Introduction
Life-limiting conditions are those for which there is no possibility of cure, where death is inevitable at some point in time during childhood or young adulthood (McNamara-Goodger and Feudtner, Reference McNamara-Goodger, Feudtner, Goldman, Hain and Liben2012; Fraser and Parslow, Reference Fraser and Parslow2018). Pediatric palliative care (PC) is a multidisciplinary approach that improves the quality of life of this population and their families through preparing them for an anticipated death through providing physical, psychosocial, and spiritual supports, as well as managing complex conditions during the end of life (EoL; Chambers, Reference Chambers2018). The number of children, adolescents, and young adults with life-limiting conditions is increasing as a result of increased survival and earlier recognition of life-limiting diagnoses. The United Kingdom data suggest that 66.4 per 10,000 individuals in pediatric populations aged from 0 to 19 years were living with life-limiting conditions in 2017, and the prevalence is likely to rise to 84.2 per 10,000 by 2030 (Fraser et al., Reference Fraser, Gibson-Smith and Jarvis2020). However, it was estimated that only 18.6% of them actually received PC before death (Widger et al., Reference Widger, Davies and Rapoport2016).
The majority of this population goes through a prolonged period of inpatient admission, primarily in intensive care unit (ICU) settings during EoL, and die in the hospital (Gao et al., Reference Gao, Verne and Peacock2016; DeCourcey et al., Reference DeCourcey, Silverman and Oladunjoye2018). Only 58% of children, adolescents, and young adults with cancer actually died at home as their place of preference (Stilwell et al., Reference Stilwell, Bhatt and Mehta2020). A growing body of evidence demonstrates that receiving early PC improves the quality of life and symptom control, facilitates earlier advanced care planning or planned withdrawal of ventilator support outside the ICU setting, and enables a choice in the place of death (PoD) outside of hospital (Abel et al., Reference Abel, Pring and Rich2013; Laddie et al., Reference Laddie, Craig and Brierley2014; Mitchell et al., Reference Mitchell, Morris and Bennett2017). Nevertheless, several patient and family-related factors influence the EoL decision-making and the PoD (Allen, Reference Allen2014; Foster et al., Reference Foster, Whitehead and Maybee2016). Thus, there is limited scientific evidence by which to illustrate the impacts of PC on children, adolescents, and young adults with life-limiting conditions in terms of their healthcare circumstances and outcomes during the EoL.
Recent systematic reviews suggest that access to PC is associated with reduced time in hospital and less invasive treatments (Marcus et al., Reference Marcus, Santos and Ciapponi2020; Tayloy et al., Reference Taylor, Booth and Beresford2020; Zuniga-Villanueva et al., Reference Zuniga-Villanueva, Widger and Medeiros2020). However, these reviews specifically evaluated the outcomes of specialist PC services, which were developed mainly in the USA, Canada, the UK, and across Europe (Knapp et al., Reference Knapp, Woodworth and Wright2011). Thus, the evidence base is not yet able to offer a universal PC model, especially in the case of non-Western countries. Furthermore, these reviews aggregated the results for patients at earlier stages of their illness. The evidence of the impact on EoL care and the PoD, including studies on PC without further subdivisions of the general and specialist levels of services, remains unclear. The aim of this study is to determine the impact of PC on EoL care and PoD for children, adolescents, and young adults with life-limiting conditions.
Methods
A systematic review was conducted to evaluate the academic literature related to PC in order to determine if the use of PC compared with not using it impacts on EoL care and the PoD. This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocol (PRISMA-P) guidelines (Moher et al., Reference Moher, Shamseer and Clarke2015).
Inclusion and exclusion criteria
We included studies if their target population was children up to 25 years of age with a life-limiting condition; the exposure of interest was self-described as “palliative care” and/or comprised at least two components of PC, including general and/or specialist PC, which means PC delivered by a healthcare team who are/are not PC specialists, as defined by the National Consensus Project for Quality Palliative Care (2018). The researchers provided a comparison between PC exposure and at least one of the outcomes of admission in terms of acute care beds, length of hospital stay, receiving cardiopulmonary resuscitation (CPR) before death, PoD, and the intensity of EoL care. Publications in English, Mandarin, or Bahasa were considered based on the language competencies of the reviewers.
