With a history from the 14th century(Reference Cano-Marquina, Tarín and Cano1), coffee is one of the most consumed beverages in the world(Reference Cornelis2). The daily coffee consumption prevalence of Korean adults increased from 54·6 % in 2001 to 65·7 % in 2011 and from 29·1 % in 2001 to 43·3 % in 2011 for those who drank two or more cups of coffee per d(Reference Je, Jeong and Park3). The average daily intake of coffee by Korean adults was 1·78 cups per d in 2017(Reference Kim and Kim4). In 2019, the average daily intake of coffee (107·5 g) was the largest among total beverages (314·9 g) in the general Korean population(Reference Han5). Epidemiological studies have shown that coffee consumption lowers the risk of various chronic diseases including CVD, type 2 diabetes, some cancers and overall mortality(Reference O’Keefe, DiNicolantonio and Lavie6–Reference Poole, Kennedy and Roderick9). However, the biological mechanisms that explain the association between coffee consumption and chronic diseases remain unknown.
Chronic inflammation is involved in the pathogenesis of various chronic diseases, including CVD, type 2 diabetes and cancer(Reference Golia, Limongelli and Natale10–Reference Crusz and Balkwill12). C-reactive protein (CRP) is an acute-phase protein that is produced and secreted by hepatocytes through transcriptional control by pro-inflammatory cytokines(Reference Sproston and Ashworth13). High-sensitivity C-reactive protein (hsCRP) is a suitable biomarker for evaluating chronic low-grade inflammation. Dietary factors are some of the determinants of inflammatory biomarkers(Reference Galland14). Coffee compounds, such as chlorogenic acid and caffeic acid, have strong antioxidant capacity both in vivo and in vitro (Reference Spagnol, Assis and Brunetti15,Reference Agunloye, Oboh and Ademiluyi16) . Coffee extracts, including caffeine and kahweol, may relieve inflammation in animals(Reference Endesfelder, Strauß and Bendix17–Reference Chou, Kao and Yue20). Coffee consumption is inversely associated with risk of CVD(Reference Chieng and Kistler21), certain types of cancer(Reference Zhao, Li and Feng22–Reference Micek, Godos and Lafranconi24), Parkinson’s disease(Reference Qi and Li25), depression(Reference Grosso, Micek and Castellano26) and mortality(Reference Grosso, Micek and Godos27), which have been also associated with higher CRP levels(Reference Osimo, Baxter and Lewis28–Reference Godos, Biondi and Galvano35). Taken together, coffee consumption and inflammatory markers such as CRP levels appear to be related.
Although an inverse association between coffee consumption and inflammatory diseases has been reported(Reference Rodríguez-Artalejo and López-García36–Reference Contaldo, Santarpia and Pasanisi38), results suggesting an association between coffee consumption and CRP levels have been inconsistent(Reference Hang, Kværner and Ma39–Reference Stutz, Ahola and Harjutsalo55). Classifying coffee types is important for conducting studies based on coffee consumption that considers only coffee constituents. Instant coffee mix, containing sugar and/or non-dairy creamers, accounts for a large portion of the Korean coffee market(Reference Je, Jeong and Park3). Non-coffee constituents in the instant coffee mix may offset the potential protective effects of coffee. The consumption of instant coffee mix was positively associated with metabolic syndrome(Reference Kim, Cho and Jacobs56), weight gain and insulin resistance(Reference Je, Jeong and Park3). However, a few studies classified the coffee variety(Reference Hang, Kværner and Ma39,Reference Cornelis and van Dam43,Reference Zampelas, Panagiotakos and Pitsavos44,Reference Lopez-Garcia, van Dam and Qi51) , whose results were conflicting, and Korean studies on this topic did not carry out analyses considering coffee types across coffee consumption categories(Reference Youn-Jung, Keun-Mi and Seung-Pil41,Reference Joo-Hyun, Young-Sung and Han-Ul42,Reference Kong, Woo and Kim53) . In addition, the overall effect of coffee intake on health is still unclear. Heavy coffee consumption may be associated with an increased risk of CVD(Reference Zhou and Hyppönen57). A significant amount of diterpenes in unfiltered coffee may affect the LDL receptor and lead to extracellular accumulation of cholesterol(Reference Grosso, Godos and Galvano58).
Therefore, we aimed to evaluate the association between coffee consumption and high CRP levels in Korean adults by considering coffee type, using the data from the Korea National Health and Nutrition Examination Survey (KNHANES).
Materials and methods
Study population
We conducted this study based on data from the seventh period (2016–2018) of KNHANES, which is a cross-sectional survey representing the South Korean population. For the KNHANES, which was performed by the Korea Centers for Disease Control and Prevention (KCDC) under the Korean Ministry of Health and Welfare, a stratified, multistage, clustered and probability sampling design was used to collect data representing non-institutionalised civilians in Korea. The KNHANES includes three parts: a health interview, health examination and nutrition survey. The KNHANES methods were previously described in detail(Reference Kweon, Kim and Jang59).
