Type 2 diabetes mellitus (T2DM) in adolescence is rapidly becoming one of the new worldwide public health challenges of the 21st century( Reference Viner, White and Christie 1 ). According to the report of International Diabetes Federation, the number of Chinese people with diabetes ranks first (109·6 million) in the world, and the sharply increasing onset of diabetes in children is a matter of great concern( Reference International Diabetes Federation 2 ). T2DM was rarely seen in children 20 years ago, however this has changed due to increasing detection, rising obesity and sedentary lifestyle( Reference Nadeau and Dabelea 3 ). Increasing T2DM prevalence in children and adolescents has been observed in developed and developing countries( Reference Nadeau and Dabelea 3 – Reference Oester, Kloppenborg and Olsen 7 ). Meanwhile, as one of the important indicators of altered glucose metabolism, impaired fasting glucose (IFG) has been an emerging issue in children and adolescents over the past three decades( Reference Smyth and Heron 8 ). It has been suggested that IFG in childhood significantly increases their later risk of T2DM and CVD( Reference American Diabetes Association 9 ).
Fu et al. ( Reference Fu, Liang and Gong 6 ) observed the prevalence of diabetes and prediabetes in boys was significant higher than in girls. But their results are still controversial. Other studies conducted in Chinese children did not observe that sex difference( Reference Wu, Zhong and Yu 10 ). Moreover, these two studies observed the diabetes prevalence in high socio-economic regions seemed to be greater than regions with low socio-economic level( Reference Fu, Liang and Gong 6 , Reference Wu, Zhong and Yu 10 ). However, it remains unknown what led to these differences. With limited medical resources( Reference Zhang 11 ), it is of great significance to prioritise intervention on the critical factors contributing to these differences for reducing the burden of diabetes and improving health in childhood.
In view of these, using data of a Chinese national survey, the present study investigated the prevalence of diabetes mellitus (DM) and IFG, and the disparities in sex and region, among children and adolescents aged 6–17 years, and further explored potential factors contributing to these gaps.
Methods
Study design and population
A national multicentred school-based health lifestyles intervention against obesity was performed from September 2013 to June 2014. The details of the programme have been described elsewhere( Reference Chen, Ma and Ma 12 ). The programme involved participants aged 6–18 years from ninety-four primary and secondary schools in seven provinces in China, including Chongqing, Hunan, Guangdong, Liaoning, Ningxia, Shanghai and Tianjin, and a total of 16 746 blood samples were collected. In the present study, participants aged 6–17 years were included (n 16 633), however those with extremely low concentration of fasting plasma glucose (FPG, <3·0 mmol/l) (n 171) or missing records of height or weight (n 28) were excluded. As a result, 16 434 participants were involved into the final analysis.
Ethnic statements
This study was approved by the Medical Ethical Committee of Peking University Health Science Center (IRB00001052-13034), and written informed consents were obtained from all the children and their parents.
Data collection and measurements
All investigators in this programme were trained to measure and record relevant information utilising standardised procedures. Self-reported information of participants’ demographic characteristics, dietary behaviour, physical activity and family history of DM (defined as the presence of DM in at least one parent or grandparent) was collected through questionnaires. FFQ was used to collect the intake frequencies of fruit, vegetables, sweet beverages, meats, fried foods and milk in the past 7 d. The threshold of two servings per d was used to categorise fruit (about 200 g per serving) and vegetables (about 150 g per serving) intake, and threshold of one serving per d was used to categorise meats (about 100 g per serving) and milk (about 250 ml per serving) intake. Questionnaire was also used to collect information of sedentary time, which included time of being seated or lying 1 d divided into two groups. The International Physical Activity Questionnaire Short Form was used to assess physical activity level, which was categorised into three groups (light, moderate and vigorous physical activity)( Reference Craig, Marshall and Sjostrom 13 ).
The anthropometric measurements were collected. Height and weight were measured according to a standardised procedure. A portable stadiometer (model TZG; Jiangyin Hongya Science and Education Equipment Co., Ltd) was used to measure height, and a scale (model RGT-140; Wuxi Weighing Apparatus Factory Co., Ltd) was used to measure fasting body weight. Participants were asked to wear underclothes only and to stand straight without shoes. Height is accurate to 0·1 cm and weight to 0·1 kg. Both height and weight were measured twice and the average values were used to calculate the BMI (weight/height2, kg/m2).