Studies were excluded where the type of intervention was exclusively on only one component of comprehensive PC (e.g., providing opioid and sedation medications or advanced care planning) or included a mixed sample of adults aged over 25 years. Assessments of EoL care were not included if data in the last year of life could not be fully determined. Outcomes of the presence of limitations related to resuscitation orders were not included if the congruence of the presence of limitations of resuscitation orders and the actual CPR attempted at the time of death could not be fully determined. Cross-sectional descriptive studies and qualitative studies were excluded.
Search strategy
The following online databases were searched: Medline, EMBASE, PubMed, Cochrane Library, CINAHL, Airiti, GARUDA Garba Rujukan Digital, and OpenGrey from 2010 to February 5, 2020, where a series of pediatric PC in various settings had been implemented over time. The search terms used included the recognized Medical Subject Heading (MeSH) and Embase subject headings (Emtree) with various combinations of the terms children, adolescents, young adults, palliative, end-of-life, place of death, and specific diagnoses from the International Classification of Disease (ICD10) coding framework (Fraser et al., Reference Fraser, Miller and Hain2012) (Supplementary Tables 1–5 in the Supplementary File for search strategy).
Screening and selection
Retrieved references were imported using the EndNote. S.-C.L. and D.Y. screened and selected all articles by title and abstract independently using a prespecified screening and selection tool. Disagreements were discussed with M.-C.H. until a consensus was reached.
Quality assessment
The quality of the individual studies was critically graded by applying the Joanna Briggs Institute (JBI) appraisal tools based on appropriate checklists. The checklists included assessment of the methodological quality of the study and any possibility of bias in its design, procedures, or analyses, scored as “yes” (2), “unclear” (1), “no” (0), or “not applicable.” The summary scores for each study were derived by calculating the total scores of relevant items. S.-C.L. and D.Y. independently assessed all papers. Any disagreement was resolved through a discussion with M.-C.H.. Scores were expressed as a percentage, where >80% was considered strong; >70% was considered good; >50% was considered adequate, and ≤50% was considered limited. We did not exclude studies based on a quality assessment.
Data extraction
Data extraction was conducted by one reviewer (S.-C.L.), using an extraction form piloted on three eligible studies. Key study characteristics, including the country of origin, setting, sample data, participant characteristics, intervention vs. control, outcomes, and measurement tools, were extracted. For this study, home and community PC was defined as any type of the availability of palliative home care services, 24-h on-call service, or a 24-h outreach pediatric PC team.
If the intervention effects were completely reported, pooled effect estimate (e.g., odds ratio and 95% confidence intervals) would be calculated using the random effects model in RevMan 5.4.1 where possible. Fisher's method for combining p-values or vote counting based on the direction of effects was used if there was minimal reporting of the data beyond p-values and the direction of effect, or the type of effect measure varied across the studies (McKenzie and Brennan, Reference McKenzie, Brennan, Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch2019).
Results
Study selection
A total of 6,461 studies were identified through electronic database searches, and seven studies were identified through reference lists. After duplicates were removed, title/abstract screening, and full-text screening, 15 studies met the eligibility criteria. A detailed illustration of the study selection process is presented in Figure 1.

Fig. 1. PRISMA flow diagram.
Risk of bias
The critical appraisal scores ranged between 45% and 95%. Seven of the 15 studies were judged to be of strong quality (Thienprayoon et al., Reference Thienprayoon, Lee and Leonard2013; Smith et al., Reference Smith, Andrews and Bratton2015; Ullrich et al., Reference Ullrich, Lehmann and London2016; Fraser et al., Reference Fraser, Fleming and Parslow2018; Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019; Spraker-Perlman et al., Reference Spraker-Perlman, Tam and Bardsley2019) (Tables 2 and 3).
Study characteristics
Characteristics of the included studies are presented in Table 1. Of the 15 studies, nine were conducted in the USA, and one each was conducted in Canada, France, Germany, New Zealand, Singapore, and the UK. The studies were published from 2013 to 2019 as cohort studies (n = 14) and case series (n = 1).
Table 1. Baseline characteristics of the included studies (n = 15)

HI-EoL: high-intensity end-of-life care; PC: palliative care; PoD: place of death; PPC: pediatric palliative care; RCoh: retrospective cohort; SPPC: specialist pediatric palliative care.
a Information on the relevant data source, year of data, and sample included in the review.
