The scope of perioperative medicine
Perioperative medicine describes the practice of patient-centred, multidisciplinary, and integrated medical care of patients from the moment of contemplation of surgery until full recovery (Reference Grocott and MythenGrocott & Mythen, 2015). This encompasses the three stages of surgical care: preoperative, intraoperative, and postoperative.
This definition covers a wide range of patients with many different conditions, ranging from a low-risk, young, healthy person undergoing minor surgery in an ambulatory care setting to a high-risk older person with multiple co-morbidities undergoing major and complex surgery.
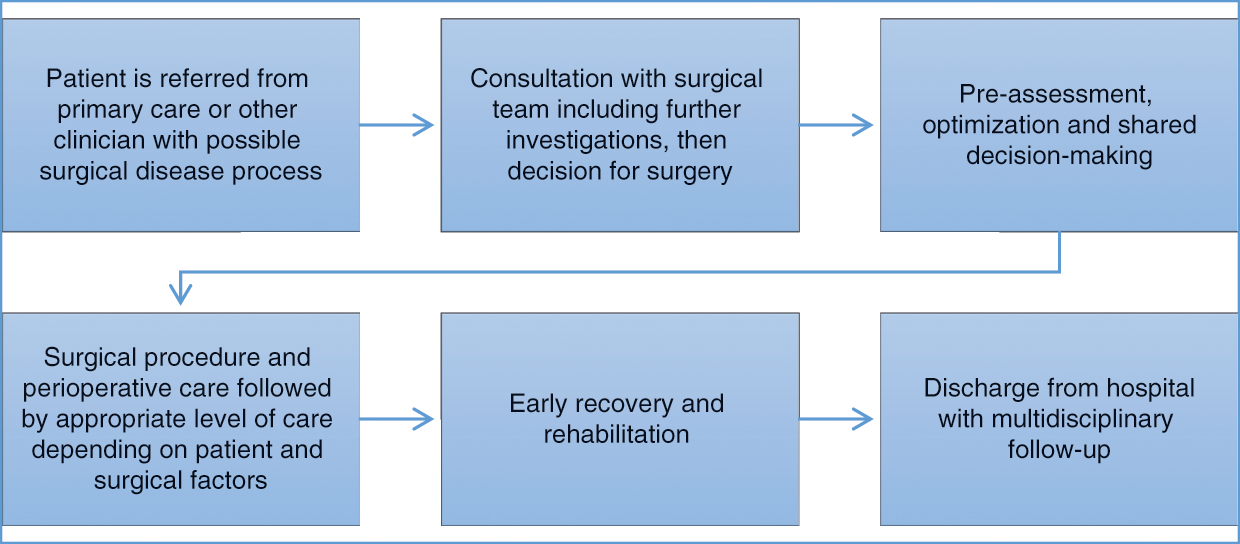
Perioperative care also involves a range of settings and disciplines. For the purpose of this chapter, it is taken as encompassing the period after a person with a possible surgical condition is referred to hospital by a primary care provider or ambulatory specialist, through traditional perioperative care, most commonly undertaken within a hospital, to their discharge and full recovery, as shown in Figure 8.1.

Figure 8.1 Patient pathway for elective surgery
Historically, the care provided to the surgical patient has been focused on the type of procedure being undertaken and the immediate recovery period, under the responsibility of an individual practitioner, a surgeon. It has typically been viewed in isolation from other elements of the patient’s experience, with little coordination and communication either within or beyond the hospital setting. However, reflecting a number of emerging factors that will be explored in this chapter, this model of care is being transformed to one that is individualized, coordinated, and delivers high quality care centred on the needs of the patient. This is particularly the case for high-risk patients with complex medical and social needs, undergoing major elective or emergency surgery. A major driver of the evolving model of perioperative care is the fact that patients with significant co-morbidities are increasingly being referred for surgical treatment. In the past these people would have been considered too high risk, or would have had a shorter life expectancy as a result of their medical conditions.
Over the last 10 to 20 years there has been a paradigm shift in the way that surgical patients are managed, driven by a mix of wider societal and clinical factors. During this time demand for surgery has risen considerably. According to OECD data, for example, in Denmark the rate of hip replacements was 140 per 100 000 population in 1996 but rose to 215 per 100 000 population in 2010 (OECD, 2015). A similar increase was observed in other western European countries, such as the Netherlands, but the increase was more pronounced in some of the southern European countries such as Greece, where it rose from 33.6 per 100 000 population in 1996 to 152 per 100 000 population in 2010.
During this time productivity has increased, driven in part by the increase in day-case surgery. The average length of stay (ALOS) (all causes) decreased across many European countries between 2000 and 2010. For example, the decline in the Netherlands was from 8.5 to 5.6 days, in the United Kingdom from 9.5 to 7.4 days, and in Greece from 8 to 6.6 days.
There is, however, considerable inter-country variation in length of stay. For example, the ALOS (for all types of patient) in Sweden was 15% lower than in the United Kingdom in 2011, with France having an ALOS 20% lower and Norway 36% lower. There are many reasons for this, reflecting different health system structures, organizations and economic contexts; however, the variation suggests that there may be opportunities to reduce length of stay in some settings, thereby potentially increasing productivity and making better use of available capacity. For example, widespread uptake of enhanced recovery programmes which combine a range of techniques to facilitate early discharge, and improvements in surgical techniques and care pathways which allow ambulatory surgery to be performed, both have the potential to dramatically impact productivity and efficiency. The impact of these changes could be significant; if ALOS in England fell by 15% by 2023, for example, with no further reductions in beds and all other things being equal, the NHS could treat around 18% more acute patients than it did in 2013/14 – an average annual increase of around 1.6% (Reference AlderwickAlderwick et al., 2015). However, as the cost of a patient recovering in a hospital bed is much less than that of undertaking a surgical procedure, the total cost would increase, possibly substantially.