Data were collected from 24 269 participants in 2016 (n 8150), 2017 (n 8127) and 2018 (n 7992). Among them, we included 20 166 participants who completed all three parts (health interview, health examination and nutrition survey) in this study. Then, we excluded the following participants who were not suitable for this study in turn: 8459 subjects who were < 19 or ≥ 65 years old; 670 subjects who self-reported diagnosis of stroke, myocardial infarction/angina, renal failure or cancer; seventy-six subjects who were pregnant; 219 subjects who had missing information on hsCRP levels; 232 subjects who had no fasting status at blood test (< 8 h); 108 subjects who had extreme total energy consumption per d (≤ 500 or > 6000 kcal/d); one subject who drank extremely large amount of coffee per d (> 100 g/d coffee powder); and 1064 subjects who had missing information on alcohol consumption, smoking status or physical activity. We included 9337 subjects (2994 in 2016, 3084 in 2017 and 3259 in 2018), including 5297 women and 4040 men in this study.
When analysed by coffee types, the following subjects were additionally excluded from the study population in turn: 269 subjects who drank lattes consisting of coffee powder and milk without sugar or cream because the number of the subjects was too small to be included in the analyses by coffee types; thirty subjects who consumed black coffee, sugar and/or cream, and other foods at the same time because we could not determine whether sugar and/or cream was added to black coffee or other foods; and 627 subjects who consumed both black coffee and coffee with sugar and/or cream in 1 d. We included 8411 subjects (2729 in 2016, 2730 in 2017 and 2952 in 2018), including 4679 women and 3732 men, in the analyses by coffee types. A flow chart of the selection criteria for eligible study population is shown in Fig. 1. Informed consent was obtained from each person in the survey, and the formal ethics approval for the KNHANES dataset was provided by the Centers for Disease Control and Prevention’s Institutional Review Board. (IRB no. 2018–01–03-P-A).
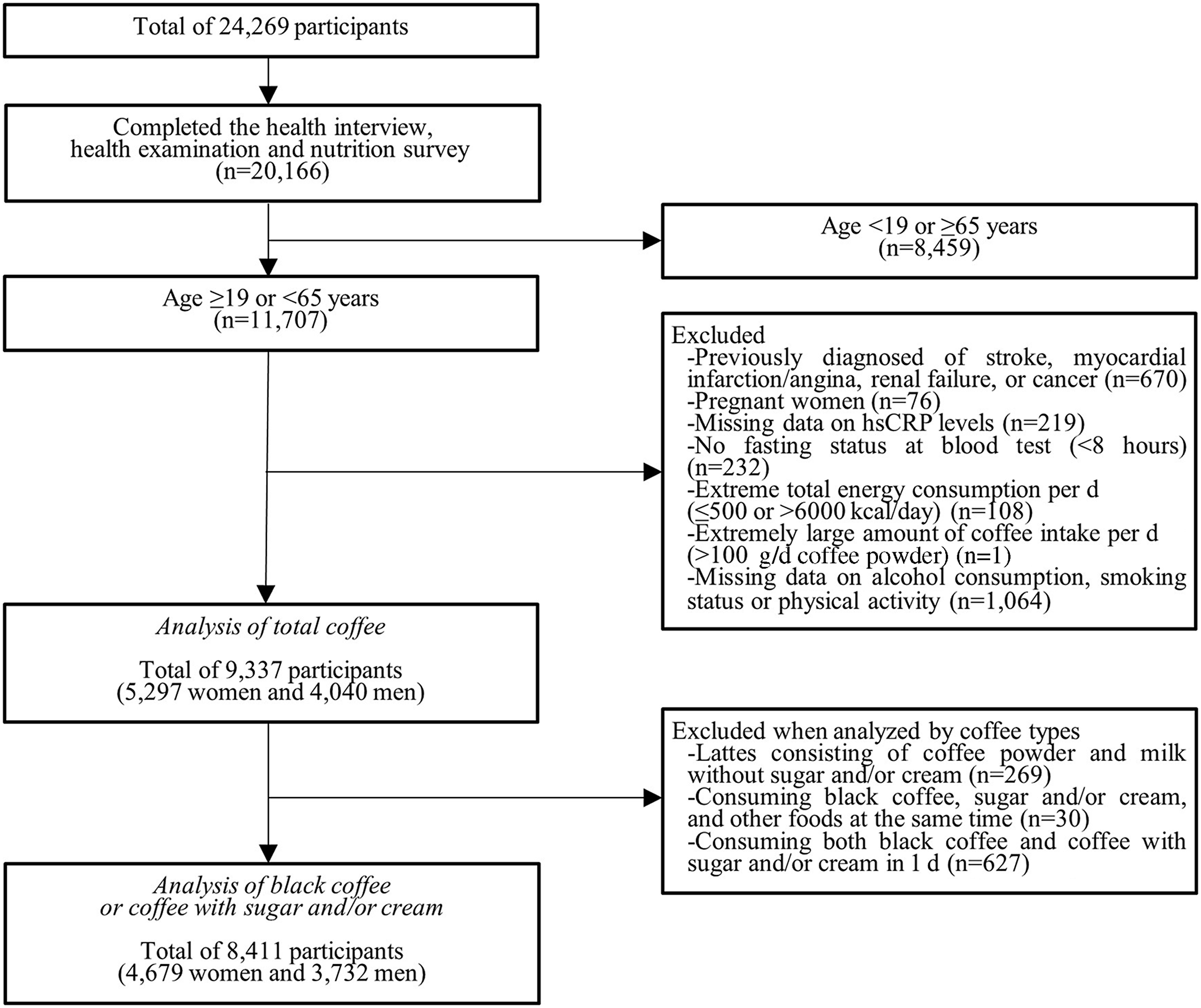
Fig. 1. Study participants included in the study after the exclusion criteria. hsCRP, high-sensitivity C-reactive protein.
