INTRODUCTION
Referral bias occurs as a result of systematic selection of patients from tertiary-care centres for inclusion in studies as the clinical features of patients presenting to tertiary-care referral centres with a particular illness differ from those in the community or general population [Reference Melton1]. Population-based studies lack referral bias as they include all patients with the disease of interest within a well-defined geographic area whether they present to primary-, secondary- or tertiary-care centres for medical care.
Only a few previous studies have evaluated the influence of referral bias on the clinical features of patients with infectious diseases, including infective endocarditis [Reference Steckelberg2, Reference Kanafani3]. We have previously demonstrated the effect of referral bias on the in vitro antimicrobial resistance rates of Pseudomonas (Ps.) aeruginosa bloodstream isolates in age- and gender-matched referral and population-based cohorts [Reference Al-Hasan4]. In the current study, we evaluated the influence of referral bias on the demographic and microbiological characteristics of patients with Gram-negative bloodstream infection (BSI) by comparing a referral cohort of patients with Gram-negative BSI to a population-based cohort within the same geographic area and time period. We aimed to (i) determine the influence of age and gender on referral patterns and (ii) examine the impact, if any, of the microbiological aetiology of Gram-negative BSI on referral.
MATERIALS AND METHODS
Settings
Olmsted County is located in southeastern Minnesota and has a population of 124 277 according to the 2000 census [5]. With the exception of a lower prevalence of persons who inject illegal drugs, a higher prevalence of middle-class persons and a higher proportion of persons employed in the healthcare industry, the population characteristics of Olmsted County residents are similar to those of USA non-Hispanic whites [Reference Steckelberg2, Reference Melton6]. The Rochester Epidemiology Project (REP) is a unique medical records-linkage system that encompasses care delivered to residents of Olmsted County, Minnesota [Reference Melton6]. The microbiology laboratories at Mayo Medical Center and Olmsted Medical Center are the only two laboratories in Olmsted County. These two medical centres are geographically isolated from other urban centres as previously described [Reference Al-Hasan4, Reference Tleyjeh7], which increases the likelihood that residents get their healthcare at the local facilities, rather than seeking healthcare at a distant geographic location.
There are three hospitals in Olmsted County. The two Mayo Clinic-affiliated hospitals, St Mary's and Rochester Methodist, are large tertiary-care centres that combine for over 1950 licensed beds and provide care for both referral and local patients in a wide variety of medical and surgical subspecialties. The third hospital, Olmsted Medical Centre, is a community-based hospital that provides primary care to local residents.
Case ascertainment
The population-based and referral cohorts were defined based on patient residency status, rather than the hospital where they received care. The population-based cohort consists of local (limited to Olmsted County residents) patients who were treated at any of the three hospitals. The referral cohort consists of patients who lived outside Olmsted County and received care at any of the three hospitals.
All residents of Olmsted County, Minnesota, were eligible for inclusion in the population-based cohort of the study as we used complete enumeration of the Olmsted County population from 1 January 1998 to 31 December 2007. After the institutional review boards of Mayo Medical Center (Rochester, Minnesota) and Olmsted Medical Center approved the study, we used the microbiology laboratory databases at both institutions to identify all episodes of Gram-negative BSI during the study period. Using the REP tools, we identified residents of Olmsted County, Minnesota, for inclusion in the population-based cohort; patients living outside Olmsted County formed the referral cohort. The referral cohort could be either self-referred or physician-referred to Mayo Medical Center hospitals for management of Gram-negative BSI. The primary investigator (M.N.A.) reviewed the medical records of all patients to confirm the diagnosis, determine patient residency status and obtain demographic and microbiological features.
Case definition
Gram-negative BSI was defined as the growth of any aerobic Gram-negative bacillus in a blood culture. Monomicrobial Gram-negative BSI was defined as the growth of only one species of Gram-negative bacillus in a blood culture. Coagulase-negative staphylococci, Corynebacterium spp. and Propionibacterium spp. were considered blood culture skin contaminants when isolated with Gram-negative bacilli, and in cases where any of these were recovered, the BSI was not designated as polymicrobial.