Currently about 10 million patients undergo a surgical procedure in the English NHS each year, with consistent rises year on year, with a 27% increase seen in the number of surgical admissions between 2003/4 and 2013/14 (Royal College of Surgeons of England, 2017). The cost of elective (non-emergency) surgical care to the system is £16 billion (€20 billion). Out of these, around 250 000 patients are characterized as high risk (see below for a discussion of risk), representing 15% of all those who require inpatient surgical care and 80% of post-operative deaths (Royal College of Anaesthetists, 2016). Much of this risk is due to pre-existing long-term conditions and complex care needs, with the number of people living with multiple long-term conditions increasing steeply with age (Reference BarnettBarnett et al., 2012) and thus growing with an ageing population. For example, the 1.25 million people in the United Kingdom aged 85 or older are expected to treble in number over the next 35 years, and across Europe to rise from 5.1% of the population in 2014 to 12.2% by 2070 (Reference WilkinsonWilkinson et al., 2012; Eurostat, 2017). In England the number of people with multiple long-term conditions was expected to reach 2.9 million out of a population of 53 million by 2018 (5.5%) (Department of Health, 2012).
Improvements in perioperative management and surgical techniques have increased the numbers of people with pre-existing conditions deemed eligible for surgery and have, paradoxically, also been driven by the need to reduce the risk of complications. However, in many countries there is evidence of implicit ageism, with older people less likely to receive surgical interventions (Reference MarguliesMargulies et al., 1993). For example, a 2014 report showed that in England there was a 37-fold difference in rates of breast excision in patients with breast cancer over the age of 65, depending on where they live (Royal College of Surgeons & Age UK, 2014). This has led to calls to focus on physiological rather than chronological age (Reference KowdleyKowdley et al., 2012). However, among those who do receive surgery, older physiological age may be associated with a greater risk of complications which, when superimposed upon their already compromised physical state, mean that they may experience significant reductions in survival in the medium and longer term, and in their ability to return to their pre-operative function. Consequently, it is increasingly important that the scope of perioperative care extends beyond the immediate period of recovery from the acute effects of surgery.
This calls for a model of care that extends across specialties and professional groups and over time. Although the concept of perioperative medicine has been in use for more than a decade, until recently it has been applied only in a few selected areas and, even then, often incompletely. One area where it has been used is in cardiac surgery, where many facilities have established mechanisms to deliver efficient, multidisciplinary, patient-centred care. In contrast, most surgical specialties lack a unified approach to the prevention and management of perioperative surgical, medical, psychological, and social complications.
This is changing, with new models of perioperative care that emphasize improvement and consistency of outcomes for patients after surgery (Reference Kehlet, Delaney and HillKehlet, Delaney & Hill, 2015). These are fundamentally multidisciplinary, led by professionals who can take a system-wide approach and who can be drawn from a range of medical specialties, but most often anaesthesia, surgery, geriatric or internal medicine. This chapter will explore these models in detail and suggest opportunities and barriers to their future development.
The role of perioperative care
Perioperative medicine aims to deliver the best, multidisciplinary, person-centred care before, during and after surgery. There is a natural tendency to focus on major surgical interventions for the highest risk patients; however, the evolving models of care can be of benefit to the entire surgical population. As the vignettes in Box 8.1 reveal, there is huge variation in the perioperative care provided.
Box 8.1 Patient stories: traditional versus integrated care
Patient story: traditional non-integrated care
Stan is 72 years old with a history of high blood pressure and diabetes. He is a heavy smoker. He goes to his primary care provider as he has been losing weight recently and suffering with stomach pains. His GP refers him urgently to a surgeon, who does some further tests and confirms that Stan has bowel cancer. He recommends that he undergoes surgery and a few days later Stan comes back to the hospital to the pre-assessment clinic (PAC) and sees an anaesthetist, who is concerned that he may have chronic airways disease and that his diabetes is poorly controlled. The anaesthetist refers the patient back to the GP for further investigations but due to the urgent need for surgery, Stan arrives on the day of the operation without record of these tests. The surgery goes well and the cancer is removed; however, two days later Stan develops a chest infection and spends three days in the high dependency unit. His recovery is further complicated by a wound infection. After six weeks Stan is discharged from hospital to a rehabilitation facility and then home, where he requires carers three times per day.
Patient story: integrated care
Ruby is 81 years old with a history of cardiac disease and chronic kidney impairment. Following her complaints of symptoms of abdominal pain and bloating, her GP orders some blood tests and scans which raise the suspicion of ovarian cancer. She is referred to a “one-stop shop” clinic which takes place in a local health centre; there, she has a consultation with a surgeon and cancer specialist who offer her surgery and chemotherapy. On the same day she has further tests to assess her fitness for surgery, followed by a consultation with a cardiologist and an anaesthetist, where a shared decision is made to proceed to surgery. Records are kept electronically and shared with Ruby’s care providers. She is supported by a specialist cancer nurse who provides her with a single point of contact and coordinates her care. The operation goes well and she is electively cared for in the high dependency unit in order to provide early detection and treatment of any complications. Ruby’s recovery is uneventful and she returns home 10 days after the surgery, to begin chemotherapy shortly afterwards.
Perioperative care is often poorly coordinated, with weak systems of communication, focused on the individual practitioner and existing organizational structures. At worst, vital information about the patient is not shared between practitioners, resulting in untimely or delayed care and errors. Also, patients may undergo procedures in circumstances where they have not been made fully aware of the implications, resulting in dissatisfaction, poor outcomes, and a worsening of their general health status. However, where good perioperative medicine exists, the care provided is focused on the needs of the patient, employing individualized care pathways. The care is well coordinated and timely, and patients share in the decision-making process. Models of care vary (as explored below) but have common themes:
Multidisciplinary: involving doctors (both primary and secondary care), nurses, allied health care professionals, such as physiotherapists, occupational therapists, speech and language therapists and dieticians, social workers and administrative staff.
Crossing organizational interfaces: particularly primary care, secondary care and social care (Reference JohnsonJohnson et al., 2013).