Laboratory tests
Venous blood samples were collected after 8 h of overnight fasting. Fasting glucose was tested by a qualified biomedical analyses company using glucose oxidase method.
Definitions
The recommendations of American Diabetes Association( 14 ) were used to define IFG and DM. A FPG concentration of ≥126 mg/dl (7·0 mmol/l) was defined as DM, while an FPG between 100 and 125 mg/dl (5·6–6·9 mmol/l) was classified as IFG, and glucose <5·6 mmol/l was defined as normal.
The Chinese BMI percentile criterion for screening overweight and obesity in children and adolescents was used to define the overweight and obesity among students aged 6–17 years( Reference Ji 15 , Reference Li, Zong and Ji 16 ). Participants with age- and sex-specific BMI ≥95th percentile were defined as obesity, while those with age- and sex- specific 85th≤BMI<95th were defined as overweight( Reference Ji 15 , Reference Li, Zong and Ji 16 ).
According to the recommendation of WHO on the age ranges of children and adolescents, the threshold of 10 years old was used to categorise the participants aged 6–17 years into children (6–9 years) and adolescents (10–17 years)( Reference Kuruvilla, Bustreo and Kuo 17 ).
Statistical analysis
Statistical analysis was performed with SPSS 20.0 (IBM Corporation). The WHO growth chart (2007) was used to calculate z-scores for height, weight and BMI( Reference de Onis, Onyango and Borghi 18 ). Dunnett’s test was used to compare the differences for continuous variables, and Cohen’s d equation was used to calculate effect size (r). The χ 2 test was used to compare the differences for proportions, and the φ coefficient (r φ ) was used to calculate effect size. The Wilson method was used to estimate 95 % CI since the prevalence estimates were close to zero( Reference Brown, Cai and Dasgupta 19 ). A logistics regression model with the backward likelihood ratio method was used to explore the influencing factors of IFG. A two-sided P<0·05 was considered as statistical significance.
Results
Characteristics of study population
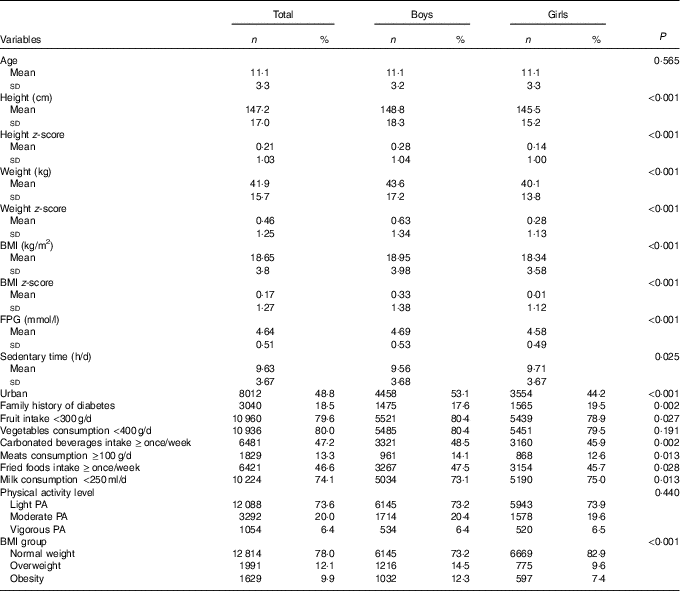
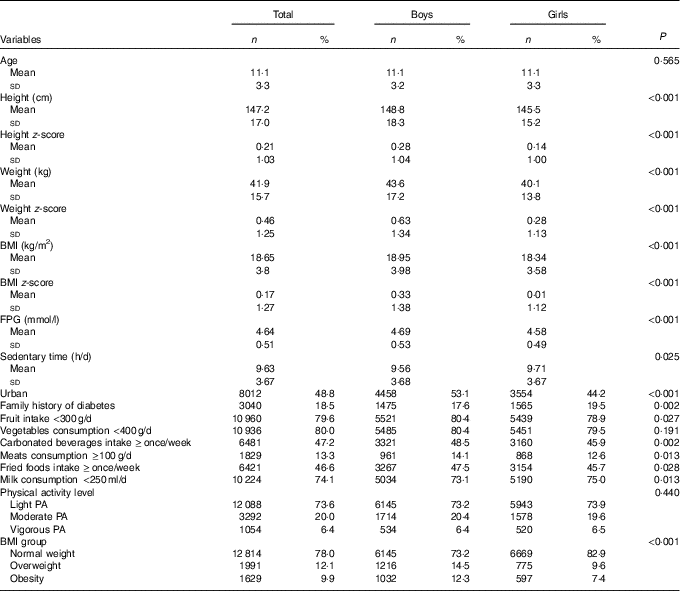
A total of 8393 (51·1 %) boys and 8041 (48·9 %) girls aged 6–17 years participated in the study. Compared with boys, girls had lower BMI and FPG, reported higher frequency of fruit intake and lower frequencies of fried foods, milk, meats and carbonated beverages intake (P<0·05, Table 1). However, boys reported less frequently a family history of diabetes and sedentary time than girls (P<0·05, Table 1).
