in sites ranging 700 km across the Antarctic Peninsula and South Shetland Islands (Guillemin et al. Reference Guillemin, Dubrasquet, Reyes and Valero2018, Ocaranza-Barrera et al. Reference Ocaranza-Barrera, González-Wevar, Guillemin, Rosenfeld and Mansilla2019). Specimens collected from the Balleny Islands and Ross Sea are part of this Antarctic Iridaea species (Fig. S5).
Kallymeniaceae
'Pugetia'
Habitat: One scuba sample from the Balleny Islands was collected at 15 m depth; dredge samples from the Balleny Islands and Possession Islands range from 90 to 772 m depth.
Representative specimens: Balleny: WELT A023669 - Buckle Island, RV Tangaroa, 10.iii.2006. Ross Sea: WELT A026002 - Possession Island, RV Tangaroa, ii.2001.
General comments: The identification of this material is provisional.
Phyllophoraceae
Phyllophora antarctica A.Gepp & E.S.Gepp
Type locality: Coulman Island, off Cape Wadsworth, British Antarctic Expedition.
Habitat: Subtidal and widespread, one of only three seaweeds reported from Cape Evans McMurdo Sound, the southernmost site for benthic macroalgae, where it is the dominant primary producer (Schwarz et al. Reference Schwarz, Hawes, Andrew, Norkko, Cummings and Thrush2003, Norkko et al. Reference Norkko, Thrush, Cummings, Funnell, Schwarz, Andrew and Hawes2004, Thrush et al. Reference Thrush, Dayton, Cattaneo-Vietti, Chiantore, Cummings and Andrew2006). Samples examined came predominantly from scuba collections from 15 to 20 m depth, and most material was fragmentary.
Distribution: East Antarctica: Ross Sea (Ross Island, McMurdo Sound, Victoria Land).
West Antarctica: Antarctic Peninsula.
Representative specimens: Ross Sea: WELT A034411-14 - McMurdo Jetty, Kelley, 8.xii.2014; WELT A23650-51 - Faraglione, Italica, 20.ii.2004.
General comments: As previously observed by Fredericq & Ramirez (Reference Fredericq and Ramirez1996), phylogenetic analysis of Antarctic samples of Phyllophora antarctica (now including material from the Ross Sea; Fig. S6) indicates that members of this taxon form a clade distinct from that of Phyllophora crispa (Huds.) P.S.Dixon, and further work is required to determine the correct generic placement for this taxon.
Phyllophorella sp. (Fig. 5b)
Habitat: Subtidal, two scuba collections from 15 m depth and one dredge sample from 63 to 85 m depth.
Representative specimens: Balleny: WELT A023659 - Wendy's Rock, Borradaile Island, Shears, 22.ii.2006.
General comments: A sequence from WELT A023659 (ON211594) forms a clade with Phyllophorella. This substantially extends the known distribution of this genus: the three species of Phyllophorella described to date are all known solely from Peru (Calderon & Boo Reference Calderon and Boo2016) (Fig. S6).
HALYMENIALES
'Halymeniaceae'
Description: Dark red brown thalli to ~30 cm in height.
Habitat: Subtidal, two scuba collections from 15 to 16 m depth, dredge samples from 63 to 1570 m depth.
Representative specimens: Balleny: WELT A023675 - Wendy's Rock, Borradaile Island, Shears, 22.ii.2006.
General comments: The identification of this species is provisional. The thalli collected by scuba are well developed, whereas the dredge samples consist of fragments and torn portions of blades.
PLOCAMIALES
Plocamiaceae
Plocamium sp.
Habitat: Epilithic or epiphytic to 60 m; strongly shade-adapted deep-water or undergrowth alga with strong cold adaptation; at the Balleny Islands, this species was widespread and recorded from all sites visited, with scuba records from ~15 to 20 m depth; dredge samples from Ross Sea and Balleny Islands include samples collected from 2 to 120 m and up to 1500 m depth.
Distribution: East Antarctica: Ross Sea, Balleny Islands.
Representative specimens: Balleny: WELT A023657 - Young Island, RV Tangaroa, 11.iii.2006. Ross Sea: WELT A034421 - Franklin Island, Wilcox, 13.ii.2012; WELT A023681 - Adélie Cove, Italica, 22.ii.2004; WELT A026021-A026022 - Possession Island, RV Tangaroa, ii.2001.