Well led: this could be by doctors from different specialties, including anaesthesia, surgery, acute medicine, cardiology, geriatrics and others. Most commonly, anaesthetists lead perioperative teams since they are the most numerous hospital specialty and their current training model makes them natural candidates to do so. However, the interdisciplinary nature of good care means that there should be an emphasis on deploying the skills and expertise available in order to achieve optimal patient outcomes.
Robust communication: through the provision of a single point of contact for patients, surgeons and primary care providers, facilitated where possible by technology that enables the secure collection and exchange of patient data.
Evidenced-based with continual improvements in quality driven by robust audit data.
Patient-centred: respecting patients’ autonomy, listening to and respecting their wishes, and keeping them informed and involved with their care are the key tenets of patient-centred care which is now widely seen as an essential component of gold standard practice (Reference Epstein and StreetEpstein & Street, 2011). This model of care is gradually superseding its antiquated predecessors – doctor- and disease-centred care – and represents a paradigm shift from the patriarchal style of medicine which was practised for much of the 20th century.
Using appropriate technology: at present the majority of perioperative care is delivered in a visit-based system with the patients travelling to a hospital/clinic to be reviewed by the health professional who provided the index treatment. With the rise of digital health platforms and the ever-increasing availability of technology, there is potential for increasing amounts of perioperative care to be delivered remotely in a home-based system.
Multidisciplinary assessment and optimization – models of care
Geriatrician-led
Pre-operative CGA provided by a consultant geriatrician-led MDT involves multidomain assessment and optimization of the condition of the high-risk or older surgical patient (Reference PartridgePartridge et al., 2014; Reference MougMoug et al., 2016). This is particularly important given the increased frequency of risk factors and adverse post-operative outcomes in the older patient. The MDT can also support the surgical teams with post-operative medical care, focusing on functional optimization and discharge planning for both emergency and elective patients.
There is growing evidence that CGA is associated with improved process and outcomes such as decreased length of stay, reduction in delays and cancellations, and reduction in medical complications. One example is the Proactive care of Older People undergoing Surgery team (POPS) at Guy’s and St Thomas’ NHS Foundation Trust in London (Reference DhesiDhesi, 2012). The POPS team was designed to improve perioperative care and planning, address problems with poor rehabilitation and delayed discharges, and reduce the high rates of post-operative medical complications in elderly patients.
The Medical Research Council framework for complex interventions (Reference CraigCraig et al., 2008) was used to create, implement, and evaluate the POPS team. It is a geriatrician-led MDT which includes anaesthetic and surgical teams, therapists, social workers, and nursing staff. Patients with multiple co-morbidities, frailty and/or cognitive impairment are identified and referred to the POPS team. A CGA is then performed and a personalized perioperative care plan generated. Pre-operatively, risk factors and co-morbid conditions are identified and optimized, discussions are held with the patient, their family and the MDT to aid shared decision-making, and the appropriate level of post-operative care is determined. Post-operatively, regular geriatrician reviews and ward rounds take place and cases are discussed at POPS MDT meetings; there is also close communication between the POPS team and community/social services, facilitating quick and effective discharge to the community.
Around 1000 elective patients are seen by the POPS team annually, and the team also reviews any appropriate patients admitted to the surgical wards as an emergency. The impact of this service has been impressive, with significant reductions in medical complications, including pneumonia and delirium, pressure sores and delayed mobilization, and in length of stay in hospital (Reference Dhesi and SwartDhesi & Swart, 2016). Similar findings were obtained with the Systematic Care Older Patients undergoing Elective Surgery (SCOPES) service at Nottingham University Hospitals NHS Trust in England (Reference Dhesi and SwartDhesi & Swart, 2016).
Anaesthetist-led
Patients are triaged (based on estimated perioperative risk of mortality), with higher-risk patients attending an anaesthetist-led clinic. The clinician employs a range of clinical assessment and physiological testing (e.g. cardiopulmonary exercise testing) to provide an objective assessment of the risks and benefits of surgery. The clinic is supported by a range of health care professionals to provide expert advice and support (including organ specialists, therapists, and allied health care professionals).
Assessment of fitness for surgery
The assessment of fitness for surgery, and therefore risk of post-operative complications, is fundamental to perioperative care. There is strong evidence for an association of objectively measured fitness with outcomes from major surgery: in general, fitter people do better and this is perhaps even more important than chronological age (Reference SnowdenSnowden et al., 2013).
Assessing fitness allows an assessment of risk, thereby facilitating a discussion leading to a shared decision about whether and how the patient should proceed to treatment. Simple methods have been used to gauge cardiorespiratory fitness, for example using patient questionnaires to ascertain the person’s maximal level of daily activity and the 6-Minute Walk Test where the distance walked by the person predicts morbidity and mortality.
Reliably and objectively testing and quantifying fitness is increasingly becoming a prerequisite for major elective surgery, particularly in those patients known to have risk factors such as chronic diseases or obesity. Cardiopulmonary exercise testing (CPET) uses an incremental exercise test (usually on a treadmill or exercise bike) to generate safe, accurate, and repeatable data that correspond with the demands of major surgery on the body (Reference Carlisle J and SwartCarlisle & Swart, 2007).
Barriers to greater use of CPET include the costs of setting it up, the routine operation of the equipment, and the need for skilled expertise to conduct the assessments and interpret the test results. Often the test is conducted by physiologists, supported by clinicians. Many anaesthetists are now trained to make these assessments and there is growing recognition that the cost of managing post-operative deterioration in patients who have not been thoroughly assessed and their condition optimized often outweighs the costs of providing the tests.
Assessment of fitness can be done as part of comprehensive pre-operative screening. This can be nurse-led and most hospitals in England also have consultant anaesthetist-led clinics to assess more complex patients. At this point in the patient journey blood investigations and assessments of the function of other body systems (heart, lungs and kidneys) are also done and patients may be referred for specialist opinions.