Participant characteristics
The study samples ranged from 60 to 24,342 for a total of 36,148 subjects. All studies considered both boys and girls. Subject aged from seven of the 15 studies ranged from 0 to 4 years old (67.7%), 5 to 14 years old (19.0%), and 15 to 25 years old (13.3%) (Chang et al., Reference Chang, MacLeod and Drake2013; Keele et al., Reference Keele, Keenan and Sheetz2013; Snaman et al., Reference Snaman, Kaye and Lu2017; Fraser et al., Reference Fraser, Fleming and Parslow2018; Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019; Spraker-Perlman et al., Reference Spraker-Perlman, Tam and Bardsley2019). Diagnoses of the subjects included neonatology/chromosome disorder (27.6%), respiratory disease (15.1%), hematology/oncology disease (15.4%), cardiology/circulatory disease (12.6%), neurological disease (8.1%), gastrointestinal/liver disease (5.4%), immunology/infectious disease (4.4%), trauma, and other (11.4%).
Intervention/control characteristics
Eleven studies reported that PC was implemented in an inpatient hospital setting (Chang et al., Reference Chang, MacLeod and Drake2013; Keele et al., Reference Keele, Keenan and Sheetz2013; Smith et al., Reference Smith, Andrews and Bratton2015; Vern-Gross et al., Reference Vern-Gross, Lam and Graff2015; Osenga et al., Reference Osenga, Postier and Dreyfus2016; Ullrich et al., Reference Ullrich, Lehmann and London2016; Snaman et al., Reference Snaman, Kaye and Lu2017; Fraser et al., Reference Fraser, Fleming and Parslow2018; Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019; Spraker-Perlman et al., Reference Spraker-Perlman, Tam and Bardsley2019); two studies implemented services in an inpatient hospice setting (Thienprayoon et al., Reference Thienprayoon, Lee and Leonard2013; Fraser et al., Reference Fraser, Fleming and Parslow2018), and 11 studies implemented services in home and community settings (Chang et al., Reference Chang, MacLeod and Drake2013; Thienprayoon et al., Reference Thienprayoon, Lee and Leonard2013; Friedrichsdorf et al., Reference Friedrichsdorf, Postier and Dreyfus2015; Vern-Gross et al., Reference Vern-Gross, Lam and Graff2015; Osenga et al., Reference Osenga, Postier and Dreyfus2016; Ullrich et al., Reference Ullrich, Lehmann and London2016; Chong et al., Reference Chong, De Castro Molina and Teo2018; Fraser et al., Reference Fraser, Fleming and Parslow2018; Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019; Zernikow et al., Reference Zernikow, Szybalski and Hübner-Möhler2019). The control groups in all studies included subjects with either no PC team involvement or no active PC orders.
Outcome characteristics
Data on demographics, hospital admission, LoS, CPR at the time of death, PoD, and intensity of EoL care were extracted from national databases, regional databases, medical records, and surveys. Significance was defined as p < 0.05.
Number of admissions in acute care beds
The combination of p-values in three studies suggests that there was strong effect of PC in reducing the number of hospital admissions in at least one study (p < 0.001) (Chong et al., Reference Chong, De Castro Molina and Teo2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019; Zernikow et al., Reference Zernikow, Szybalski and Hübner-Möhler2019). Children and adolescents aged < 18 who received home-based PC had fewer hospital admissions during their last year of life, but no differences were found in the number of emergency visits (Chong et al., Reference Chong, De Castro Molina and Teo2018). Studies also varied in terms of whether or not they found differences in the number of hospital admissions in the last month of life for children, adolescents, and young adults with cancer (Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019; Zernikow et al., Reference Zernikow, Szybalski and Hübner-Möhler2019) (Tables 2 and 3).