Table 1 Characteristics of the Chinese children and adolescents aged 6–17 years (Numbers and percentages; mean values and standard deviations)
FPG, fasting plasma glucose; PA, physical activity.
Sex and age disparities in prevalence of impaired fasting glucose and diabetes mellitus
The overall prevalence of IFG and DM was 1·89 (95 % CI 1·67, 2·08) and 0·10 (95 % CI 0·05–0·15) %, respectively (Table 2).
Table 2 Fasting glucose level (mmol/l) and prevalence of impaired fasting glucose (IFG) in Chinese children, stratified by sex and age group (Mean values and standard deviations; numbers, percentages and 95 % confidence intervals)
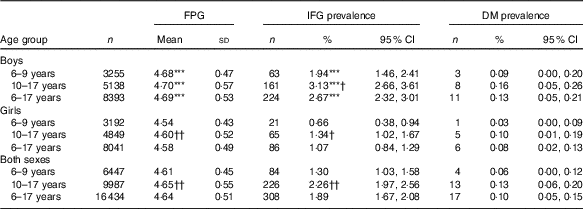
DM, diabetes mellitus; FPG, fasting plasma glucose.
*** P<0·001 between boys and girls.
†P<0·05 between 6–9 years group and 10–17 years group; †† P<0·01 between 6–9 years group and 10–17 years group.
Compared with girls, boys had higher FPG concentration and IFG prevalence in total population and in all age subgroups (P<0·001, Table 2). However, this study did not observe any statistically significant difference in DM prevalence between boys and girls (P>0·05, Table 2).
Compared with children, adolescents had higher IFG prevalence (2·26 v. 1·30 %, r φ 0·034, P<0·05) in boys (3·13 v. 1·94 %, r φ 0·036, P<0·05) and girls (1·34 v. 0·66 %, r φ 0·032, P<0·05). Similarly, adolescents had higher FPG concentration than children (4·65 v. 4·61 mmol/l, r 0·040, P<0·01) in girls (4·60 v. 4·54 mmol/l, r 0·063, P<0·01), but not in boys (4·70 v. 4·68 mmol/l, r 0·019, P>0·05). However, the difference of DM prevalence was not statistically significant between children and adolescents (P>0·05, Table 2).
Region disparities in prevalence of impaired fasting glucose and diabetes mellitus
Overall, compared with rural population, urban population had higher FPG concentration (4·65 v. 4·62 mmol/l, r 0·029, P<0·001) and DM prevalence (0·15 v. 0·05 %, r φ 0·016, P<0·01, Table 3). After stratification by age subgroups, only urban adolescents had a higher FPG concentration than their rural peers (4·68 v. 4·62 mmol/l, r 0·055, P<0·001), and rural children had a higher prevalence of IFG than their urban peers (1·83 v. 0·78 %, r φ 0·047, P<0·001) (Table 3).