Historically, pre-operative testing was largely performed on the day before surgery and it was left to the admitting junior doctor to decide which tests should be performed, leading to significant variability in pre-operative testing and creating the potential for significant patient harm. With the shift towards PACs this process has become more rigorous and standardized, with significant improvements to patient care. PAC is now widely accepted as the gold standard of care across Europe, exemplified by a law passed in 1994 in France which stipulates that a PAC visit must be completed at least two days before any admission for elective anaesthesia (Reference Flynn and SilvayFlynn & Silvay, 2012). However, the PAC approach has its own pitfalls as there is a tendency towards over-testing and delays to treatment as incidental abnormalities are followed up and investigated further. The recognition of risk of patient harm due to unnecessary investigations, and delayed definitive treatment of the initial pathology, have led to a trend of more selective pre-operative testing (Reference FeelyFeely et al., 2013; Reference Bohmer, Wappler and ZwisslerBohmer, Wappler & Zwissler, 2014).
This is exemplified by the joint recommendations from the German societies of Anaesthesiology, Internal Medicine, and Surgery (DGAI, DGIM, and DGCH), published in 2010. These recommendations highlight the importance of precise medical history and examination, and suggest a standardized scheme to identify factors which may necessitate further testing. If there are no such factors and the procedure to be performed is low risk, the authors claim that no further testing is needed. The recommendations address patient- and procedure-specific indications for pre-operative testing such as laboratory tests, electrocardiogram, X-ray, echocardiogram, pulmonary function and extended cardiac testing. The aim is to reduce unnecessary investigations which have been shown to have no beneficial effect on perioperative patient safety, thereby streamlining the pre-operative assessment process and reducing costs and delays to treatment. A national survey of German anaesthesiologists performed in 2013 suggests that the recommendations have been effective, with 39.1% of anaesthetists stating that they now conduct fewer ancillary tests (Reference Dhesi and SwartDhesi & Swart, 2016).
Risk optimization and lifestyle modification
Once a comprehensive assessment of risk has been undertaken, and as part of the multidisciplinary approach, measures to optimize the chances of a good outcome from surgery can be decided in collaboration with the patient. Through liaison with other professionals, control of chronic diseases such as diabetes, asthma and heart disease can be optimized. In addition, lifestyle advice can be given and other services can be sign-posted, including smoking cessation (Reference McKee, Gilmore and NovotnyMcKee, Gilmore & Novotny, 2003), alcohol reduction, weight loss, and dietary and nutrition advice.
Recently, the concept of “prehabilitation” has been adopted; this consists of a group of interventions that are introduced into the patient pathway pre-operatively, aimed at enhancing a person’s ability to withstand the stress of major surgery and achieving lasting beneficial effects on recovery (Reference GillisGillis et al., 2014). Although the choice of timing must be balanced with the risk of delaying surgery (particularly in cases where cancer is suspected or diagnosed), it is evident that improvement in pre-operative fitness will optimize the chances of a successful outcome from surgery.
One major intervention, with increasing evidence of benefit (although not consistently), is exercise therapy (Reference Snowden and MintoSnowden & Minto, 2015). There is overwhelming evidence that physical activity improves the health of people with chronic conditions and also prevents many common diseases (Academy of Medical Royal Colleges, 2015). This is also true in the context of the pre-operative phase but it is important that an exercise programme achieves a high level of adherence, with support from the appropriate health professionals. Several studies have shown significant improvements in length of stay and reductions in post-operative complications following cardiac surgery in patients who have used prehabilitation programmes (Reference HoogeboomHoogeboom et al., 2014). There is also some evidence that prehabilitation can benefit patients undergoing thoracic, abdominal and major joint surgery, particularly in high-risk patients with poor pre-operative condition (Reference HoogeboomHoogeboom et al., 2014).
Unfortunately, the current body of evidence surrounding prehabilitation is skewed towards low-powered randomized controlled trials in healthy individuals, whereas the greatest benefit is likely to be seen in high-risk patients. Furthermore, there is a lack of consensus regarding the most efficacious exercise programme, for example whether it should be resistance or aerobic training, and whether it should be delivered in a hospital or home-based environment (Reference HoogeboomHoogeboom et al., 2014).
Pre-operative risk assessment and shared decision-making
The concept of shared decision-making (SDM) is attracting increasing attention in many countries (Reference BlancBlanc et al., 2014). It represents a shift from antiquated paternalistic medicine to a patient-centred model, and is especially pertinent in the field of perioperative medicine as decisions surrounding surgeries can have life-changing consequences.
SDM is defined as “a broad term that describes [a] collaborative effort between the physician and patient to make an informed clinical decision that enhances the chance of treatment success as defined by each individual patient’s preferences and values” (Reference Slover, Shue and KoenigSlover, Shue & Koenig, 2012). It involves the provider offering information on possible treatment modalities, including risks, benefits and alternatives, and the patient sharing their relevant values and preferences. A mutual decision can then be made on a treatment plan most likely to deliver the best outcome with respect to these factors, whether it is a choice between different types of surgery, or a choice between surgery and conservative management. This type of patient empowerment has several benefits, including decreased indecision and decisional conflict, and improved patient knowledge and participation in treatment decisions. It allows for care to be tailored to the needs of individual patients and can increase patient satisfaction.
Box 8.2 Patient story: shared decision-making
Anil is 78 years old and undergoes routine screening for abdominal aortic aneurysm (AAA). This reveals that he has an 8 cm aneurysm and so is referred to a vascular surgeon. Since the risk of it rupturing is around 50% per year, Anil is offered an open surgical repair and is then seen in a PAC. Anil also has heart failure and emphysema, and his health has been deteriorating for a while. In the clinic he undergoes a CPET, among other tests, which reveals that he has a poor physiological reserve. Following this, he has an hour-long discussion with an anaesthetist, where the risks and benefits of having surgery are discussed. Anil understands that he is at high risk of complications if he has surgery, and will be unlikely to get back to his pre-operative level of function. Following a period of time to reflect and discuss with his family, he returns to the clinic and decides, along with his care providers, not to proceed with surgery and instead to adopt a conservative approach.
SDM has been shown to affect patient decision-making, with a tendency to choose more conservative therapeutic options, particularly in orthopaedic patients (Reference Slover, Shue and KoenigSlover, Shue & Koenig, 2012). It has also been postulated that it can improve equity in health care, as the physician is beholden to explain alternative treatments that may have been unknown to certain groups of patients (Reference ElwynElwyn et al., 2010).