Table 2. Critical appraisal results of eligible studies using the JBI critical appraisal checklist for cohort studies (n = 14)
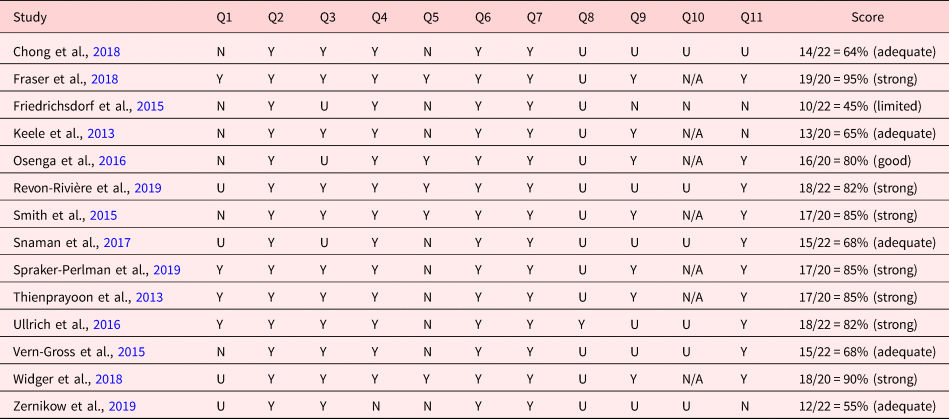
Y, yes; U, unclear; N, no; N/A, not applicable.
Y=2; U=1; N = 0 points
Q1= Were the two groups similar and recruited from the same population?
Q2 = Were the exposures measured similarly to assign people to both exposed and unexposed groups?
Q3 = Was the exposure measured in a valid and reliable way?
Q4 = Were confounding factors identified?
Q5 = Were strategies to deal with confounding factors stated?
Q6 = Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)?
Q7 = Were the outcomes measured in a valid and reliable way?
Q8 = Was the follow-up time reported and sufficient to be long enough for outcomes to occur?
Q9 = Was follow up complete, and if not, were the reasons to loss to follow up described and explored?
Q10 = Were strategies to address incomplete follow-up utilized?
Q11 = Was appropriate statistical analysis used?
Table 3. Critical appraisal results of eligible studies using the JBI critical appraisal checklist for case series (n = 1)

Y, yes; U, unclear; N, no
Y=2; U=1; N = 0 points
JBI critical appraisal checklist for case series:
Q1 = Were there clear criteria for inclusion in the case series?
Q2 = Was the condition measured in a standard, reliable way for all participants included in the case series?
Q3 = Were valid methods used for the identification of the condition for all participants included in the case series?
Q4 = Did the case series have consecutive inclusion of participants?
Q5 = Did the case series have complete inclusion of participants?
Q6 = Was there clear reporting of the demographics of the participants in the study? Q7 = Was there clear reporting of clinical information of the participants?
Q8 = Were the outcomes or follow-up results of cases clearly reported?
Q9 = Was there clear reporting of the presenting site(s)/clinic(s) demographic information?
Q10 = Was statistical analysis appropriate?
A consistent finding across studies was that children, adolescents, and young adults who received inpatiant hospital PC, either with or without the provision of home and community PC, were less likely to be admitted to the ICU (Keele et al., Reference Keele, Keenan and Sheetz2013; Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019). Among children, adolescents, and young adults who died for any reason, those who received PC had a 71% reduction in risk of ICU admission (Keele et al., Reference Keele, Keenan and Sheetz2013); for those who died with cancer, receiving PC was associated with a 6.25-fold decrease in the odds of ICU admission (OR, 0.16; 95% CI 0.13–0.20) (Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019). If those who died due to treatment-related causes (e.g., acute toxicities) were excluded, there was a 3.33-fold decrease in the odds of ICU admission during the last month of life. However, general PC had no impact (Widger et al., Reference Widger, Sutradhar and Rapoport2018). These results are presented in Table 4.
Table 4. Description of the included papers comparing number of admissions in acute care beds for people with PC involvement vs. those without it (n = 5)
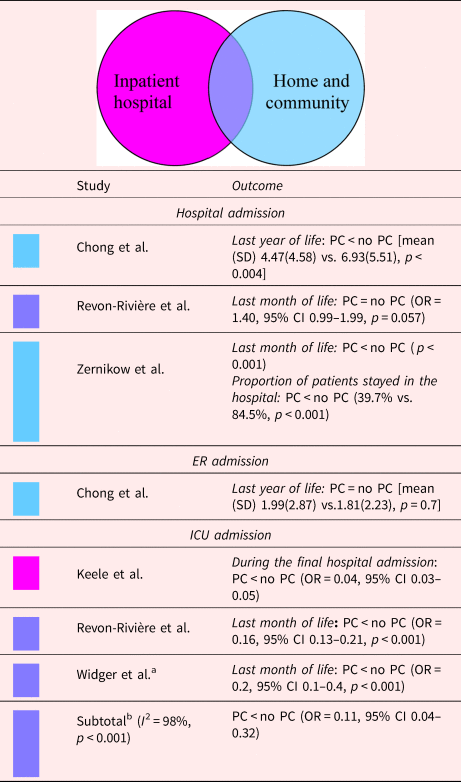
CI, confidence interval; OR, odds ratio; PC, palliative care.