Table 3 Differences in mean level of fasting glucose (mmol/l) and prevalence of impaired fasting glucose (IFG) between urban and rural children (Mean values and standard deviations; numbers, percentages and 95 % confidence intervals)
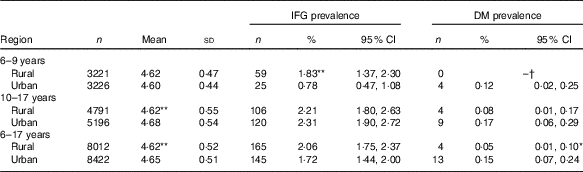
DM, diabetes mellitus.
*P<0·01 between rural and urban; ** P<0·001 between rural and urban.
†‘–’, no data.
Influencing factors for impaired fasting glucose
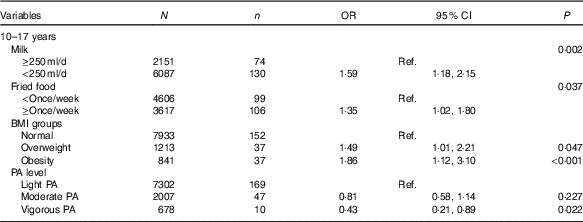
Table 4 shows influencing factors of IFG in adolescents. Although BMI groups and self-reported information, including intake frequencies of fruit, vegetables, carbonated beverages, meats and fried foods, sedentary time, physical activity and record of family history of diabetes were tested, only four factors were associated with IFG in adolescents, but not in children using the backward likelihood ratio method. Adolescents who reported consuming milk <250 ml/d (OR=1·59, 95 % CI 1·18, 2·15; P=0·002) and fried foods ≥once/week (OR=1·35, 95 % CI 1·02, 1·80; P=0·037) had higher risk of IFG than those who reported consuming milk >250 ml/d and fried foods <once/week. Moreover, compared with normal weight adolescents, overweight (OR=1·49, 95 % CI 1·01, 3·10; P=0·047) and obese adolescents (OR=1·86, 95 % CI 1·12, 3·10; P<0·001) were associated with higher risk of IFG. However, self-reported vigorous physical activity was associated with lower risk of IFG (OR=0·43, 95 % CI 0·21, 0·89; P=0·022).
Table 4 Factors analysis of impaired fasting glucose in Chinese adolescents aged 10–17 years (Numbers, odds ratios and 95 % confidence intervals)
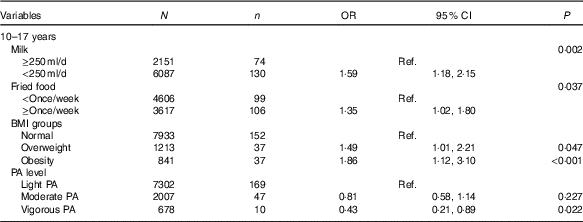
Ref., reference; PA, physical activity.
Region and sex differences in factors for impaired fasting glucose
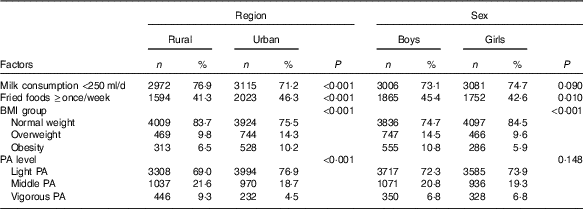
Table 5 presents region and sex differences in potential factors for IFG. Compared with rural adolescents, more urban adolescents reported they consumed fried foods ≥once/week (46·3 v. 41·3 %, P<0·001), while less urban adolescents reported they consumed milk <250 ml/d (71·2 v. 76·9 %, P<0·001) and performed vigorous physical activity (4·5 v. 9·3 %, P<0·001). In addition, the obesity prevalence of urban adolescents was higher than that in their rural peers (10·2 v. 6·5 %, P<0·001).
Table 5 Factors related to the region and sex disparities in impaired fasting glucose among adolescents aged 10–17 years (Numbers and percentages)
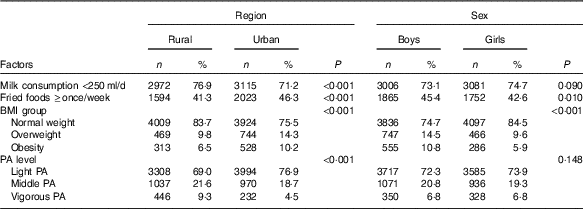
PA, physical activity.