Assessment of coffee consumption
We used the 24-h recall data from the KNHANES to assess coffee consumption. We used the intake for the tertiary food code. The intake for the tertiary food code is obtained in KNHANES by calculating the conversion coefficient based on the reference solid content because a risk of misinterpretation of intake exists by simply adding the intakes of foods in different states. In the KNHANES, the reference solid food for the intake of the tertiary food code of coffee is in the form of coffee powder. One instant black coffee powder (2·75 g) is defined as the dry weight of one cup of coffee. We converted > 0 to 2·75 g, > 2·75 to 8·25 g and > 8·25 g to ≤ 1 cup, > 1 to 3 cups (2–3 cups) and > 3 cups, respectively.
We classified coffee into black coffee and coffee with sugar and/or cream. The amount of sugar and/or cream is included in the intake for the tertiary food code of coffee with sugar and/or cream. Therefore, we investigated the coffee powder content (%) of each coffee with sugar and/or cream product so that we included only the amount of coffee powder in the coffee intake. If the name of the coffee product was not presented or we could not find the coffee powder content (%) of the coffee product, we used the median value of the coffee powder content (%) of the remaining coffee products.
Assessment of C-reactive protein levels
The hsCRP level was tested in the KNHANES by Immunoturbidimetry using a Roche Cardiac C-reactive Protein High Sensitive (Roche)(Reference Joo-Hyun, Young-Sung and Han-Ul42). In asymptomatic adults, the CRP level of 2·2 mg/l or above was considered elevated(Reference Joo-Hyun, Young-Sung and Han-Ul42,Reference Visser, Bouter and McQuillan60) . In the KNHANES 2016, if the hsCRP level was above 20·00 mg/l or less than 0·15 mg/l, then the HE_hsCRP variable, which shows the hsCRP level, was indicated as 20·01 mg/l or 0·149 mg/l, respectively. In the KNHANES 2017–2018, when the hsCRP level was above 20·00 mg/l or below 0·15 mg/l, HE_hsCRP_etc, which was a newly created categorical variable, had a value of > 20·00 or < 0·15, respectively. To ensure data consistency, when the hsCRP level was greater than 20·00 mg/l or less than 0·15 mg/l, we replaced > 20·00 and < 0·15 mg/l of the HE_hsCRP_etc code with 0·149 and 20·01 mg/l of HE_hsCRP code in the data from 2017 and 2018, respectively.
Confounding variables
Demographic and lifestyle factors such as age, sex, education level, household income, alcohol consumption, smoking status, physical activity, sleep duration and diet quality were obtained using a self-reported questionnaire or by personal interview. Education level was classified into elementary school or lower, middle school or high school, and college or higher. Household income was categorised into quartiles, such as lowest, lower-middle, upper-middle and highest. We multiplied the collected frequency of alcohol consumption by the collected amount of alcohol drank, then calculated the servings of alcohol consumption per d. Then, we categorised alcohol consumption into < 1, 1–< 3 and ≥ 3 servings per d. We grouped smoking status into non-smoker, past smoker and current smoker. We classified physical activity as low and high. We considered high physical activity as at least 75 min of vigorous activity per week, at least 150 min of moderate activity per week or at least 150 min of a combination of vigorous and moderate activity per week (vigorous activity for 1 min was counted as moderate activity for 2 min). We classified sleep duration into < 6, 6 to < 8 and ≥ 8 h per d. We divided diet quality into low and high, which was assessed via a modified diet quality index for Koreans (DQI-K)(Reference Lim, Lee and Shin61,Reference Na, Kim and Chung62) . According to cut-point references such as Korean dietary reference intakes (KDRI), the DQI-K points of ‘0 and 1’ or ‘0, 1, and 2’ were assigned to each of the eight factors: daily protein, cholesterol, wholegrain, fruit, vegetable and Na intake, and percent of energy from fat and saturated fat. The sum of the DQI-K point of each factor from each participant ranges from 0 to 9. DQI-K scores of 0–4 and 5–9 reflect high-diet quality and low-diet quality, respectively(Reference Lim, Lee and Shin61,Reference Na, Kim and Chung62) .