Shading depicts the PC model.
a Data on the sample with all cause of death.
b Random-effects meta-analysis on the association between PC and the number of admissions in the ICU.
Length of hospital stay
The combination of p-values in five studies suggests that there was an effect in reducing the LoS in the EoL in at least one study (p = 0.011, five studies) (Keele et al., Reference Keele, Keenan and Sheetz2013; Smith et al., Reference Smith, Andrews and Bratton2015; Osenga et al., Reference Osenga, Postier and Dreyfus2016; Chong et al., Reference Chong, De Castro Molina and Teo2018; Spraker-Perlman et al., Reference Spraker-Perlman, Tam and Bardsley2019). Even an analysis restricted to studies where during intervention, only inpatient hospital PC was delivered, suggested an increased likelihood of shorter LoS in the PC group in at least one study (p = 0.0167, three studies) (Keele et al., Reference Keele, Keenan and Sheetz2013; Smith et al., Reference Smith, Andrews and Bratton2015; Spraker-Perlman et al., Reference Spraker-Perlman, Tam and Bardsley2019) (Table 5).
Table 5. Description of the included papers comparing length of hospital stay for people with palliative care involvement vs. those without it (n = 5)
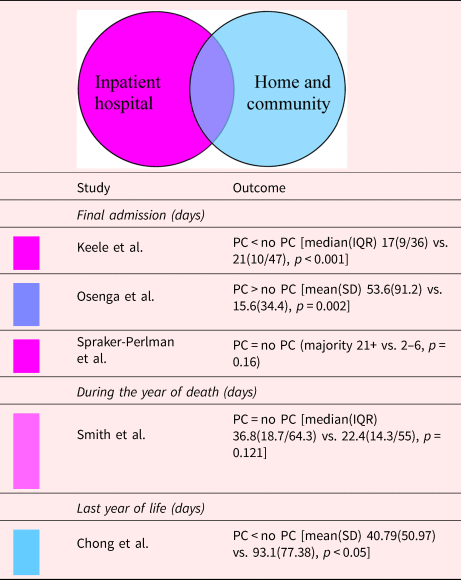
IQR, interquartile range; PC, palliative care; SD, standard deviation.
Shading depicts the PC model.
aData on the sample with all cause of death.
However, of the five studies examining LoS, in two of them, no differences in terms of cumulative LoS were observed in the last admission, last month, or last year of life [40% (95% CI 5–85%), p = 1.625] (Keele et al., Reference Keele, Keenan and Sheetz2013; Chong et al., Reference Chong, De Castro Molina and Teo2018). Only one study excluded those who were hospitalized <5 days before death (Keele et al., Reference Keele, Keenan and Sheetz2013). Four of five studies did not exclude patients who died quickly after hospital admission, which may have created bias since those who received PC were more likely to have complex, critical illnesses with longer disease trajectories; on the contrary, those with short LoS or unexpected death usually lacked access to PC (Keele et al., Reference Keele, Keenan and Sheetz2013; Osenga et al., Reference Osenga, Postier and Dreyfus2016). Therefore, the association between PC and LoS tended to be biased.
CPR at the time of death
Of the three studies examining CPR at the time of death, all but one showed that patients who underwent hematopoietic stem cell transplants were less likely to undergo CPR at the EoL (p = 0.03) (Ullrich et al., Reference Ullrich, Lehmann and London2016). However, studies on infants, or adolescents, and young adults, with a median age of 1.3 months and 17.3 years, respectively, found no difference in terms of CPR attempted at the time of death (Osenga et al., Reference Osenga, Postier and Dreyfus2016; Snaman et al., Reference Snaman, Kaye and Lu2017). Even though two of the three studies did find the median resuscitation discussion to be 5 days earlier (p < 0.001) or increased do-not-resuscitate (DNR) orders in place in patients followed by a PC team (AOR = 7.92, 95% CI 2.02–31.12) (Osenga et al., Reference Osenga, Postier and Dreyfus2016; Ullrich et al., Reference Ullrich, Lehmann and London2016). This result is presented in Table 6.
