Migraine, a recurrent disabling headache accompanied by chronic and episodic manifestations, is one of the most complex neurological disorders (Goadsby et al., Reference Goadsby, Lipton and Ferrari2002). Multiple cross-sectional studies (Ashina et al., Reference Ashina, Serrano, Lipton, Maizels, Manack, Turkel and Buse2012; Buse et al., Reference Buse, Manack, Serrano, Turkel and Lipton2010; Camarda et al., Reference Camarda, Pipia, Taglialavori, Di Fiore, Camarda and Monastero2008; Fuller-Thomson et al., Reference Fuller-Thomson, Schrumm and Brennenstuhl2013; Mercante et al., Reference Mercante, Peres, Guendler, Zukerman and Bernik2005; Molgat & Patten, Reference Molgat and Patten2005; Rist et al., Reference Rist, Schurks, Buring and Kurth2013; Zwart et al., Reference Zwart, Dyb, Hagen, Odegard, Dahl, Bovim and Stovner2003) have consistently observed that migraine often co-occurs with depression, a psychological disorder characterized by dramatic decline in both mental and physical conditions (Remick, Reference Remick2002).
Also supported by some Caucasian population-based longitudinal studies (Breslau et al., Reference Breslau, Davis, Schultz and Peterson1994; Breslau et al., Reference Breslau, Schultz, Stewart, Lipton, Lucia and Welch2000; Breslau et al., Reference Breslau, Lipton, Stewart, Schultz and Welch2003; Modgill et al., Reference Modgill, Jette, Wang, Becker and Patten2012), the association between migraine and depression is considered to be bi-directional, with migraine or severe headache increasing up to a 3-fold higher population relative risk (RR) of suffering depression, and vice versa. Another longitudinal study (Mongini et al., Reference Mongini, Keller, Deregibus, Raviola, Mongini and Sancarlo2003) also revealed a potential bi-directional association between depression and migraine frequency and severity, while one U.S. study (Swartz et al., Reference Swartz, Pratt, Armenian, Lee and Eaton2000) failed to repeat these results, possibly due to the influence of other comorbid psychological disorders, such as panic disorder, and social phobia, which were jointly analyzed in the study.
Although repeatedly observed, little is known about the mechanisms underlying the association between migraine and depression. An increased RR (i.e., RR > 1) of one trait, evaluated from twin and family samples, has been identified in relatives of probands reporting the same trait, for both migraine (Cologno et al., Reference Cologno, De Pascale and Manzoni2003; Kalfakis et al., Reference Kalfakis, Panas, Vassilopoulos and Malliara-Loulakaki1996; Lemos et al., Reference Lemos, Castro, Barros, Sequeiros, Pereira-Monteiro, Mendonca and Sousa2009; Russell & Olesen, Reference Russell and Olesen1995; Stewart et al., Reference Stewart, Staffa, Lipton and Ottman1997; Stewart et al., Reference Stewart, Bigal, Kolodner, Dowson, Liberman and Lipton2006; Thomsen et al., Reference Thomsen, Olesen and Russell2003) and depression (Barker et al., Reference Barker, Copeland, Maughan, Jaffee and Uher2012; Merikangas et al., Reference Merikangas, Cui, Heaton, Nakamura, Roca, Ding and Angst2014; Schreier et al., Reference Schreier, Hofler, Wittchen and Lieb2006; Sullivan et al., Reference Sullivan, Neale and Kendler2000; Vandeleur et al., Reference Vandeleur, Merikangas, Strippoli, Castelao and Preisig2014). These results provide strong evidence on familial aggregation in migraineurs and depressive patients. The variation in estimated RRs among these studies is likely due to the different diagnostic approaches and study populations. For instance, the lifetime prevalence of migraine was calculated as 20–28% (Al-Hashel et al., Reference Al-Hashel, Ahmed, Alroughani and Goadsby2014; Bicakci et al., Reference Bicakci, Bozdemir, Over, Saatci and Sarica2008; Moens et al., Reference Moens, Johannik, Verbeek and Bulterys2007) based on the ID migraine™ Screener criteria (Lipton et al., Reference Lipton, Dodick, Sadovsky, Kolodner, Endicott, Hettiarachchi and Harrison2003) but reduced to approximately 12% (Buse et al., Reference Buse, Manack, Fanning, Serrano, Reed, Turkel and Lipton2012; Lipton et al., Reference Lipton, Stewart, Diamond, Diamond and Reed2001) when based on the International Headache Society (IHS) criteria (Headache Classification Committee of the International Headache, 2013); and Caucasian populations had significant higher migraine and depression morbidity than African and Asian populations (Riolo et al., Reference Riolo, Nguyen, Greden and King2005; Stewart et al., Reference Stewart, Lipton and Liberman1996).