Statistical analysis
We used PROC SURVEYFREQ and PROC SURVEYREG (SAS Institute) procedures to estimate the prevalence and mean of demographic and lifestyle factors. We used the PROC SURVEYLOGISTIC procedure to calculate multivariable-adjusted OR and 95 % CI of high CRP levels according to coffee consumption. An OR of less than or greater than 1·0 means that coffee consumption was inversely or positively associated with high CRP levels, respectively. If the 95 % CI includes 1·0, then the OR is not considered statistically significant(Reference Tenny and Hoffman63,Reference Szumilas64) . We adjusted model 1 for age (continuous) and sex (women and men). We additionally adjusted model 2 for BMI (kg/m2, continuous), daily total energy intake (kcal/d, continuous), education level (elementary school or lower, middle school or high school, and college or higher), household income (lowest, lower-middle, upper-middle and highest), alcohol consumption (> 1, 1–< 3 and ≥ 3 servings/d), smoking status (non-smoker, past smoker and current smoker), physical activity (low and high), sleep duration (< 6, 6 to < 8 and ≥ 8 h/d), DQI-K (low-diet quality (scores of 5–9) and high-diet quality (scores of 0–4)), HDL-cholesterol (mg/dl, continuous), fasting plasma glucose (mg/dl, continuous), TAG (mg/dl, continuous), total cholesterol (mg/dl, continuous), systolic blood pressure (mmHg, continuous), diastolic blood pressure (mmHg, continuous) and leucocyte count (thous/μl). When selecting covariates for a multivariable model, we consulted the previous studies on this topic and the characteristics of the study subjects(Reference Hang, Kværner and Ma39–Reference Zampelas, Panagiotakos and Pitsavos44). We conducted all statistical analyses with SAS 9.4 software (SAS Institute). We considered a two-tailed P value less than 0·05 to indicate statistical significance.
Results
General characteristics
Table 1 shows the general characteristics of Korean adults by total coffee consumption. Compared with people in the lowest group (non-drinkers), those in the highest group of total coffee consumption (> 3 cups/d) were more likely to be older and men and to have higher physical activity. Those in the highest category of total coffee consumption also had higher plasma glucose and diastolic blood pressure and lower systolic blood pressure than those in the lowest category. Adults who consumed more total coffee had higher daily energy intake, education level, household income and diet quality, and shorter sleep duration. Frequent total coffee drinkers were likely to be current smokers. Those who drank more total coffee also had higher total cholesterol and leucocyte concentrations.