The detailed blood culture methods used have been described elsewhere [Reference Uslan8, Reference Al-Hasan, Eckel-Passow and Baddour9]. Blood cultures were processed using standard microbiology techniques according to the Clinical and Laboratory Standards Institute (CLSI). The microbiology laboratories at the Mayo Medical Center, Rochester and Olmsted Medical Center are certified by the College of American Pathologists.
Statistical analysis
Multivariable logistic regression analysis was used to examine the impact of age, gender and calendar year on referral patterns of patients with Gram-negative BSI. Age was categorized into five groups (0–18, 19–39, 40–59, 60–79, ⩾80 years). Multivariable logistic regression analysis was also used to evaluate the effect of microbiological aetiology of Gram-negative BSI on referral, after adjusting for age group and gender. Odds ratios (OR) with 95% confidence intervals (CI) were calculated to indicate the strength of association with referral. JMP (version 8.0, SAS Institute Inc., USA) was used for statistical analysis. The level of significance for statistical testing was defined as P<0·05 (two-sided).
RESULTS
We identified 846 Olmsted County residents with first episodes of monomicrobial Gram-negative BSI between 1998 and 2007. The median age of patients in this population-based cohort was 68 years [interquartile range (IQR) 47–81] and 56·5% were females.
During the same time period, 2919 patients with first episodes of monomicrobial Gram-negative BSI living outside Olmsted County were treated at Mayo Medical Center hospitals in Rochester, Minnesota. The median age of patients in this referral cohort was 63 years (IQR 49–74) and 44·0% were females.
There was no detectable change in referral patterns over calendar years 1998 to 2007 (OR 0·99, 95% CI 0·97–1·01 per year). Table 1 demonstrates the influence of age group and gender on referral of patients with Gram-negative BSI. Compared to the reference age group of 0–18 years, elderly patients aged ⩾80 years were less likely to be referred (OR 0·43, 95% CI 0·30–0·62). Females with Gram-negative BSI were also less likely to be referred than males (OR 0·63, 95% CI 0·53–0·74).
Table 1. Demographic characteristics of patients with Gram-negative bloodstream infections in referral and population-based cohorts
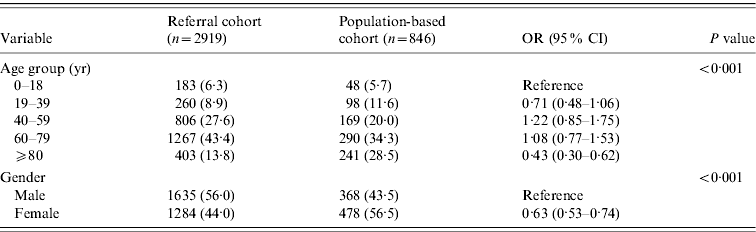
OR, Odds ratio; CI, confidence interval.
Data are given as number (%) unless otherwise specified.
Microbiology
Escherichia coli was the most common Gram-negative bacillus that caused BSI in both cohorts, but it was underrepresented in referral patients (adjusted OR 0·50, 95% CI 0·43–0·58). It contributed to over one-half (54·0%) of episodes of Gram-negative BSI in the population-based cohort, but only 34·8% of episodes in the referral cohort (Table 2). Proteus mirabilis was also underrepresented in referral patients, even after adjusting for age group and gender (adjusted OR 0·49, 95% CI 0·30–0·82). In contrast, Ps. aeruginosa, Enterobacter cloacae, Serratia marcescens and Stenotrophomonas maltophilia were overrepresented in the referral cohort. S. maltophilia was 18 times more likely to be reported as a cause of Gram-negative BSI from a tertiary-care referral centre compared to a population-based cohort. Despite being an extremely uncommon cause of Gram-negative BSI in the general population contributing to only 0·1% of cases, it was the eighth most common Gram-negative bacillus causing BSI in the referral cohort accounting for 2·3% of cases.