Significant familial aggregation of one trait indicates the presence of shared genetic and/or environmental factors in susceptibility of the trait. A considerable genetic contribution can be further determined after excluding environmental factors. Because both monozygotic (MZ) and dizygotic (DZ) twin pairs share similar living resources and environment, they are considered to experience the same environmental factors. Therefore, the observation of higher RRs within MZ twin pairs compared to DZ twin pairs provides evidence for a genetic contribution in susceptibility of the trait. For example, a significant higher RR for prostate cancer within male MZ pairs (RR = 12.3, 95% CI = 8.4–18.1) compared to male DZ pairs (RR = 3.1, 95% CI = 1.9–4.9; Lichtenstein et al., Reference Lichtenstein, Holm, Verkasalo, Iliadou, Kaprio, Koskenvuo and Hemminki2000), indicates a significant contribution of genetic factors for the risk of prostate cancer.
The heritability of migraine and depression are both estimated at approximately 50% (Levinson, Reference Levinson2006; Mulder et al., Reference Mulder, Van Baal, Gaist, Kallela, Kaprio, Svensson and Palotie2003), indicating both genetic and environmental factors play an important role in their development. Therefore, extending RR analyses in MZ and DZ twin pairs across both migraine and depression provides a natural experiment to determine the existence of shared genetic components between the two traits. Ours is the first such study to use a large population-based Australian twin sample to examine the association between migraine and depression.
In this article, we first examine the familial aggregation of migraine, depression, and their co-occurrence. We next compare relative risks estimated in MZ twin pairs to those estimated in DZ twin pairs to provide evidence for the contribution of genetic factors towards their risk.
Materials and Methods
Samples
As shown in Figure 1, participants were drawn from three Australian twin cohorts based at QIMR Berghofer Medical Research Institute (Heath et al., Reference Heath, Howells, Kirk, Madden, Bucholz, Nelson and Martin2001; Wright & Martin, Reference Wright and Martin2004). Subjects with migraine and depression status were selected and constituted the ‘merged migraine sample’ (N = 38,279) and ‘merged depression sample’ (N = 60,170) respectively. Definitions of migraine and depression were homogenized across the cohorts. Subjects also answered questions regarding demographic characteristics (e.g., sex, date of birth, zygosity) via semi-structured telephone interview and/or questionnaire. After combining the two merged samples and removal of non-twins and twins with missing status of either migraine or depression, a total of 5,319 twin pairs (2,456 MZ and 2,863 DZ pairs) remained for analysis.

FIGURE 1 The flow chart of selected twin sample.
Assessment of Migraine
Migraine symptom information ranged from single-answer self-report (yes or no) of migraine, using the ID Migraine™ Screener (Lipton et al., Reference Lipton, Dodick, Sadovsky, Kolodner, Endicott, Hettiarachchi and Harrison2003) — three questions shown to accurately identify 93% of people with migraines — to detailed IHS diagnostic criteria (International Classification of Headache Disorders, ICHD-3; Headache Classification Committee of the International Headache, 2013). For the collection of detailed ICHD-3 diagnostic criteria (see Table 1), participants answering ‘yes’ to ever having ‘migraine or recurrent attacks of headache’ (screening positive), then answered a number of questions relating to their symptoms. Diagnoses were determined for the two major varieties of migraine: 1.1 migraine without aura (MO) and 1.2 migraine with aura (MA, primarily comprising 1.2.1 typical aura with migraine headache), which account for 90–95% of all IHS migraines (Launer et al., Reference Launer, Terwindt and Ferrari1999).