Although SDM is increasing and is seen as the gold standard of patient care, uptake has been limited, due in part to the perception that it is an expensive and time-consuming endeavour that requires an investment in training. However, the impact on consultation time is usually minimal, and the growth of digital technology means that decision-making aids can be produced and disseminated at relatively low cost (Reference ElwynElwyn et al., 2010).
Box 8.3 Torbay Hospital Clinic shows financial viability
The surgical risk assessment and SDM clinic at Torbay Hospital, South Devon Healthcare NHS Foundation Trust, UK, is an excellent example of SDM in operation. Approximately 900 high-risk patients per annum are referred to the SDM clinic to have a comprehensive risk assessment and an in-depth consultation regarding their treatment options. The aim is to empower patients to make more informed decisions on their care and to allow perioperative care planning including allocation of resources such as high dependency and intensive therapy units. This model has been shown to be financially viable, with an estimated £382 (€480) reduction in total cost of care for high-risk patients undergoing bowel cancer resection.
Care bundles – enhanced recovery
In recent years enhanced recovery programmes (ERP) have become increasingly popular, with a substantial body of evidence demonstrating their ability to improve post-operative outcomes and to reduce length of stay. Common components of ERP include pre-operative counselling, planning, and nutrition, usually delivered in an outpatient clinic setting, and after the patient has been admitted to hospital intra-operative management such as guided fluid therapy, maintenance of normothermia (a normal state of temperature) and use of minimally invasive approaches. Post-operatively, initiatives such as early mobilization, prompt resumption of normal diet, innovative analgesic techniques and proactive discharge planning are employed.
Enhanced recovery after surgery (ERAS) for colorectal surgery was first described by Professor Henrik Kehlet in Denmark during the 1990s (Reference FearonFearon et al., 2005). The principles of this programme are shown in Figure 8.2. Subsequently the same elements have been applied to other surgical specialties, including orthopaedics and gynaecology, and have developed into the international ERAS society with centres of excellence in Canada, Denmark, France, Spain, Sweden and the United Kingdom.

Figure 8.2 Components of enhanced recovery after surgery (ERAS) pathway
Note: NSAIDs: non-steroidal anti-inflammatory drugs
There have been several studies showing improved outcomes, such as length of stay and morbidity, when ERAS is used (Reference AdaminaAdamina et al., 2011). Despite this body of evidence, uptake of the programme and adherence to its principles have been relatively low; patient-, staff- and practice-related factors as well as a lack of resources have been suggested as potential barriers to entry which must be overcome if widespread implementation is to be achieved (Reference Segelman and NygrenSegelman & Nygren, 2014). Furthermore, there is a paucity of evidence on the effect of ERAS on patient-related outcomes such as quality of life and cost-effectiveness, and furtherresearch is indicated in these areas to quantify the true value of ERAS and other ERPs.
Research on post-operative outcomes in orthopaedic patients after use of ERPs has been promising; however, there is a wide variation in the components of the programmes evaluated, with substantial variations in results (Reference IbrahimIbrahim et al., 2013). Development and widespread implementation of a standardized enhanced recovery protocol would help in disseminating best practice. Reference StowersStowers et al. (2014) suggested a protocol for enhanced recovery after hip and knee arthroplasty described in Table 8.1 below, which shares many features and principles of the ERAS protocol for colorectal surgery while being tailored towards the needs of patients undergoing orthopaedic surgery.
Table 8.1 A proposed enhanced recovery protocol for elective total hip and knee arthroplasty
| Pre-operative care |
|
| Intra-operative care |
|
| Post-operative care |
|
Ambulatory surgery
Over the past 20 years there has been a significant increase in productivity driven by the rise in proportion of operations performed as ambulatory cases. Since 2005 England has employed financial incentives to switch to ambulatory surgery, driven by the rollout of a system of payment by results (PbR) for all elective procedures. As day-case patients cost less to treat than patients who stay overnight as inpatients (in 2013/14 the average day-case cost was £698 (€872) and the average inpatient-case cost was £1367 (€1708)), the increasing number and proportion of day cases has helped to reduce overall costs per case. In effect, by treating more patients as day cases, by 2013/14 the NHS had saved around £2 billion (€2.5 billion), equivalent to an average saving over the 15 years since 1998/9 of around 1.4% per year of the total spend on elective day and inpatient care (Reference ApplebyAppleby, 2015).
Several other factors have facilitated the shift towards day surgery including: cultural change, availability of regional anaesthesia, faster-acting anaesthetic, analgesic (pain-killer) and antiemetic (anti-sickness) drugs, organizational improvements, i.e. day-case units, minimally invasive surgery, and changing patient expectations.
Workforce
Current anaesthetic workforce model
In most countries anaesthetists form the largest single hospital medical specialty and their skills are used in all aspects of patient care (Royal College of Anaesthetists, 2016). While the perioperative anaesthetic care of the surgical patient is the core of specialty work, the scope of anaesthetic practice can extend to:
The pre-operative preparation of surgical patients
The resuscitation and stabilization of patients in the ED;
Pain relief in labour and obstetric anaesthesia;
Intensive care medicine, although increasingly this is becoming a specialty in its own right with a separate training and accreditation structure;
Varying age groups: neonatal, paediatric and adult;
Transport of acutely ill and injured patients;
Pain medicine;
The provision of sedation and anaesthesia for patients undergoing various procedures outside the operating theatre.
In the main, services are delivered by specialists, placing large demands on the current workforce in some countries. With an ageing population, many with multiple co-morbidities and requiring more complex surgical procedures, there are projections of at least a 25% increase in demand in the United Kingdom by 2033 (Centre for Workforce Intelligence, 2015).
Non-physician anaesthetists
In some European countries anaesthesia is currently delivered by non-physicians, albeit with supervision by consultants (Reference VickersVickers, 2000). Box 8.4 illustrates some examples.