General comments: Various species of Plocamium have been reported from many locations in Antarctica (e.g. Oliveira et al. Reference Oliveira, Pellizzari, Medeiros, Yokoya, Gómez and Huovinen2020) and a comparative systematic study is required to clarify the identity of material from Antarctica and neighbouring regions. Previously, Plocamium specimens from the Balleny Islands and the Ross Sea were referred to as Plocamium cartilagineum (L.) P.S.Dixon. It is clear that Antarctic material does not belong to this taxon: Saunders & Lehmkuhl (Reference Saunders and Lehmkuhl2005) confirmed that P. cartilagineum is restricted to the North Atlantic. Phylogenetic analysis of rbcL sequence data shows that there is a strongly supported clade of specimens from the Balleny Islands, South Shetland Islands and the Antarctic Peninsula (Fig. S7).
Sarcodiacae
Trematocarpus?
Description: A single blade fragment, dark red-brown.
Habitat: Dredged from 70 to 120 m depth
Representative specimens: Balleny: WELT A023667 - Buckle Island, RV Tangaroa, 9.iii.2006.
General comments: This placement is provisional. The material was previously placed as Sarcodia sp. but differs from all other specimens examined, and when further material becomes available it should be compared with Trematocarpus antarcticus (Hariot) Fredericq & R.L. Moe.
RHODYMENIALES
Rhodymeniaceae
Hymenocladiopsis sp.
Habitat: Subtidal, known from a single specimen collected by scuba at 15 m depth.
Representative specimens: Balleny: WELT A023631 - Wendy's Rock, Borradaile I., Shears, 22.ii.2006.
General comments: The specimen collected at the Balleny Islands is provisionally placed in this genus pending further material for study. The type species of the genus, Hymenocladiopsis crustigena (now Hymenocladiopsis prolifera (Reinsch) M.J.Wynne), has an extensive crust holdfast and grows at depths of 5–30 m along the Antarctic Peninsula as far south as northern Marguerite Bay and in the South Shetland and South Orkney islands (Moe Reference Moe1986).
Rhodymenia sp.
Habitat: Subtidal, two specimens collected by scuba at 15 m depth from two sites.
Representative specimens: Balleny: WELT A023670 - Cape Smythe, Sturge Island, Shears, 19.ii.2006.
General comments: The identification of this material is provisional. Rhodymenia coccocarpa (Mont.) M.J. Wynne (previously known as Rhodymenia subantarctica R.W.Ricker) has been reported from West Antarctica.
Discussion
In this account, we report a total of 30 species, 27 from the Balleny Islands and 18 from the Ross Sea. Earlier treatments of the flora reported a total of 11 species from the Balleny Islands (Zaneveld Reference Zaneveld1968, Zaneveld & Sanford Reference Zaneveld and Sanford1980, Wagner & Zaneveld Reference Wagner and Zaneveld1988), and 37 species have been reported previously from the Ross Sea (Cormaci et al. Reference Cormaci, Furnari, Scammacca, Faranda, Guglielmo and Ianora2000). The samples from the Ross Sea that are included in this report were gathered largely opportunistically yet include five new records for the region: Antarcticothamnion polysporum, Callithamnion sp., Elachista antarctica, Notophycus fimbriatus and ‘Pugetia’.
In order to interpret these findings and compare them with previously published accounts, it is important to consider the reliability of the taxonomy and systematic understanding of the flora. Three of the records reported here are placed provisionally within families of the red algae based on the material available (Delesseriaceae, Dumontiaceae, Halymeniaceae), and we have also recorded as yet undetermined non-geniculate coralline algae. A further 10 taxa have been provisionally placed in genera, requiring further work before their placements are confirmed and/or they can be identified to the species level. In some cases, the record in this report is based on a very small number of samples that may be incomplete or sterile, precluding more thorough documentation until further collections are available (e.g. Hymenocladiopsis sp., ‘Pugetia’ sp.). For the taxa reported here, five of the records are based on a single collection, and a further five records are based on two to three collections, clearly indicating that collections have not yet reached saturation in terms of flora coverage.
For some of the taxa presented here, recent taxonomic and phylogenetic investigations have been conducted, and we consider the identifications are soundly based (e.g. Phycodrys antarctica, Prasiola crispa, Tethysphytum antarcticum; Lin & Nelson Reference Lin and Nelson2010, Heesch et al. Reference Heesch, Sutherland and Nelson2012, Sciuto et al. Reference Sciuto, Moschin, Alongi, Cecchetto, Schiaparelli and Caragnano2021). For other groups of taxa, the published literature is not reliable, with significant underpinning nomenclatural and systematic issues that are far from resolved (e.g. non-geniculate coralline algae). Sequence data have confirmed the need for further work on the genera Ballia and Iridaea and on the correct generic placement of species currently recorded as Phyllophora antarctica, Phyllophorella sp. and Palmaria decipiens in Antarctica.