TABLE 1 The Survey Questions of IHS-Based Migraine and DSM-Based Depression

After careful merging of all available migraine information, lifetime diagnoses for migraine were made subject to data availability, according to: (1) IHS ICHD-3 MO/MA diagnostic criteria, (2) the ID Migraine™ Screener, or (3) self-reported migraine. Hence, migraine status was measured in four categories according to these three criteria: non-migraine, self-report migraine (i.e., participants with positive status of self-reported measurement but negative or unknown status of the other two criteria), ID migraine (i.e., participants with positive status ID migraine™ Screener criteria but negative or unknown IHS-based migraine status) and IHS migraine (i.e., participants with positive status of IHS-based migraine). The ‘broad’ migraine status (i.e., any migraine) was defined when participants had reported at least one positive migraine status; and the ‘narrow’ migraine status was strict to the IHS migraine status.
Assessment of Depression
Participants were first asked two screening questions: ‘Has there ever been two weeks or more when you were depressed or down most of the day, nearly every day?’ and ‘Has there ever been two weeks or more when you were a lot less interested in most things or unable to enjoy the things you used to enjoy, most of the day, nearly every day?’ With at least one positive response, participants then answered additional questions (see Table 1). Lifetime depression was diagnosed according to the third and revised edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R; American Psychiatric Association, 1994) criteria: during a 2-week period, participants who had positive responses of more than five symptoms were diagnosed as suffering major depressive disorder (MDD) and participants who had 2–4 positive responses were diagnosed as suffering minor depressive disorder (MiDD). Therefore, in our study, depression status was measured in three categories: non-depression, MiDD, and MDD. Participants with either MiDD or MDD were defined to have the ‘broad’ depression status (i.e., any depression), and the MDD status was used as the ‘narrow’ depression status.
Analyses
Of the total 5,319 twin pairs included in the target sample, we stratified them into five subgroups according to sex and zygosity: female MZ pairs, male MZ pairs, female DZ pairs, male DZ pairs, and opposite sex DZ pairs. RRs were calculated as the statistical ratio comparing the frequency of a target disorder occurring in co-twins of probands and that occurring in co-twins of health controls, which was calculated from the cross-tabulations of proband–co-twin pairs (see supplementary Tables S1–S6). RR for migraine and depression was estimated in co-twins of twin probands reporting migraine or depression to evaluate their familial aggregation and co-occurrence.
Age at onset for migraine and depression was not available, therefore the ‘survey age’, representing the age when subjects participated in the survey, was used to adjust for potential age effects. To investigate whether age would influence RR estimation, logistic regression analyses were performed to calculate the effect of age in four analyses (i.e., migraine in co-twins of probands reporting migraine, depression in co-twins of probands reporting depression, migraine in co-twins of probands reporting depression, and depression in co-twins of probands reporting migraine). Probands were randomly selected as the first interviewed twin for the same-sex MZ/DZ pairs; while for the opposite sex DZ pairs, RR estimates were obtained for selecting the female twin as well as the male twin as proband, to estimate cross-sex RRs.
We estimated RRs and their 95% confidence intervals (CIs) for migraine (i.e., self-report migraine, ID migraine, IHS migraine, and any migraine) and depression (i.e., MDD and any depression) to assess their familial aggregation. We then separately estimated the RRs of migraine in co-twins of probands with depression and the RRs of depression in co-twins of probands with migraine, for broad diagnosis (i.e., any migraine and any depression) and narrow diagnosis (i.e., IHS migraine and MDD) respectively. All the analyses were performed using either SPSS (v22) or Rstudio (RStudio Team, 2014).