Compared with girls, more boys reported they consumed fried foods ≥once/week (45·4 v. 42·6 %, P=0·010). Moreover, obesity prevalence in boys was significant higher than in girls (10·8 v. 5·9 %, P<0·001).
Discussion
Using data of a national survey conducted in Chinese children and adolescents aged 6–17 years, the present study observed the prevalence of IFG and DM was 1·89 and 0·10 %, respectively. Generally, boys had higher FPG concentration and IFG risk than girls, urban children and adolescents had higher FPG concentration and DM prevalence than their rural peers. This may be partly attributed to the higher obesity prevalence and unhealthy dietary behaviour in boys and those living in urban regions than those in girls and in rural regions. Regarding the limited medical resources( Reference Zhang 11 ), populations with higher burden of IFG and DM should be prioritised, and interventions aimed to reduce obesity and improve dietary behaviour could be helpful to bridge these gaps.
Consistent with the previous Chinese study( Reference Fu, Liang and Gong 6 ), the prevalence of IFG (1·89 %) and DM (0·10 %) in Chinese children and adolescents was lower than those in other countries. For example, Fu et al.( Reference Fu, Liang and Gong 6 ) studied diabetes in clinical settings and found the prevalence of DM was 0·11 % in Chinese children. Several surveys conducted in other countries showed that the prevalence of DM was 0·4 % in Nigeria children and adolescents aged 2–19 years( Reference Agbre-Yace, Oyenusi and Oduwole 20 ), 0·3 % in American adolescents aged 10–19 years( Reference Dabelea, Mayer-Davis and Saydah 21 ) and 0·45 % in the Saudi Arabian population aged 0–18 years( Reference Al-Rubeaan 22 ). Additionally, the present study observed Chinese children and adolescents had a lower IFG prevalence than their peers from Saudi Arabia( Reference Al-Rubeaan 22 ) and America( Reference Li, Ford and Zhao 23 ) (1·89 v. 6·12 and 16·1 %, respectively).
A significant sex difference in FPG concentration and IFG prevalence was observed in this study. Boys had a 0·11 mmol/l higher FPG concentration than girls. Meanwhile, the prevalence of IFG and DM in boys was 1·5 and 0·6 times higher than in girls, respectively. A similar difference was also found in the Uganda study( Reference Bahendeka, Wesonga and Mutungi 24 ), which reported that DM prevalence in boys and girls was 1·6 (95 % CI 0·8, 2·6) and 1·1 (95 % CI 0·6, 1·7) %, respectively. We speculated that the difference of overweight/obesity prevalence between Chinese boys and girls might contribute to the sex gap in IFG. Overweight/obesity was positively associated with IFG, and the prevalence of overweight/obesity in boys was 58 % higher than in girls, which had been found in this study.
Region difference in FPG concentration and DM prevalence was found in the present study. Urban children and adolescents had a higher DM prevalence than their rural peers. The FPG level in urban participants was also higher than that in rural counterparts, but the difference was very small. We speculated that the differences in frequency of fried foods consumption and obesity prevalence between urban and rural children and adolescents might be associated with the region differences. We found that consuming fried foods ≥once/week and overweight/obesity were positively associated with IFG, and the proportion of consuming fried foods ≥once/week and overweight/obesity prevalence in urban children and adolescent was higher than in their rural peers. In addition, we observed rural children aged 6–9 years had 1·35 times higher IFG prevalence than their urban peers. Although the potential mechanism remains highly speculative, we cannot exclude that it may be associated with some of the rural children aged 6–9 years not truly fasting.
IFG is a risk state before diabetes, which is important in the prevention and control strategies for diabetes. In the present study, we found the IFG was negatively associated with milk consumption in children aged 10–17 years, which was also detected by the Health Professionals Follow-up Study( Reference Choi, Willett and Stampfer 25 ) and a 10-year follow-up study among 37 187 women( Reference Liu, Choi and Ford 26 ). They estimated that one serving of dairy foods daily was associated with an annual reduction in diabetes incidence of 9·0 % in men and 4·0 % in women. The mechanisms underlying the associations between dairy consumption and DM include the high content of Ca and vitamin D in dairy foods( Reference Pittas, Lau and Hu 27 ) and the positive effect of dairy foods and Ca intake on weight control( Reference Zemel, Donnelly and Smith 28 ).