Table 2. Distribution of the ten most common pathogens causing Gram-negative bloodstream infections in the referral and population-based cohorts
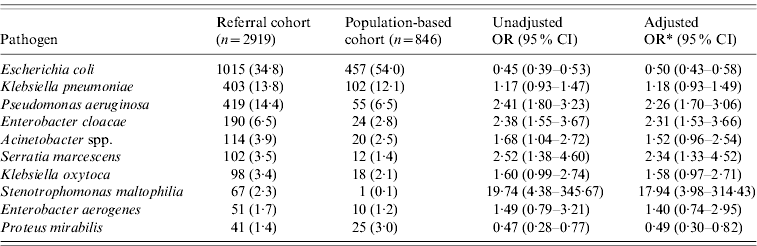
OR, Odds ratio; CI, confidence interval.
Data are given as number (%) unless otherwise specified.
* Odds ratios in this column are adjusted for age group and gender.
DISCUSSION
The majority of studies of BSI published in the medical literature were performed at large tertiary-care centres; related population-based studies have been scant. The emphasis on institutional studies and rarity of population-based studies has provided a perception that the demographic, microbiological and clinical characteristics of patients with Gram-negative BSI treated at tertiary-care centres have been applicable to all settings. Institutional studies from tertiary-care centres systematically select patients with certain characteristics who are more likely to be referred for care at these centres. The differences in the results of institutional and population-based studies of Gram-negative BSI have been mostly overlooked and often attributed to geographical and time variations. This study is the first to directly compare a referral and population-based cohort of patients with Gram-negative BSI within the same geographic area and during the same period of time.
In this study, we demonstrated a difference in the demographic features of referral patients with Gram-negative BSI and those in the general population. Elderly patients with Gram-negative BSI were less likely to receive care at tertiary-care referral centres. This underrepresentation of elderly patients in studies performed at tertiary-care centres has been previously described, for example, in other infectious conditions, including infective endocarditis, and non-infectious conditions, such as Alzheimer's disease [Reference Steckelberg2, Reference Kokmen10]. It is conceivable that elderly patients were less likely to be referred to tertiary-care centres due to the wishes of patients and family not to pursue more aggressive medical care. It is also possible that elderly patients prefer to seek medical care in local primary- and secondary-care centres closer to home and family.
Females were also less likely to be referred for tertiary-care centres for management of Gram-negative BSI. Female patients with Gram-negative BSI are more likely than males to have a urinary tract primary source of infection [Reference Al-Hasan11]. It is conceivable that females with Gram-negative BSI due to acute pyelonephritis were less likely to be referred to tertiary-care centres due to the less complex management requirements of their illness compared to patients with other sources of infection, such as liver abscesses, for example, who might need surgical or interventional radiological procedures that might be available only at large tertiary-care centres.
The microbiological distribution of Gram-negative bacilli that caused Gram-negative BSI was also different between referral patients and those in the community. Despite the fact that E. coli was the most common pathogen causing BSI in several population-based studies from different continents [Reference Madsen12–Reference Gosbell, Newton and Sullivan18], most institutional studies have reported that Staphylococcus aureus has been the most common cause of BSI [Reference Diekema, Pfaller and Jones19–Reference Douglas24]. This observation was also previously demonstrated locally. In a cross-sectional population-based study in Olmsted County, Minnesota, E. coli was the most common microorganism causing BSI contributing to 25% of cases followed by S. aureus (17%) [Reference Uslan8]. On the other hand, in a previous institutional study including all episodes of BSI at Mayo Clinic (Rochester, Minnesota), S. aureus was the most common microorganism followed by E. coli, accounting for 17% and 12% of cases, respectively [Reference Cockerill25]. Our current study suggests that the underestimation of E. coli as a cause of BSI in tertiary-care referral centres probably explains this phenomenon. It is not surprising that patients with S. aureus BSI were more likely to present for medical care at tertiary-care centres than those with E. coli BSI. S. aureus BSI is more likely than E. coli BSI to cause more serious complications such as infective endocarditis, epidural abscesses and deep surgical-site infections that often require surgical interventions that may not be available at local primary- and secondary-care centres [Reference Corey26]. On the other hand, the urinary tract is predominantly the most common source of infection in patients with E. coli BSI [Reference Al-Hasan11]. Therefore, patients infected with this organism are likely to receive care at their local primary- and secondary-care centres, as transfers to tertiary-care centres for this indication may not be warranted in many cases.