Results
Demographics
As shown in Table 2, based on 10,638 individuals (6,584 females and 4,054 males) from the twin sample, the lifetime prevalence was estimated at 45% for any migraine and 42% for any depression. Consistent with the previous findings (Arroyo-Quiroz et al., Reference Arroyo-Quiroz, Kurth, Cantu-Brito, Lopez-Ridaura, Romieu and Lajous2014; Bierut et al., Reference Bierut, Heath, Bucholz, Dinwiddie, Madden, Statham and Martin1999; Buse et al., Reference Buse, Manack, Fanning, Serrano, Reed, Turkel and Lipton2012; Kendler et al., Reference Kendler, Neale, Kessler, Heath and Eaves1992), the lifetime prevalence of IHS MO/MA migraine and DSM-III-R-based MDD was estimated at 14% and 34% respectively. Generally, females showed higher lifetime prevalence than males; the differences were small for self-report migraine but increased for ID migraine and especially IHS migraine. Both any depression and MDD also demonstrated higher lifetime prevalence in females than in males, whereas the lifetime prevalence of MiDD was higher in males compared to females.
TABLE 2 Lifetime Prevalence of Migraine and Depression Based on Australian Twin Sample
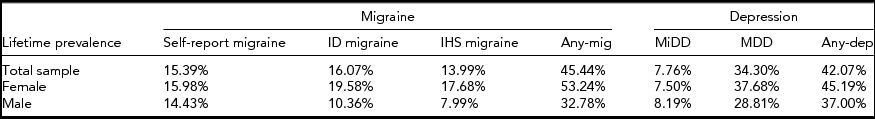
Any-mig = Any migraine; Any-dep = Any depression.
The mean ‘survey age’ was estimated at 36±11 years, ranging from 18 to 89 years. The logistic regressions under all four analyses indicated that age does not significantly influence risk for migraine and depression in our sample (p value >.05). Therefore, we present RRs without age adjustment.
Familial Aggregation
As shown in Table 3, we observed a significantly increased RR (RR = 1.73, 95% CI: 1.62–1.85) for any migraine in co-twins of probands reporting any migraine, compared with co-twins of non-migraine controls. The RR for any migraine was significantly higher within MZ pairs (RR = 2.07, 95% CI: 1.88–2.27) compared to DZ pairs (RR = 1.47, 95% CI: 1.34–1.62). Similarly, regardless of migraine diagnosis (i.e., self-report migraine, ID migraine, IHS migraine, or any migraine), an increased RR was consistently found in the total sample, and a significant higher RR was calculated within MZ pairs compared to DZ pairs, although RRs calculated in DZ pairs for self-report migraine and ID migraine were not statistically significant, most likely due to their reduced diagnostic reliability and smaller sample size. In addition, most RRs calculated within same-sex DZ pairs were slightly higher than those calculated within DZ opposite-sex pairs, suggesting the presence of sex-specific effects in familial aggregation. Furthermore, RR for migraine increased in line with the reliability of migraine diagnosis, with no significant difference in RR between self-report migraine and ID migraine, but a significantly higher RR for IHS migraine. This pattern of results was observed in all zygosity subgroups.
TABLE 3 Relative Risks (and 95% CI) of Single-Trait and Number of Proband–Co-Twin Pairs in Different Twin Samples

CI = confidence interval; Any-mig = any migraine; Any-dep = any depression; DZ F–M pairs = opposite sex DZ pairs with female as probands; DZ M–F pairs = opposite sex DZ pairs with male as probands.
Regarding depression, although any depression comprised two types of depression, MDD and MiDD, because of the relatively small number of MiDD patients (N = 826 participants), we focused on results for MDD and any depression. Similar to migraine, an increased RR for depression in co-twins of probands reporting depression was observed for both MDD (RR = 1.75, 95% CI: 1.63–1.89) and any depression (RR = 1.53, 95% CI: 1.43–1.63) in the total sample. These RRs significantly increased to 2.35 (95% CI: 2.10–2.62) for MDD and 1.89 (95% CI: 1.72–2.08) for any depression in MZ pairs, and significantly decreased to 1.36 (95% CI: 1.23–1.51) for MDD and 1.27 (95% CI: 1.17–1.39) for any depression in DZ pairs, respectively. RRs calculated in all zygosity subgroups also remained significant, and a slightly higher RR was observed within same-sex DZ pairs compared to DZ pairs. Also similar to migraine, the RRs in DZ pairs for the narrow diagnosis (MDD) were higher although not statistically significant, compared to RRs for the broader any depression.