Table 1. Characteristics of study population according to total coffee consumption in Korean adults aged 19–64 years*
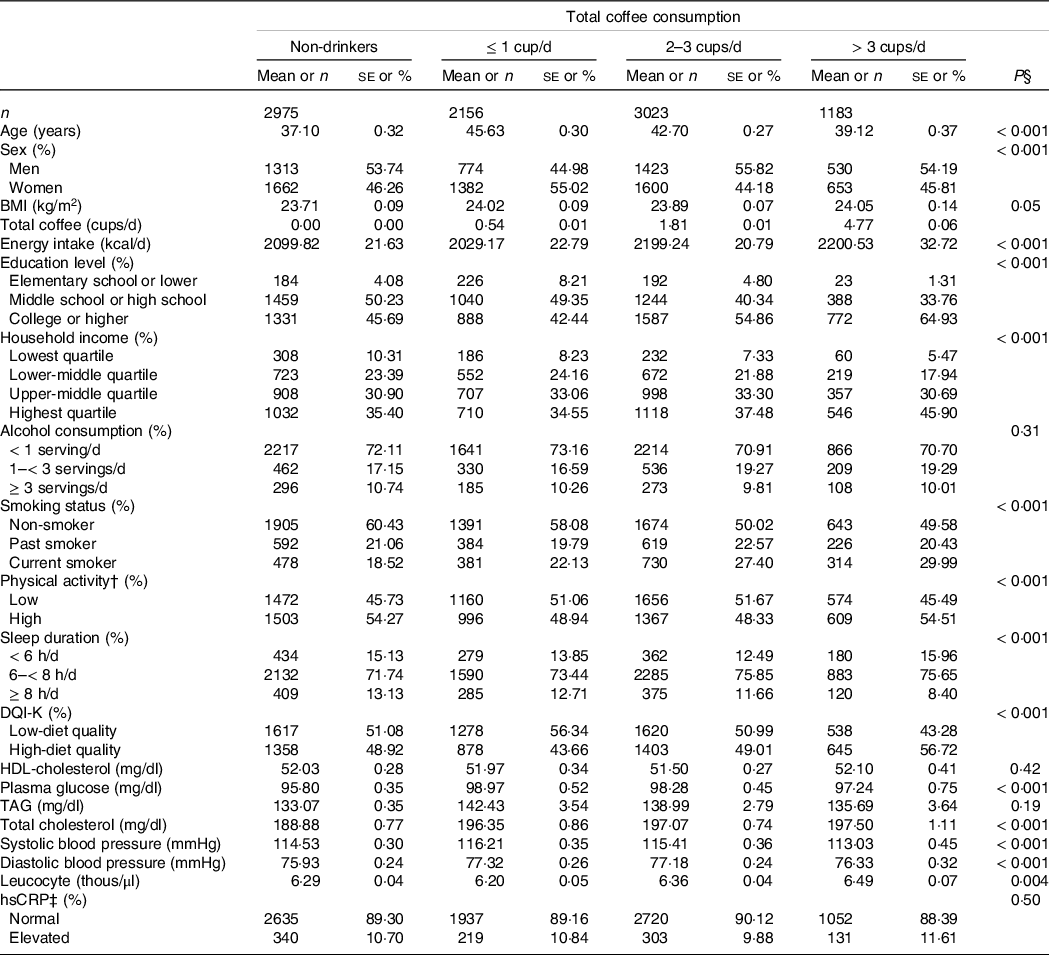
DQI-K, a modified diet quality index for Koreans; hsCRP, high-sensitivity C-reactive protein.
* Values are presented as mean and standard errors for continuous variables, and number and weighted % for categorical variables.
† High physical activity was defined as at least 75 min of vigorous activity per week, at least 150 min of moderate activity per week, or at least 150 min of a combination of vigorous and moderate activity per week.
‡ hsCRP was considered elevated if 2·2 mg/l or more.
§ P-values obtained from the χ 2 test for categorical variables and from PROC SURVEYREG procedure for continuous variables.
Coffee consumption and high C-reactive protein levels
The results of multivariable analyses on the association between coffee consumption and high CRP levels in Korean adults are shown in Table 2. After the multivariable adjustment, we found that adults who consumed 2–3 cups/d of total coffee had 17 % lower odds of high CRP levels than non-drinkers (OR = 0·83, 95 % CI 0·69, 0·99). By type of coffee, the inverse association was stronger in adults consuming black coffee (OR = 0·61, 95 % CI 0·45, 0·84), while the inverse association was much weaker in those consuming coffee with sugar and/or cream (OR = 0·92, 95 % CI 0·74, 1·14). More than three cups/d of heavy coffee consumption was not significantly associated with high CRP levels.
Table 2. Multivariable adjusted OR for high CRP levels according to coffee consumption and types in Korean adults aged 19–64 years
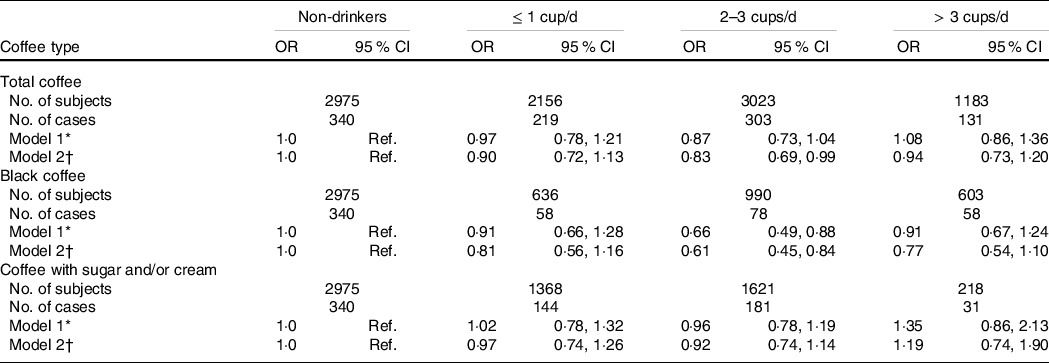
CRP, C-reactive protein; Ref., reference; DQI-K, a modified diet quality index for Koreans.
* Model 1 was adjusted for age and sex.
† Model 2 was adjusted for age, sex, BMI, daily total energy intake, education level, household income, alcohol consumption, smoking status, physical activity, sleep duration, DQI-K, HDL-cholesterol, fasting plasma glucose, TAG, total cholesterol, systolic blood pressure, diastolic blood pressure and leucocyte count.