Algal diversity has been observed previously to decrease by midway along the western Ross Sea coast in the Terra Nova Bay region (Cormaci et al. Reference Cormaci, Furnari and Scammacca1992), and in the southern Ross Sea the species diversity is low, with three red macroalgae dominating habitats (Iridaea sp., P. antarctica and non-geniculate corallines; Miller & Pearse Reference Miller and Pearse1991). The observations and collections presented here support these earlier accounts, with the five new records for the Ross Sea sampled from the Outer Ross Sea region (71–72°S), indicating that further biodiversity surveys in the wider Ross Sea are warranted. The flora of West Antarctica is much better known than that of East Antarctica.
A relatively high proportion of the macroalgal flora recorded from the Antarctic region is considered to be endemic to Antarctica: ~35% of the total flora. This includes 44% of the Ochrophyta and 36% of the Rhodophyta (Wiencke et al. Reference Wiencke, Amsler, Clayton, de Broyer, Koubbi, Griffiths, Raymond, Udekem d'Acoz and Van de Putte2014). This high degree of endemism is attributed to the isolation of the continent and the ‘significant tectonic, oceanographic and climatic changes’ that have affected the region over the past 50 million years (discussed by Guillemin et al. Reference Guillemin, González-Wevar, Cárdenas, Dubrasquet, Garrido, Montecinos, Gómez and Huovinen2020). Molecular sequence data and analyses are providing insights into the relationships of the floras of Antarctica, southern South America and some of the peri-Antarctic islands, including enabling the recognition of cryptic species (e.g. Hommersand et al. Reference Hommersand, Moe, Amsler and Fredericq2009, Billard et al. Reference Billard, Reyes, Mansilla, Faugeron and Guillemin2015, Pellizzari et al. Reference Pellizzari, Silva, Silva, Medeiros, Oliveira and Yokoya2017, Dubrasquet et al. Reference Dubrasquet, Reyes, Sanchez, Valdivia and Guillemin2018, Reference Dubrasquet, Garrido, Bruning, Reyes and Guillemin2021, Ocaranza-Barrera et al. Reference Ocaranza-Barrera, González-Wevar, Guillemin, Rosenfeld and Mansilla2019). The impact of glaciation has been investigated for selected macroalgae on the Antarctic Peninsula, revealing evidence of genetic bottlenecks associated with the Last Glacial Maximum ~18,000 years ago, with low genetic diversity and evidence of recent population expansion (Guillemin et al. Reference Guillemin, Dubrasquet, Reyes and Valero2018, Reference Guillemin, González-Wevar, Cárdenas, Dubrasquet, Garrido, Montecinos, Gómez and Huovinen2020). Guillemin et al. (Reference Guillemin, González-Wevar, Cárdenas, Dubrasquet, Garrido, Montecinos, Gómez and Huovinen2020) speculated on the role of glacial refugia in the peri-Antarctic islands south of the Antarctic Polar Front (APF), including referencing the Balleny Islands as an area that warrants further sampling.
Macroalgae form significant beds along the coastal regions of Antarctica to depths of 100 m, contributing as both primary producers and as ecosystem engineers. The flux of materials from these extensive beds provides an important source of energy supporting a significant proportion of secondary production (Marina et al. Reference Marina, Salinas, Cordone, Campana and Moreira2018, Quartino et al. Reference Quartino, Saravia, Campana, Deregibus, Matula, Braso, Momo, Gómez and Huovinen2020). Momo et al. (Reference Momo, Cordone, Marina, Salinas, Campana, Valli, Gómez and Huovinen2020) summarized the critical role played by macroalgae in Antarctic coastal food webs. Drift or stranded macroalgae play a key part in these food chains. To date, 39 species of macroalgae have been recorded drifting, stranding or floating in Antarctica, including some crossing the APF (Macaya et al. Reference Macaya, Tala, Hinojosa, Rothäusler, Gómez and Huovinen2020). The ecological isolation of Antarctica is predicted to be strongly influenced by changes in the frequency and intensity of storms, with an increasing number of incursions crossing the APF predicted (Fraser et al. Reference Fraser, Morrison, Hogg, Macaya, van Sebille and Ryan2018), Fraser et al. (Reference Fraser, Morrison, Rojas, Gómez and Huovinen2020) evaluated the biogeographical processes influencing the floras of the Antarctic and sub-Antarctic, focusing on the role of buoyant macroalgal rafts and their contributions to species dispersal.