Table 6. Description of the included papers comparing CPR at the time of death for people with PC involvement vs. those without it (n = 3)
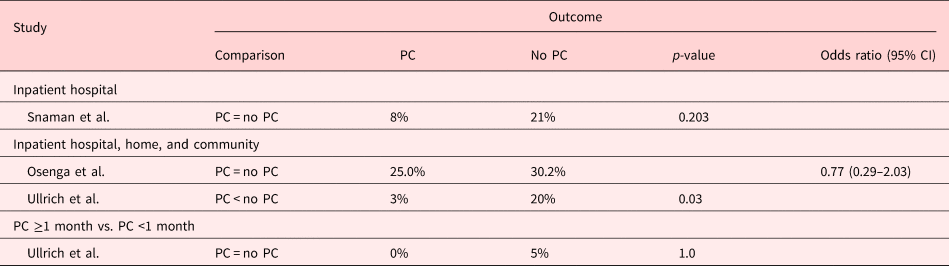
CI: confidence interval; PC: palliative care.
Place of death
Even though PC was accessible in inpatient, home, and community settings, the examined studies varied in terms of whether the patients in the PC group were less likely to die in a medical setting (i.e., hospital and ICU), or more likely to die in the community (i.e., home, hospice, and home hospital services). Four of six studies indicated that the PC group had a smaller proportion of deaths in a medical setting [67% (95% CI 22–96%), p = 0.7] (Chang et al., Reference Chang, MacLeod and Drake2013; Thienprayoon et al., Reference Thienprayoon, Lee and Leonard2013; Fraser et al., Reference Fraser, Fleming and Parslow2018; Widger et al., Reference Widger, Sutradhar and Rapoport2018); four of six studies showed PC to lead to a greater proportion of deaths in the community [67% (95% CI 22–96%), p = 0.7] (Thienprayoon et al., Reference Thienprayoon, Lee and Leonard2013; Friedrichsdorf et al., Reference Friedrichsdorf, Postier and Dreyfus2015; Fraser et al., Reference Fraser, Fleming and Parslow2018; Zernikow et al., Reference Zernikow, Szybalski and Hübner-Möhler2019) (Table 7). In the case of children in the PICU, those who died after receiving PC at discharge who had either transferred hospitals or had moved into the community had an 8.06 times greater chance of not dying in a medical setting compared with those who were not discharged for PC (Fraser et al., Reference Fraser, Fleming and Parslow2018). However, if restricted to cancer patients, the possibility of dying in the hospital was comparable regardless of whether there was PC in inpatient, home, and community settings (Thienprayoon et al., Reference Thienprayoon, Lee and Leonard2013; Vern-Gross et al., Reference Vern-Gross, Lam and Graff2015; Ullrich et al., Reference Ullrich, Lehmann and London2016; Widger et al., Reference Widger, Sutradhar and Rapoport2018). Although home hospital services were accessible, and EoL discussions occurred earlier (median time from first discussion to death was 204 days earlier), there was no significant decrease in inpatient deaths after the implementation of PC (Vern-Gross et al., Reference Vern-Gross, Lam and Graff2015). However, if the intervention included the delivery of hospice care, PC was associated with being more likely to die at home (Thienprayoon et al., Reference Thienprayoon, Lee and Leonard2013; Fraser et al., Reference Fraser, Fleming and Parslow2018).