In the present study, we additionally observed that fried foods consumption and obesity could raise the risk of IFG, which was also reported by other studies( Reference Cahill, Pan and Chiuve 29 , Reference Alhazmi, Stojanovski and McEvoy 30 ). Frying is a common cooking method worldwide, which modifies both the foods composition and oils by the processes of oxidation, polymerisation and hydrogenation. With repeated use, oils deteriorate and increase amounts of cholesterol oxidation products and trans fatty acid( Reference Li, Ha and Wang 31 ), which have been associated with insulin resistance and diabetes( Reference de Souza, Mente and Maroleanu 32 , Reference King and Priesbe 33 ). Furthermore, obesity is characterised by excess body fat, which was associated with IFG and diabetes( Reference Neeland, Turer and Ayers 34 ).
In addition, a strong negative association between vigorous physical activity and IFG was observed among children and adolescents aged 10–17 years. Similar association has been reported by several cross-sectional studies, longitudinal studies and meta-analyses, indicating that physical activity independent of dietary or weight loss reduces the risk of T2DM in people with prediabetes( Reference Manson, Nathan and Krolewski 35 – Reference Yates, Khunti and Bull 37 ). The mechanism behind the association may be linked with physical activity-induced improvements in glucose homoeostasis which occur through acute responses and chronic adaptations( Reference Albright, Franz and Hornsby 38 – Reference Despres 41 ). However, we did not observe significant association between sedentary time and IFG in the present study (OR=0·96; 95 % CI 0·93, 1·01; P=0·087). It was inconsistent with the study of Kriska et al.( Reference Kriska, Delahanty and Edelstein 42 ), which observed overweight/obese adolescents aged 10–17 years with T2DM spent significantly more time being sedentary than their obese counterparts without T2DM (56 min/d, P<0·001). We speculated that methods of collecting sedentary time between two studies might contribute to the inconsistent association. The accelerometry was used to assess the sedentary time in the study of Kriska et al.( Reference Kriska, Delahanty and Edelstein 42 ). However, the self-report questionnaire was used to collect the sedentary time in the present study. Thus, recall bias and length of sedentary bouts in the present study could be contributed to the differences( Reference de Winter, Rioux and Boudreau 43 ).
The present study had several limitations. Firstly, we cannot distinguish type 1 DM from T2DM in this study, and the results may include both types of diabetes. Secondly, only FPG, rather oral glucose tolerance test or HbA1c, was measured. Studies found the sensitivity of intravenous fasting glucose as a screening method for DM could be relatively low, and thus, this method may not identify some individuals with impaired glucose tolerance. Thirdly, any symptomology of diabetes was not recorded in this study, such as polyuria, polydipsia and weight loss. Moreover, we did not measure the fasting insulin concentration and cannot assess the insulin resistance, such as homoeostasis model assessment-insulin resistance.
Despite those limitations, the present study using a large sample with broad representation found the prevalence of IFG and DM was still low in Chinese children. However, sex and region disparities in FPG and IFG have been observed, and interventions addressing fried foods, vigorous physical activity and obesity may contribute to bridging these gaps. Appropriate approaches should be conducted for children and adolescents with IFG or DM and prioritise interventions aimed to modify dietary behaviours and reducing burden of obesity for improving the health of Chinese children and adolescents.
Acknowledgements
The authors greatly appreciate the Educational Administration Leaderships and primary and middle school health nurses. The authors would additionally acknowledge all participants and investigators for their cooperation and efforts, especially the effort of D. M. Ma and R. Ma.
The project was supported by the Research Special Fund for Public Welfare Industry of Health (grant no. 201202010) and the Excellent Talents Fund Program of Peking University Health Science Center (BMU2017YJ002).
J. M., Y. M., J. J., J. L., X. Z., C. L., H. W., H. Z. and D. P. were co-investigators and designed the study, Z. W. carried out the initial analysis, and Y. M. and B. D. supervised the data analysis. All authors were involved in writing the manuscript and had final approval of the submitted and published versions. Z. W. took full responsibility for the whole work.
The authors declare that there are no conflicts of interest.