Table 3 shows the association between coffee consumption and high CRP levels in Korean adults by sex. After the multivariable adjustment, we found that men who consumed 2–3 cups/d of total coffee had 25 % lower odds of high CRP levels than non-drinkers (OR = 0·75, 95 % CI 0·58, 0·98), while the inverse association with 2–3 cups/d of total coffee was weaker in women (OR = 0·91, 95 % CI 0·70, 1·19). By type of coffee, 2–3 cups of black coffee was inversely associated with high CRP levels in men (OR = 0·65, 95 % CI 0·41, 1·03) and women (OR = 0·55, 95 % CI 0·36, 0·83), and the association seems slightly stronger in women. More than three cups/d of heavy coffee or consumption of coffee with sugar and/or cream were not significantly associated with high CRP levels both in men and women.
Table 3. Multivariable adjusted OR for high CRP levels according to coffee consumption and types in Korean adults aged 19–64 years by sex
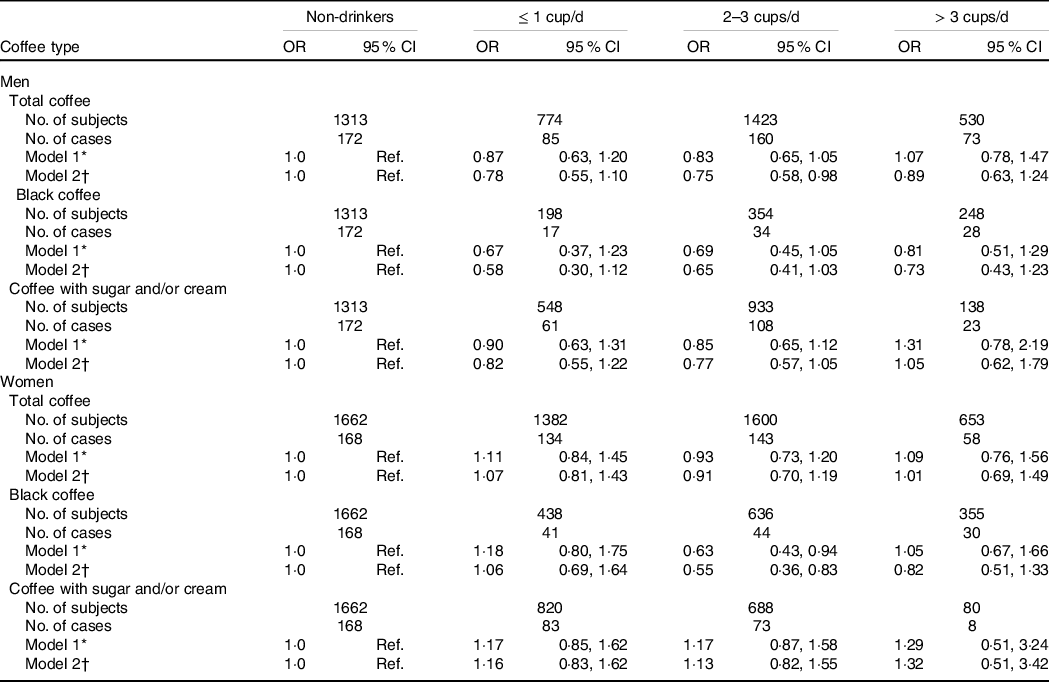
CRP, C-reactive protein; Ref., reference; DQI-K, a modified diet quality index for Koreans.
* Model 1 was adjusted for age.
† Model 2 was adjusted for age, BMI, daily total energy intake, education level, household income, alcohol consumption, smoking status, physical activity, sleep duration, DQI-K, HDL-cholesterol, fasting plasma glucose, TAG, total cholesterol, systolic blood pressure, diastolic blood pressure and leucocyte count.
Discussion
This study examined the association between coffee consumption and high plasma CRP concentrations in a total of 9337 Korean adults, using data from the KNHANES, a large nationally representative study of Korean population. In the current study, we found that 2–3 cups/d of coffee consumption was inversely associated with high CRP levels. By type of coffee, 2–3 cups/d of black coffee was associated with 39 % lower odds of high CRP levels, and the inverse association with moderate black coffee consumption was found both in men and women. However, there was no significant association of coffee with sugar and/or cream and high CRP levels.