Box 8.4 Employment of non-physicians to give anaesthetics
Sweden: Anaesthetic nurses (ANs) are all drawn from nursing backgrounds. They may enter AN training directly after graduating as a nurse, although most also have a minimum of two years’ practical nursing experience. The AN training programme lasts for one year. Physicians supervise a variable number of theatres and for the most part physicians must be present at the induction and reversal of anaesthesia.
The Netherlands: Anaesthetic nurses are drawn from either nursing backgrounds or straight from school with good exam results; the former group undergo two years’ training and the latter three years’ training. Physicians normally supervise two operating theatres and must be present at the induction and reversal of anaesthesia. An AN must be present at every anaesthetic.
The United Kingdom: The main groups eligible to commence training as a physician’s assistant (anaesthesia) or PA(A) are registered health care professionals with at least three years’ clinical experience and/or degree level studies, or graduates with a biomedical science or biological science degree. Typically PA(A)s work in a 2:1 model where there is one consultant anaesthetist supervising two PA(A)s or a trainee anaesthetist and a PA(A) simultaneously in two operating theatres. PA(A)s are also used to reduce theatre downtime, leading to increased throughput on lists and theatre utilization, pre-operative assessment, exercise testing, provision of sedation to other specialties, cardiac arrest teams, and for regional and local anaesthetic provision. This model has not, however, been widely adopted, with only around 120 PA(A)s trained by 2015, but this is projected to increase with plans by the Department for Health and Social Care to fully regulate PA(A)s.
Perioperative care workforce model
As has been outlined above, optimal perioperative care is delivered by a well led MDT, focused around the patient. In many acute care settings the components of the team already exist but are often fragmented and exist in isolation with poor communication between them. Members of the perioperative MDT include:
Doctors (both primary and secondary care), including:
➢ Anaesthetists
➢ Surgeons
➢ General practitioners
➢ Care of the Elderly physicians
➢ Specialist physicians such as diabetologists, cardiologists and respiratory physicians
➢ Radiologists
➢ Intensivists
Nursing staff
Physicians’ assistants
Allied health care professionals such as:
➢ Physiotherapists
➢ Occupational therapists
➢ Speech and language therapists
➢ Dieticians
➢ Social workers
Administrative staff
Training
High quality and well organized training is integral to the future of perioperative care. Clinical training for physician anaesthetists combines the acquisition of clinical knowledge, skills and behaviours, with a broad range of clinical leadership and management skills necessary. In addition, clinicians are now increasingly required to have at least a working knowledge of improvement science, discussed in more detail later in the chapter, and the ability to apply relevant research into their clinical practice.
As has been discussed, good quality perioperative care transcends traditional boundaries in terms of clinical specialties and across organizational forms. This requires that training adapts too, whereby clinicians from different specialties such as anaesthesia, surgery and medicine acquire similar skills and knowledge in order to collaborate more closely. Post-graduate qualifications, such as the UCL Perioperative Medicine MSc, are open to all health care professionals thus promoting true multidisciplinary working (https://www.ucl.ac.uk/surgery/courses/msc-perioperative-medicine).
Barriers to delivery of perioperative care
There is a projected shortfall of physician anaesthetists, as well as other specialties. In the United Kingdom changes to medical and nursing training has resulted in a deficit of applications for training posts, meaning that some roles within the perioperative team are left unfilled. This threatens the sustainability of the workforce and poses safety challenges in terms of rota gaps, unmet service need, and increased requirement for locum or ad-hoc positions. Recently, there has also been difficulty filling positions for higher training in anaesthesia (http://www.rcoa .ac.uk/news-and-bulletin/rcoa-news-and-statements/rcoa-links-low-fill-rates-inadequate-supply-of-trainees). However, this situation may offer an opportunity for the design and implementation of new models of care (as discussed below) and improved patient outcomes.
Good quality perioperative care transcends traditional organizational forms and systems. For example, patients will often be cared for by their primary surgical team in conjunction with other medical and non-medical specialists in primary and secondary care. This demands good communication. Due to difficulties sharing information in health systems, however, information is often not passed on or made available when it is required. This can lead to replication, waste and, at worst, error. In addition, further barriers to the provision of good quality care, as with other health care settings, include inter- and intra-provider variation, processes lacking reliability, and lack of standardization. Addressing these issues is best done using improvement science principles (see below).
The future
Health care systems are facing challenges from the ageing population with a greater prevalence of chronic co-morbid conditions, and the opportunities to intervene provided by advances in medicine. However, with these challenges comes the opportunity to innovate and implement transformational change to the way that we deliver perioperative care. Consideration also needs to be given to the appropriateness of costly, complex surgical therapies, and whether centrally funded health care systems should be expected to provide these with the possible consequence of less available resources for more established therapies with proven cost-effectiveness. Policy-makers have a responsibility to engage with the public in discussion, and as a society, in order to determine where each health care system’s priorities lie within a cost-constrained environment.
For the vast majority of patients undergoing a surgical procedure, the episode is uncomplicated with good outcomes. However, increasing numbers of patients are being exposed to greater risk through a combination of their pre-existing condition, the surgical treatment itself, or issues regarding the delivery of care. The development of the perioperative care model offers a solution that can optimize the chances of a good outcome, particularly for high-risk patients.
Excellent perioperative care is, in part, already being offered in an individualized manner with the ability to draw on expertise and resource, as and when the patient needs it. This is described in Figure 8.3.