Table 7. Description of the included papers comparing place of death for people with PC involvement vs. those without it (n = 10)
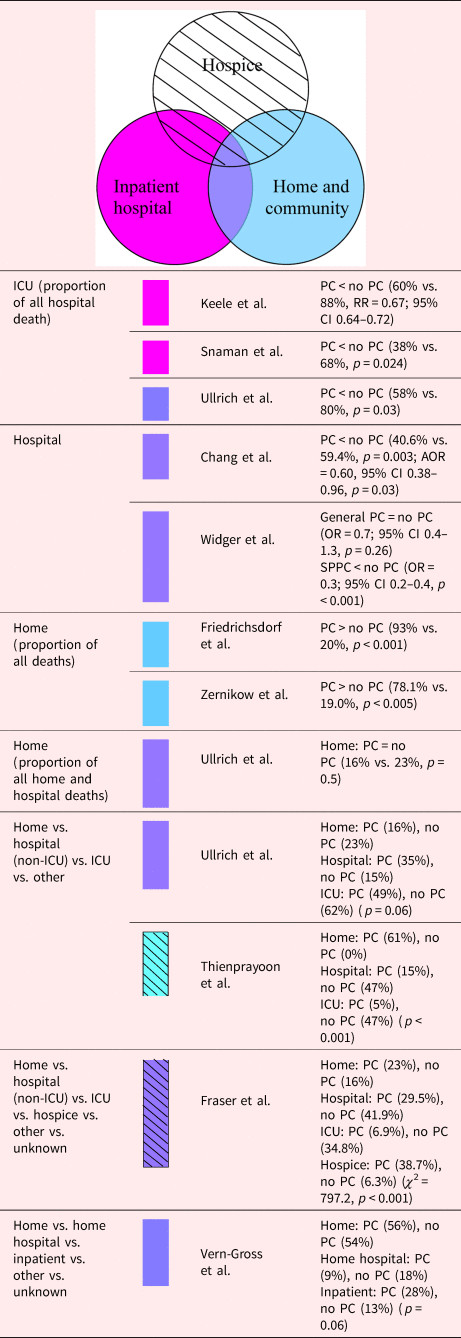
CI, confidence interval; OR, odds ratio; PC, palliative care; RR, relative risk.
Shading depicts the PC model.
In at least one study, there was strong evidence suggesting that PC in reduces the proportion of ICU deaths in all hospital deaths (p = 0.0017, two studies) (Ullrich et al., Reference Ullrich, Lehmann and London2016; Snaman et al., Reference Snaman, Kaye and Lu2017). Both with and without the delivery of inpatient hospice, home, and community PC, all four studies showed that inpatient hospital PC decreased the proportion of patients dying in an ICU (Keele et al., Reference Keele, Keenan and Sheetz2013; Ullrich et al., Reference Ullrich, Lehmann and London2016; Snaman et al., Reference Snaman, Kaye and Lu2017; Fraser et al., Reference Fraser, Fleming and Parslow2018). Furthermore, Ullrich et al. (Reference Ullrich, Lehmann and London2016) reported that there were no differences in receiving less or more than 1 month of PC before death in a cure-oriented stem cell transplantation setting.
Intensity of EoL care
Two studies reported results based on the intensity of EoL care summary scores (Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019) (Table 8). Both defined high-intensity EoL care (HI-EoL) as the occurrence of at least one of the following four indicators: intrahospital intravenous chemotherapy <14 days prior to death, ≥1 ICU admission, >1 emergency room visit, or >1 hospital admission in the last month of life. General PC was associated with a 1.67-fold decrease in the odds of HI-EoL (Widger et al., Reference Widger, Sutradhar and Rapoport2018), whereas specialist inpatient hospital PC, either with or without the provision of home and community PC, was associated with a 3.57-fold decrease in the odds of HI-EoL care during the last month of life for children aged 0–25 who died as a result of cancer (OR, 0.28; 95% CI 0.20–0.40) (Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019). However, if those who died due to treatment-related causes were included, general PC alone was not associated with a lower intensity of the EoL care. These patients were more likely to have had hematologic malignancies and were significantly less likely to have accessed specialist PC services (Widger et al., Reference Widger, Sutradhar and Rapoport2018).
Table 8. Description of the included papers comparing composite high-intensity EoL care indicator for people with PC involvement vs. those without it (n = 2)
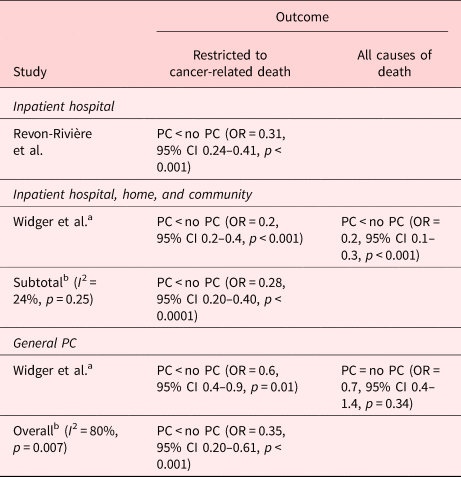
CI, confidence interval; PC, palliative care.
a Data on the sample with all cause of death.
b Random-effects meta-analysis on the association between PC and the intensity of EoL care.