Association Between Migraine and Depression
For the broad diagnoses (Table 4) in the total sample, co-twins of probands reporting any depression had a significantly increased RR (RR = 1.18, 95% CI: 1.11–1.26) for any migraine compared to co-twins of controls. The reverse was also true, with the RR for any depression significantly increased (RR = 1.12, 95% CI: 1.05–1.20) in co-twins of probands reporting any migraine compared to co-twins of controls, thus indicating a bi-directional association between the two disorders. The RR for any migraine in co-twins of probands reporting any depression was higher than the reverse. The bi-directional association became more significant within MZ pairs, with an RR for any migraine in co-twins of probands reporting any depression of 1.36 (95% CI: 1.24–1.48) and RR for any depression in co-twins of probands reporting any migraine was 1.26 (95% CI: 1.14–1.38). Similar to some of the single-trait RR estimates, the cross-trait RR estimates decreased in DZ twin pairs and became non-significant, and RRs in same-sex DZ pairs were increased compared to opposite-sex DZ pairs. Also, significantly higher cross-trait RRs were observed within MZ pairs compared to DZ pairs for any migraine in co-twins of probands reporting any depression and vice versa, suggesting the presence of shared genetic components between any migraine and any depression.
TABLE 4 Relative Risks (and 95% CI) of Cross-Trait and Number of Proband–Co-Twin Pairs in Different Twin Samples

CI = confidence interval; Any-mig: any migraine; Any-dep: any depression; DZ F–M pairs = opposite sex DZ pairs with female as probands; DZ M–F pairs = opposite sex DZ pairs with male as probands.
For the narrow diagnoses (Table 4), a bi-directional association between IHS migraine and MDD was also found and was more significant compared to the broad diagnosis in the total sample and MZ pairs. The RRs for IHS migraine in co-twins of probands reporting MDD was 1.67 (95% CI: 1.46–1.92) in the total sample and 2.23 (95% CI: 1.81–2.75) in MZ pairs; while the RRs for MDD in co-twins of probands reporting IHS migraine was 1.25 (95% CI: 1.13–1.37) in the total sample and 1.55 (95% CI: 1.34–1.79) in MZ pairs. In contrast, results in DZ pairs suggest a unidirectional association, with a significant RR for IHS migraine in co-twins of probands reporting MDD at 1.35 (95% CI: 1.13–1.62) and a non-significant RR for MDD in co-twins of probands reporting IHS migraine at 1.06 (95% CI: 0.93–1.22), respectively. Compared to DZ pairs, MZ pairs always provided significantly higher RRs for IHS migraine in co-twins of probands reporting MDD and the reverse. The difference in RRs calculated in MZ and DZ pairs was larger for the narrow diagnoses compared to the broad diagnoses. Notably, the observed risk for IHS migraine in co-twins of probands reporting MDD was considerably higher than the reverse, for total sample, MZ pairs and DZ pairs.
Discussion
Familial Aggregation of Migraine and Depression?