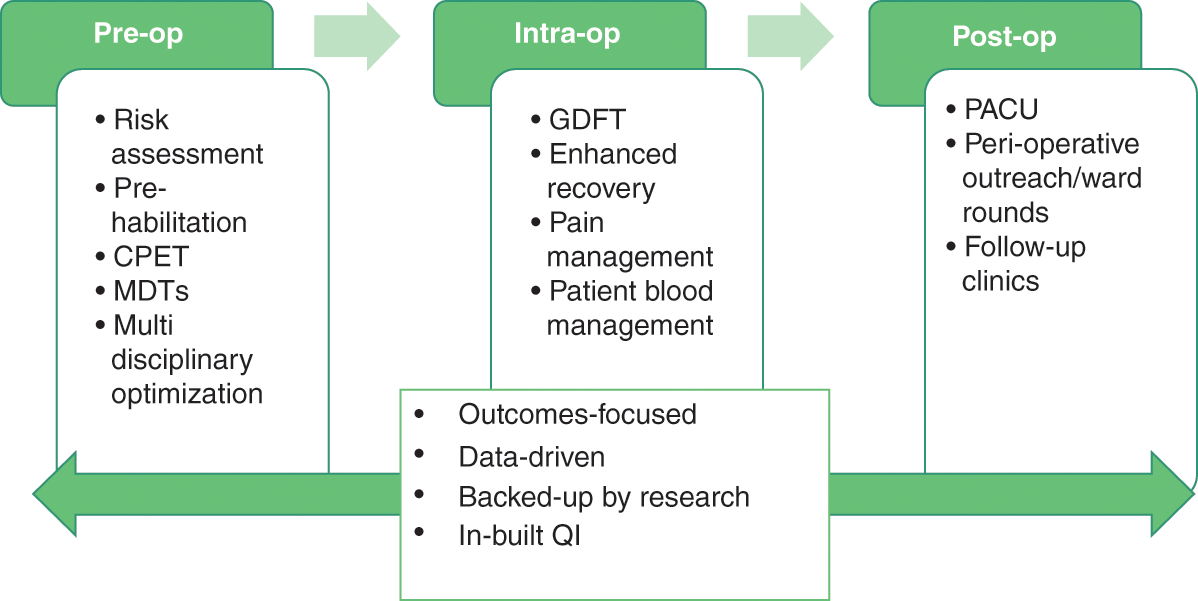
Figure 8.3 Individual perioperative care pathway
Notes: CPET: cardiopulmonary exercise testing; MDT: multidisciplinary team; GDFT: goal directed fluid therapy; PACU: post-anaesthesia care unit; QI: quality improvement
There are a number of enablers to the provision of quality, coordinated perioperative care including: technology, research and improvement science, and improved models of care.
Technology
Although the use of technology in medicine is growing, we have yet to truly tap into its full potential. Advances in genomics, telemedicine, robotics, virtual and augmented reality, artificial intelligence and electronic medical records have the potential to cause a paradigm shift in the delivery of perioperative care. As these advances in computing continue at an exponential rate, the challenge for perioperative care providers is to find new and effective ways to harness technology to improve outcomes for their patients. However, this is often costly and demands front-loaded funding. Even if this results in cost-savings and efficiencies in the medium to longer term, financial cuts mean that technology programmes face significant challenges.
Increasingly data are being digitized, which can then be analysed, shared and used to drive quality improvement. For example, powerful machine-learning algorithms could be applied to ascertain which patient, provider and procedural characteristics will impact most on their post-operative outcomes, or be used to supply live decision support to PACs enabling selection of appropriate pre-operative tests and a bespoke prehabilitation package. Digitized data can be seamlessly and securely transferred between stakeholders, including hospitals, primary care providers, research and academic institutions, and patients themselves. This increased availability of information presents manifold opportunities for research and identification of best practice, allows for safer and more efficient delivery of care through the avoidance of repetitive data gathering, and can empower patients by giving them ownership of their medical records.
Innovation in anaesthesia in the past 10 years has centred on a number of aspects:
Airway equipment, for example video laryngoscopy.
Ultrasound machines, which are now in widespread use in anaesthesia for use in diagnosis, vascular access and regional anaesthesia where needles are inserted under direct vision and local anaesthetic drugs are deposited around nerves.
The increasing profile given to human factors and systems design, particularly in the management of clinically challenging, time-sensitive situations. The Clinical Human Factors Group (CHFG), founded by Martin Bromiley, a pilot whose wife died as a direct result of medical error, is at the forefront of this (Clinical Human Factors Group, 2018); mitigation of these important sources of error and risk to patients has been increasingly recognized as having a significant impact in perioperative care. Techniques implemented to help control human factors include: application of learning from other sectors, such as the aviation industry, human factors design and engineering, and improved simulation and team working techniques (Reference Weinger and GabaWeinger & Gaba, 2014).
Drugs, especially those that enable enhanced recovery; for example, sugammadex is a novel reversal agent for some muscle relaxant drugs, although its use is limited by its relatively high cost.
Increasing awareness around the environmental impact of anaesthesia, particularly that of the volatile agents and nitrous oxide, which are greenhouse gases, is driving increased use of total intravenous anaesthesia (TIVA). There is also increasing evidence that TIVA with propofol is associated with decreased reoccurence of malignancy following cancer surgery, the mechanism of which is unclear.
Research and improvement science
The evolution of perioperative medicine needs to occur in parallel with the development of the research agenda, with a particular focus on translating discoveries and advances into meaningful changes in care delivery and outcomes for patients more rapidly. At present, basic scientists are directing their efforts at understanding the biological mechanisms underlying post-operative morbidity, and why its impact should be so sustained. Clinical triallists are evaluating interventions to mitigate adverse outcomes in pragmatic studies involving tens of thousands of patients. It is recognized that unplanned variations in structures and processes between health care providers have a significant impact on outcomes after surgery; thus initiatives within the field of improvement science are focusing on this area.
Improvement science in health care is a concept that has been generating increasing interest over the past few years, as health care providers, academics, and front-line staff look to improve care delivery and generate practical real-life learning and approaches to aid development and dissemination of best practice. However, it is still in what some authors call the “pre-paradigm phase of emergence”, which in part means there is an absence of an agreed definition (Reference Marshall, Pronovost and Dixon-WoodsMarshall, Pronovost & Dixon-Woods, 2013). Commonly the term is used to describe the application of the principles of W Edwards Deming to health care. A broader definition of improvement science is that it is a coordinated approach to quality improvement (QI), which aims to create practical learning that can make a timely difference to patient care (Reference Marshall, Pronovost and Dixon-WoodsMarshall, Pronovost & Dixon-Woods, 2013).