P. mirabilis was also underestimated in the referral cohort. This is also conceivable since P. mirabilis BSI, similar to E. coli BSI, is usually associated with a urinary tract primary source of infection [Reference Al-Hasan, Eckel-Passow and Baddour27].
The observation that S. maltophilia BSI was nearly 18-fold overestimated in the referral cohort, compared to the population-based cohort of the study, is an excellent example of referral bias. This observation highlights the importance of clearly understanding the setting of where each study is performed prior to generalizing its results to other populations. S. maltophilia BSI is usually seen in cancer patients, especially those with haematogenous malignancies, neutropenia, central venous catheters, and those receiving broad-spectrum antimicrobial agents, particularly carbapenems [Reference Paez and Costa28–Reference Paez31]. Cancer is much more prevalent in hospitalized patients at large tertiary-care centres that provide care for cancer patients, such as Mayo Medical Center, than in the general population. Therefore, S. maltophilia is a much more common cause of Gram-negative BSI in tertiary-care centre series than in population-based studies.
Other Gram-negative bacilli that are usually associated with nosocomial and healthcare-associated infections such as Ps. aeruginosa, E. cloacae and Serratia marcescens were also more common causes of BSI in tertiary-care centres than in the general population. Patients with BSI due to these microorganisms are more likely to have comorbid medical conditions requiring treatment at tertiary-care centres than those with E. coli and Klebsiella pneumoniae BSI, for example. In previous population-based studies, 78% and 92% of BSI episodes due to Ps. aeruginosa and E. cloacae were acquired in the hospital or healthcare setting [Reference Al-Hasan4, Reference Al-Hasan32], compared to only 41% and 52% of episodes of E. coli and K. pneumoniae BSI, respectively [Reference Al-Hasan11, Reference Al-Hasan33].
The primary strengths of our study were the large sample size and the inclusion of patients with Gram-negative BSI in both referral and population-based settings over a 10-year period of time.
Our study has limitations. First, the population of Olmsted County consists mainly of middle-class whites; therefore, our study results may be generalized only to communities with similar population characteristics. Second, our data were derived from one geographic area. The results of studies from multiple geographic locations might provide a more generalizable view. Finally, detailed clinical variables were not collected in all patients and thus we were unable to compare underlying medical conditions, primary source of infection, and outcomes between the referral and population-based cohorts.
In summary, patients with Gram-negative BSI presenting to tertiary-care centres have different demographic and microbiological characteristics compared to those in the general population. Gram-negative BSI surveys from tertiary-care centres tend to identify younger patients, males, and those with Gram-negative microorganisms that cause nosocomial or healthcare-associated infections. The underestimation of E. coli BSI in tertiary-care centre series resulted in differences in the most common cause of BSI in institutional studies vs. population-based investigations. Physicians should be aware of the influence of referral bias on the results of institutional surveys performed at tertiary-care centres and should consider this before generalizing results to other settings.
ACKNOWLEDGEMENTS
The study received funding from the Small Grants Program and the Baddour Family Fund at the Mayo Clinic, Rochester, Minnesota. The funding source had no role in study design. This work was made possible by research grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (National Institutes of Health, U.S. Public Health Service).
The authors thank Emily Vetter and Mary Ann Butler for providing vital data from the microbiology laboratory databases at the Mayo Clinic, Rochester and Olmsted Medical Center. The authors also thank Susan Schrage, Susan Stotz, R.N., and all the staff at the Rochester Epidemiology Project for their administrative help and support.'
DECLARATION OF INTEREST
None.