Previous Caucasian population-based family studies (Cologno et al., Reference Cologno, De Pascale and Manzoni2003; Kalfakis et al., Reference Kalfakis, Panas, Vassilopoulos and Malliara-Loulakaki1996; Lemos et al., Reference Lemos, Castro, Barros, Sequeiros, Pereira-Monteiro, Mendonca and Sousa2009; Russell & Olesen, Reference Russell and Olesen1995; Stewart et al., Reference Stewart, Staffa, Lipton and Ottman1997; Stewart et al., Reference Stewart, Bigal, Kolodner, Dowson, Liberman and Lipton2006; Thomsen et al., Reference Thomsen, Olesen and Russell2003) consistently reported familial aggregation of migraine in first-degree relatives of probands, with highly variable RRs ranging from 1.40 to 13.82, largely due to differences in migraine types (e.g., MA, familial hemiplegic migraine), diagnostic approaches (e.g., IHS-based self-report questionnaire or interview) and sample selection. Our results from DZ pairs are equivalent to proband–first-degree RR estimates from the general population, and are in line with previous RR estimates based on IHS migraine and any migraine status. By comparing our results for strict IHS migraine to results for the broader definitions of migraine, self-report migraine, and ID migraine status clearly demonstrates such expected sensitivity of RR and familial aggregation analysis of migraine to the diagnostic approach. Our finding of significantly higher RR for migraine within MZ pairs than DZ pairs provides strong evidence for a genetic contribution to migraine susceptibility.
Previous studies on measuring clustering of depression often utilized odds ratios (ORs) rather than RRs. The OR for depression in first-degree relatives of depressive probands have been calculated at approximately 2–3 in Caucasian population-based family samples (Barker et al., Reference Barker, Copeland, Maughan, Jaffee and Uher2012; Merikangas et al., Reference Merikangas, Cui, Heaton, Nakamura, Roca, Ding and Angst2014; Schreier et al., Reference Schreier, Hofler, Wittchen and Lieb2006; Sullivan et al., Reference Sullivan, Neale and Kendler2000; Vandeleur et al., Reference Vandeleur, Merikangas, Strippoli, Castelao and Preisig2014). Assuming a depression prevalence of between 20% and 50%, ORs ranging from 2 to 3 are estimated to be equivalent to RRs of approximately 1.11–1.50 (using OR to Risk Ratio Conversion; http://clincalc.com/Stats/ConvertOR.aspx). Thus, our RR estimates for depression from DZ pairs are in agreement with published findings, and support the familial aggregation of depression. Our finding of significantly higher RR for depression within MZ pairs compared to DZ pairs provides strong evidence for a genetic contribution to depression.
Although the difference in RRs between DZ F-M pairs (opposite sex DZ pairs with female as proband) and DZ M-F pairs (opposite sex DZ pairs with male as proband) for migraine and depression are not significant, the risks for migraine and depression are consistently larger in male co-twins of affected female probands compared to female co-twins of affected male probands. These results suggest the possible influence of sex-specific effects (i.e., effects expressed in one sex but not the other) that may include genetic (G), environmental (E), and/or interacting (G×E) effects.
Bi-Direction Association Between Migraine and Depression?
In line with previous findings (Breslau et al., Reference Breslau, Davis, Schultz and Peterson1994; Breslau et al., Reference Breslau, Schultz, Stewart, Lipton, Lucia and Welch2000; Breslau et al., Reference Breslau, Lipton, Stewart, Schultz and Welch2003; Modgill et al., Reference Modgill, Jette, Wang, Becker and Patten2012; Mongini et al., Reference Mongini, Keller, Deregibus, Raviola, Mongini and Sancarlo2003), results from analysis of broad diagnoses in the total sample indicate a bi-directional association between migraine and depression, with an increased RR for any migraine in co-twins of probands reporting any depression, and vice versa. The association became stronger when analyzing the more narrow diagnoses of IHS migraine and MDD.
The observed risk for migraine in relatives of probands reporting depression was considerably higher than the risk for depression in relatives of probands reporting migraine. These results were observed for both broad and narrow diagnoses, and remained when calculating RRs by averaging over selecting either twin 1 or twin 2 as proband, thus ensuring our results are robust to proband selection within twin pairs. These findings provide further support to findings from a recent analysis of single nucleotide polymorphism (SNP) genotype data indicating that patients with comorbid migraine and depression are genetically more similar to patients with only depression than patients with only migraine (Ligthart et al., Reference Ligthart, Hottenga, Lewis, Farmer, Craig, Breen and Nyholt2014).