Improvement science is built around the robust scientific assessment of QI projects, including the design, deployment, and assessment of complex multifaceted interventions. If applied correctly, it adds considerable external validity to the results of these interventions, allowing them to be taken up more rapidly by other institutions and health care systems, and breaking down silos of best practice. The process of rapid testing and improvement helps to generate confidence in the proposed changes among the stakeholders.
Furthermore, this approach helps to mitigate the risks caused by poorly planned and unscientific QI projects, which are not evidence-based, nor appropriately monitored to ensure positive impact on patients. Therefore improvement science is critical to maximizing the impact of QI interventions and effective use of resources as health care systems adjust to the demands of modern and future medicine (Reference Varkey, Reller and ResarVarkey, Reller & Resar, 2007).
There has been a lot of research looking at QI interventions in perioperative care. This is because although significant advances have been made in recent years, there are an estimated 234 million surgical procedures performed annually around the world with considerable risk of patient harm. A recent systematic review of QI research in perioperative care using techniques such as audit and feedback, Plan-Do-Study-Act (PDSA) cycles, and methodologies such as Lean Six Sigma which are used to remove waste and reduce variation, demonstrated that although there were many studies in this field, the reporting was suboptimal, leading the authors to conclude that we need to orientate research towards QI and improvement science in perioperative care and develop a comprehensive, coherent, and valid framework for the design and reporting of QI interventions in this field (Reference JonesJones et al., 2014).
Recognition at all levels of health care from policy-makers, commissioners, and organizational boards to front-line staff that QI should be part of an organization’s daily business is essential in order that a culture of continuous improvement is sustained. Improvement work performed as part of teams is most effective but in order for this to occur, it is important that time and resources are dedicated to it; however, in many instances, in part due to the sustained pressures of delivering against rising demands, QI is regarded as a non-mandatory activity.
Fundamental to developing a supportive and nurturing culture that encourages innovation and improvement is the adoption of coaching. An example of an effective health care system that has embedded coaching into its systems is the Sheffield Microsystem Coaching Academy (Sheffield Microsystem Coaching Academy, 2018).
Collaboration between academics and clinicians is flourishing with the recognition that “big data” and nationally funded audits of processes and outcomes can be used to study and deliver improvements in these outcomes.
Developing evidence can be combined with significant advances in technology, digital health, patient empowerment and anaesthetic techniques to produce gold standard models of care. These models of care and existing examples of best practice should be scaled across health care systems in order to reduce variability in standards of care delivered and to improve patient outcomes.
Improved models of care
In the immediate future efforts to improve perioperative care should include the dissemination of existing best practice – for example, enhanced recovery programmes have been shown to improve post-operative outcomes; however, their use has remained sporadic. This is a prime example of where best practice, validated by research, could be scaled to positively impact the lives of vast numbers of patients. These programmes have the potential to bring greater improvements by taking a more holistic approach, including nutrition and prehabilitation, and by utilizing the power of technology to improve patient engagement.
Perioperative care could also be rapidly improved by the uptake and dissemination of shared decision-making principles, empowering patients to take more charge of their care journeys, and putting patient preference at the centre of perioperative care. Where digital patient information resources are created, these should, where possible, be made open source and widely disseminated to spread best practice in a cost-effective manner.
As we redesign our services and meet the demands of 21st-century medicine, it is important to embrace the truest form of disruption, which is taking techniques and learning from different sectors and applying it in innovative ways to solve the problems we face. One good example of this would be the application of engineering and manufacturing principles such as lean methodology to health care systems. This would develop superior, more efficient processes, with fewer delays for the patient and higher productivity for the hospital, and consequently free up capacity to treat more patients and generate more funding (Reference Dahlgaard, Pettersen and Dahlgaard-ParkDahlgaard, Pettersen & Dahlgaard-Park, 2011), which could then be reinvested in order to fund the array of technologies discussed elsewhere in this chapter. Furthermore, when we are implementing new models of care or improving existing ones, it is important that we utilize the improvement science techniques described above in order to ensure maximum efficiency and continuous improvement, and create data with external validity.
When health care providers look further ahead and plan delivery of perioperative care in the mid-21st century, it is important that they embrace the shift towards patient-centred, home-based care, and integrate the necessary infrastructure to utilize the myriad of technological advances that are already presenting themselves (Reference RosenRosen et al., 2016).
It is possible that much preoperative assessment could be completed remotely through the use of telemedicine consultations, at home diagnostic equipment, and digital educational resources to deliver prehabilitation and relevant information for the patient. This type of remote working will free up space in hospitals and will allow health care professionals to work more efficiently, but it also require substantial staff training, organizational culture change, and investment in the necessary equipment and software to make it a reality.
The operating theatres of the future should allow for advanced surgical equipment such as robotics and imaging devices. Digital connectivity will be paramount to allow incorporation of remote multidisciplinary input, access to electronic health records, and integration of machine learning and artificial intelligence clinical decision-making and technical assistance tools. Robotic surgery has also created the interesting concept of remote operating; conceivably the principal surgeon could operate from a console thousands of miles away from the patient, allowing their expertise to be shared on a global scale. Fully autonomous robotic operating devoid of any requirement for human input is viewed by many authors as being the future of surgery, with the potential to become the standard operative modality and revolutionize perioperative care (Reference MoustrisMoustris et al., 2011).
The transition to these improved models of care will be challenging and, due to the level of infrastructural improvements required, will be likely to require substantial up-front investment. However, there are some favourable societal trends emerging, for instance the general public are increasingly becoming digitally connected, with most households in developed countries now having internet access, and smartphones and other devices being readily available. This technological environment is perfectly primed to connect patients and health care providers and can facilitate the patient-centric and home-based care of the 21st century.
Furthermore, the previously discussed challenges that health care is currently facing, with rising demand for services and financial constraints, represent significant drivers for change; the need to innovate in order to improve efficiency and modernize care delivery has never been greater. This is well demonstrated in the United Kingdom by the NHS five-year forward view policy document (NHS England, 2016), which puts innovation and new models of care at centre stage.