Consistent with the results of cross-sectional studies(Reference Hang, Kværner and Ma39,Reference Maki, Pham and Yoshida40,Reference Cornelis and van Dam43,Reference Yamashita, Yatsuya and Muramatsu46,Reference Kotani, Tsuzaki and Sano48,Reference Arsenault, Earnest and Després49,Reference Lopez-Garcia, van Dam and Qi51–Reference Jacobs, Kröger and Floegel54) and one meta-analysis study(Reference Zhang and Zhang45), we observed inverse associations between coffee consumption and high plasma CRP concentrations. Coffee consumption may be associated with several health conditions in a J-shaped relationship(Reference Grosso, Godos and Galvano58). In one meta-analyses, coffee consumption of 2·5 cups per d produced the largest reduction in CVD mortality(Reference Kim, Je and Giovannucci65). One cohort study found a non-linear association of coffee consumption with CVD and all-cause mortality, with the largest risk reduction seen at 2–3 cups/d(Reference Chieng, Canovas and Segan66). In one meta-analysis, an inverse relationship was identified between coffee consumption and risk of CVD, which was the lowest at 3–5 cups/d(Reference Ding, Bhupathiraju and Satija67). In other words, heavy coffee consumption does not further reduce risk of CVD or all-cause mortality compared with moderate coffee consumption. The certain coffee intake level of protective effect varies from study to study, which may be due to the different amounts considered one cup of coffee set in each study. These results of previous studies on the benefits of coffee at the specific level of coffee intake are consistent with our results.
For moderate black coffee consumption of 2–3 cups per d, protective effects may outweigh adverse effects, whereas for heavy black coffee consumption of more than three cups/d, adverse effects may offset protective effects(Reference Ding, Bhupathiraju and Satija67). Coffee contains various bioactive compounds, including caffeine, chlorogenic acid, caffeic acid and kahweol, which can exert protective effects on health via anti-inflammatory and antioxidant capacities(Reference Spagnol, Assis and Brunetti15–Reference Chou, Kao and Yue20). Coffee also contains bioactive components that can adversely affect health. Coffee with a significant amount of diterpenes, with known lipid-raising effects, may be associated with higher LDL-cholesterol levels(Reference Cornelis and van Dam43). Diterpenes in unfiltered coffee may affect the LDL receptor and lead to extracellular accumulation of cholesterol. However, there is no evidence that long-term coffee consumption is associated with dyslipidemia(Reference Grosso, Godos and Galvano58). In addition, caffeine increases blood pressure acutely by antagonising adenosine A1 and A2A receptors(Reference Ding, Bhupathiraju and Satija67). However, tolerance develops rapidly, and the effects of caffeine on long-term blood pressure control are minimal(Reference Chieng, Canovas and Segan66). Another reason that the lower risk of elevated CRP levels disappears with heavy coffee consumption may be the residual confounding effects of smoking, which is an important confounder for the association of coffee consumption with CVD and may bias the relative risk upwards(Reference Kim, Je and Giovannucci65,Reference Chieng, Canovas and Segan66) .
The mechanisms supporting the inverse association between coffee intake and CRP levels may be explained as follows: caffeine, a major component of coffee, has relieved inflammation or reduced CRP levels in several animal studies. Caffeine could decrease plasma CRP levels(Reference Moases Ghaffary and Abtahi Froushani68) and could mitigate pulmonary inflammation(Reference Endesfelder, Strauß and Bendix17,Reference Villanueva-García, Mota-Rojas and Miranda-Cortés18,Reference Chou, Kao and Yue20) and retinal inflammation by showing its anti-inflammatory and neuroprotective effects via A2A adenosine receptor signalling(Reference Conti, Lazzara and Romano69). Another main ingredient in coffee, kahweol, showed anti-inflammatory ability in vivo through the inhibition of COX-2 and iNOS expression at inflammatory sites(Reference Kim, Kim and Jeong19). Additionally, the chlorogenic acid in coffee may decrease the synthesis of inflammatory mediators by lowering the expression of pro-inflammatory cytokine genes, inhibiting protein tyrosine phosphatase 1B and regulating NF-κB activation(Reference Grosso, Godos and Galvano58). Plasma chlorogenic acid exhibited an inverse relationship with CRP levels(Reference Lee, Tan and Hiramatsu70). In addition, the melanoidins produced during coffee bean roasting could reduce chronic inflammation by increasing anti-inflammatory mediators(Reference Moreira, Nunes and Domingues71). However, no experimental studies have determined the association between coffee as a whole and CRP levels directly. Therefore, the underlying mechanisms need to be further examined in future studies.
In this study, the association between coffee with sugar and/or cream and high CRP levels was not significant overall. The inflammatory effect of added sugar may offset the anti-inflammatory effect of coffee. The findings of previous studies on the positive association between sugar intake and high CRP levels support this(Reference Mazidi, Kengne and Mikhailidis72,Reference Tamez, Monge and López-Ridaura73) . A review study has reported that added sugar may need to be included as a potential confounder in studies on the association between coffee consumption and health by sufficient evidence(Reference Thomas and Hodges74).