There is increasing focus being given to the changes to the biota in the Antarctic region, either through the introduction of species directly by human-mediated vectors (vessel traffic, ballast water) or through the effects of changing climate on algal seasonality, depth distributions and habitat changes and potentially influencing competitive relationships between resident species, as well as range expansions and the survival of sub- Antarctic or cold temperate species, with consequences for the native Antarctic biota (e.g. Mystikou et al. Reference Mystikou, Peters, Asensi, Fletcher, Brickle and Van West2014, Chown et al. Reference Chown, Clarke, Fraser, Cary, Moon and McGeoch2015, Schoenrock et al. Reference Schoenrock, Schram, Amsler, McClintock, Angus and Vohra2016, Pellizzari et al. Reference Pellizzari, Luiz Henrique Rosa, Yokoya, Gómez and Huovinen2020, Valdivia Reference Valdivia, Gómez and Huovinen2020). Improved baseline information about the macroalgal flora is needed to fully understand changes over time. As noted by Mystikou et al. (Reference Mystikou, Peters, Asensi, Fletcher, Brickle and Van West2014), ‘species numbers from limited collections alone cannot be considered as a reliable proxy to estimate changes in algal communities impacted by climate change over a time span of several decades’.
The full biodiversity of the Balleny Islands remains poorly known, and detailed comparisons with the floras of East and West Antarctica are not possible with the material currently available. Based on the number of taxa known from single or few collections, we suggest that more diversity will be discovered in this region when thorough and well-targeted collections are made. Based on collections currently available, many of the taxa cannot be fully documented as there is insufficient mature material available. In addition, the taxonomic resolution for the Antarctic flora as a whole requires further research. The continued use of names that are known not to apply to Antarctic species confuses the literature and inhibits analysis of relationships.
Despite the inadequacies in the data available for the Balleny Islands, it is clear that the macroalgal biodiversity of the Balleny Islands and the outer Ross Sea has been underestimated. The Balleny Islands occupy a unique position in relation to the Antarctic continent and there are some intriguing patterns of species distribution that warrant further investigation. Further targeted collections in this region would enable better definition of the biota and community structure and enable investigation of relationships and connections within the wider Antarctic region.
Acknowledgements
We acknowledge with thanks collections and field observations from the Balleny Islands provided by the Tiama research team (Nick Shears, Franz Smith and Rebecca McLeod); collections from the RV Italica; NIWA voyage collections from TAN0402 (biodiversity survey of the western Ross Sea and Balleny Islands in 2004 undertaken by the National Institute of Water and Atmospheric Research and financed by the former New Zealand Ministry of Fisheries), TAN0602 (specimens were collected as part of the project ‘Antarctic Geophysical & Scientific Studies’ funded by the New Zealand Ministry of Fisheries) and TAN0802 (research funded by the New Zealand Government under the New Zealand International Polar Year Census of Antarctic Marine Life Project). We are grateful for collections from Franklin Island provided by Sarah Wilcox, from McMurdo enabled by Brett Grant (NIWA) and Amanda Kelley (University of California) and Ross Sea samples provided by Vonda Cummings (NIWA). We thank Jenn Dalen and Antony Kusabs (Herbarium, Museum of New Zealand Te Papa Tongarewa) for their assistance with the registration of algal specimens. We thank Quantarctica and the Norwegian Polar Institute for provision of their simple base map. We also thank two anonymous reviewers for their comments.
Financial support
This work has been supported by funding from NIWA SSIF to the Coasts and Oceans National Centre, Marine Biodiversity Programme.
Author contributions
WAN processed and identified specimens, analysed data and led the writing of the manuscript. KFN processed specimens and data, contributed to the approach of the article, prepared maps and figures and prepared and edited the manuscript prior to submission. RD contributed to taxonomic identifications, performed DNA extraction, amplification, molecular analyses and data interpretation and contributed to writing the manuscript. JES contributed to sequencing and initial analyses and commented on the text.
Supplemental material
Seven supplemental figures and one supplemental table will be found at https://doi.org/10.1017/S0954102022000220.