Regardless of the diagnostic approach, the association between migraine and depression was stronger within MZ twin pairs compared to DZ pairs, thus providing strong evidence for a genetic contribution to familial aggregation of migraine and depression.
Strengths and Weaknesses
Ours is the only study to use a large population-based twin design to provide strong evidence for a genetic contribution to the familial aggregation and co-occurrence of migraine and depression. Another advantage of this study concerned the utilization of multiple approaches to diagnose migraine and depression. Comparing results from different diagnostic definitions allowed us to demonstrate both the sensitivity and validity of RR and familial aggregation analysis of migraine and depression across different diagnostic criteria.
However, there are some limitations to note. First and foremost, both migraine and depression status were diagnosed using self-reported questionnaire data, as opposed to the gold-standard of clinical-based interviews by neurologists or psychologists. Although our approach may result in some misclassification of migraine and depression status, it is not feasible to perform clinic-based interviews in samples larger enough to provide sufficient power for such familial aggregation studies. Moreover, our approach enabled narrow diagnoses of migraine and depression that satisfy clinically accepted criteria. Furthermore, our estimated lifetime prevalence of IHS migraine and DSM-III-R-based MDD are in a good agreement with published estimates (Arroyo-Quiroz et al., Reference Arroyo-Quiroz, Kurth, Cantu-Brito, Lopez-Ridaura, Romieu and Lajous2014; Bierut et al., Reference Bierut, Heath, Bucholz, Dinwiddie, Madden, Statham and Martin1999; Buse et al., Reference Buse, Manack, Fanning, Serrano, Reed, Turkel and Lipton2012; Kendler et al., Reference Kendler, Neale, Kessler, Heath and Eaves1992). Second, we did not separate IHS migraine into MO and MA. However, given our previous findings indicating a strong familial aggregation and genetic overlap between MO and MA, we believe our approach provides the most powerful and sensible use of the data to examine the relationship between migraine and depression. Third, probands of twin pairs were selected randomly as the twin who first entered the survey rather than birth order. However, considering twins essentially share the same family environment and the fact we obtain identical RRs and conclusions when selecting the second (other) twin as the proband indicates our random selection approach to be valid. Another limitation relates to our use of ‘survey age’ rather than age of onset. However, given our samples were comprised of adults past the typical age of onset, and our logistic regression analyses found no association between survey age and migraine and/or depression, we do not believe this to be an important issue.
Conclusions
In summary, this study used a large Australian population-based twin sample and found significant evidence for a genetic contribution to the familial aggregation of migraine and depression. Our findings also suggest a bi-directional association between migraine and depression, with an increased risk for depression in relatives of probands reporting migraine and vice versa. However, the observed risk for migraine in relatives of probands reporting depression was considerably higher than the reverse. These results add further support to previous studies suggesting that patients with comorbid migraine and depression are genetically more similar to patients with depression compared to patients with migraine.
A better understanding of the genetic architecture of comorbid migraine and depression has excellent potential to improve our understanding of the relationship between migraine and co-occurring depression and inform treatment strategies. For example, identifying patients most at risk of comorbid migraine and depression and chronic migraine (due to medication overuse), and identifying subgroups of patients for whom a particular treatment or treatment combination may be most effective (e.g., episodic vs. chronic migraine, depressed vs. non-depressed, those with high analgesic use). Hence, future studies should focus on characterizing and identifying the genetic and environmental factors contributing to co-occurring migraine and depression.
Acknowledgments
Huiying Zhao was supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1091816). Dale R. Nyholt was supported by an NHMRC Research Fellowship (APP0613674). This work was supported by an NHMRC project grant (APP1075175), the European Union's Seventh Framework program (2007–2013) under grant agreement no. 602633 (EUROHEADPAIN) and the US National Institutes of Health (AA07535, AA011998, AA017688, AA10249, AA13320, AA13321, AA13326, AA14041, MH66206, DA12854, DA019951).
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/thg.2016.43.