Moderate black coffee consumption showed a strong inverse association with high CRP levels, especially in women (OR = 0·55, 95 % CI 0·36, 0·83), which is consistent with the previous studies(Reference Gunter, Murphy and Cross52–Reference Jacobs, Kröger and Floegel54). A previous study reported that this association might be caused by female-specific anti-inflammatory effects of coffee(Reference Gunter, Murphy and Cross52). However, previous studies with large sample sizes have shown a significant inverse association between coffee consumption and CRP levels in both men and women(Reference Hang, Kværner and Ma39,Reference Cornelis and van Dam43) . We did not find a significant inverse association between black coffee consumption and high CRP levels in men, but the association between 2–3 cups/d of black coffee consumption and high CRP levels tended to be inverse in men (OR = 0·65, 95 % CI 0·41, 1·03). Additionally, the number of men who drank black coffee was relatively small compared with that of women. Thus, there may not be a sex difference in the association between coffee consumption and high CRP levels.
Our study has some strengths and limitations. To the best of our knowledge, this is the first study using the KNHANES to examine the relationship between coffee consumption and high CRP levels according to the coffee type, separately in men and women. Because this study was based on nationally representative data of the Korean population, our findings can be generalised to Koreans. To reduce the influence of diet and lifestyle factors as potential confounders, we tried to adjust for dietary quality using DQI-K, as well as health behaviours such as alcohol consumption, smoking status, physical activity and sleep duration. Nonetheless, our findings should be interpreted with caution because our study design was cross-sectional, so the sequence of events cannot be inferred, and causal inferences are limited. Those with morbid conditions with highly elevated CRP levels may have reduced coffee consumption(Reference Hyppönen and Zhou75), so we excluded participants with diseases such as stroke, myocardial infarction/angina, renal failure or cancer from the study population. In addition, we could not consider the information on other types of coffee (e.g. caffeinated coffee, decaffeinated coffee, Dutch coffee, etc.), and the amount of caffeine in coffee or caffeine intake through the consumption of other foods such as green tea, black tea or energy drink, because of the limited data or the insufficient number of subjects to analyse. Decaffeinated coffee may have anti-inflammatory effects similar to those of caffeinated coffee due to the action of bioactive constituents other than caffeine(Reference Hang, Kværner and Ma39,Reference Cornelis and van Dam43,Reference Lopez-Garcia, van Dam and Qi51) . Additionally, studies have shown that decaffeinated coffee as well as caffeinated coffee are inversely associated with risk of CVD, cardiovascular mortality and all-cause mortality(Reference Chieng, Canovas and Segan66,Reference Lopez-Garcia, van Dam and Li76,Reference Liu, Li and Shen77) . Another study reported that the inverse association between coffee consumption and cardiometabolic markers or all-cause mortality was not modified by genetic factors affecting caffeine metabolism(Reference Cornelis and van Dam43,Reference Chieng, Canovas and Segan66) . As our study was conducted based on a single measurement of hsCRP levels, random measurement errors would likely attenuate the findings towards the null. Previous studies that measured diet multiple times during study follow-up recorded relatively stable coffee consumption patterns and indicated that a single measurement of coffee intake may reflect medium- to long-term coffee consumption(Reference Gunter, Murphy and Cross52).
Our results differ from those of several previous studies(Reference Youn-Jung, Keun-Mi and Seung-Pil41,Reference Joo-Hyun, Young-Sung and Han-Ul42,Reference Zampelas, Panagiotakos and Pitsavos44,Reference Rebello, Chen and Naidoo47,Reference Moua, Hu and Day50,Reference Stutz, Ahola and Harjutsalo55) examining the relationship between coffee consumption and CRP levels. We found that moderate black coffee consumption of 2–3 cups per d was inversely associated with high CRP levels in Korean adults. The differences may be due to analysis by coffee type, selection of study subjects according to strict criteria and adjustment for diet quality using DQI-K. High-sensitivity CRP levels were measured in the KNHANES. The findings of one meta-analysis suggested that hsCRP was more sensitive than standard CRP in demonstrating the anti-inflammatory capacity of coffee(Reference Zhang and Zhang45).
In conclusion, moderate black coffee consumption of 2–3 cups per d was inversely associated with high CRP levels in Korean adults. These findings contribute to suggesting the pathobiological mechanisms supporting the inverse association between coffee consumption and the risk of chronic diseases such as CVD. Further prospective studies are necessary in large populations to determine the association between various types of coffee consumption and high CRP levels.
Acknowledgements
The authors thank the participants and staff of seventh period (2016–2018) of KNHANES for their valuable contributions.
This work was supported by the National Research Foundation of Korea funded by the Korean government (grant number NRF–2021R1F1A1050847). The National Research Foundation of Korea had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors’ responsibilities were as follows – conceptualisation: Y. J. and S. C.; formal analysis: S. C.; investigation: Y. J. and S. C.; data curation: Y. J. and S. C.; funding acquisition: Y. J.; writing – original draft preparation: S. C.; and writing – review and editing: Y. J. All the authors have read and agreed to the published version of the manuscript.
There are no conflicts of interest.