Discussion
This review examined the impact of PC on EoL care and PoD in children, adolescents, and young adults with life-limiting conditions based on different models of care. The results demonstrated that those who receive PC are less likely to be admitted to ICUs, are less likely to receive HI-EoL care, and are less likely to die in ICUs. However, there was less evidence of effects of PC on LoS in the EoL, receiving CPR at the time of death, and the proportion of hospital and home deaths. Several possible explanations exist, including the fact that the symptom burden may be better managed in a medical setting than in the community during EoL (Clark et al., Reference Clark, Connolly and Clapham2016; Zernikow et al., Reference Zernikow, Szybalski and Hübner-Möhler2019) or that access to PC may have an impact on decisions to receive less intervention-focused care aimed at extending life (e.g., ICU care) but not on medical care during the EoL. This also suggests that intensivists and those providing acute care need to incorporate effective PC communication skills and improve team cohesiveness with the primary clinical team and specialist PC team (Richards et al., Reference Richards, Starks and O'Connor2018).
Regarding the circumstances of death, mixed results were observed for the impact of CPR attempts at the time of death in the PC group. There was a lack of homogeneity across these studies in terms of the ages and diagnoses included in the samples, model of care, and the time between PC involvement and death. Although all studies indicated finding PC to have benefits in terms of promoting DNR orders in place (Osenga et al., Reference Osenga, Postier and Dreyfus2016; Ullrich et al., Reference Ullrich, Lehmann and London2016; Snaman et al., Reference Snaman, Kaye and Lu2017), its impact on reductions in the number of pediatric patients undergoing CPR at the time of death at the request of the patient and/or caregiver showed little consensus. Comparable to previous studies (Mack et al., Reference Mack, Chen and Boscoe2015; Johnston et al., Reference Johnston, Alvarez and Saynina2017), studies in this review revealed that studies on toddlers, as well as adolescents and young adults with cancer, indicated that PC has little impact on reducing the number of cases with CPR attempts at the time of death (Osenga et al., Reference Osenga, Postier and Dreyfus2016; Snaman et al., Reference Snaman, Kaye and Lu2017). Several possible explanations exist, including the fact that adolescents and young adults have better competency related to expressing their desire to accept invasive treatments out of a desire to sustain their lives (Hinds et al., Reference Hinds, Drew and Oakes2005). In goals of care and level of hope in patients receiving stem cell transplants may differ from those with complex chronic conditions, which has implications for EoL care decision-making.
Although previous studies have shown that an increase in home-based PC resources may reflect an increase in home deaths (Webber et al., Reference Webber, Viola and Knott2019; Håkanson et al., Reference Håkanson, Öhlén and Kreicbergs2017), the impact of inpatient, home, and community PC on increasing the number of home deaths is inconsistent in this review. However, studies where the intervention included the delivery of hospice care reported PC to be associated with being more likely to die at home as compared with no PC (Thienprayoon et al., Reference Thienprayoon, Lee and Leonard2013; Fraser et al., Reference Fraser, Fleming and Parslow2018). Complex symptoms may be better dealt with in hospitals or hospices than would be the case at home. The differences in the impact of PC on PoD may also related to the distance from a tertiary center (Kassam et al., Reference Kassam, Sutradhar and Widger2017), different health system infrastructures, policies, socio-economic conditions (Håkanson et al., Reference Håkanson, Öhlén and Kreicbergs2017), and cultural differences.
Limitations of the included studies
Although this study adds to the literature through a systematic review intended to provide evidence for the association of PC with five important outcomes, the results should be interpreted cautiously given the lack of experimental evidence and the heavily reliance on unadjusted estimates based on observational studies. In 11 studies, there were concerns about the clarity of the inclusion and exclusion criteria or about similarities in the characteristics of the two groups under consideration (Chang et al., Reference Chang, MacLeod and Drake2013; Keele et al., Reference Keele, Keenan and Sheetz2013; Friedrichsdorf et al., Reference Friedrichsdorf, Postier and Dreyfus2015; Smith et al., Reference Smith, Andrews and Bratton2015; Vern-Gross et al., Reference Vern-Gross, Lam and Graff2015; Osenga et al., Reference Osenga, Postier and Dreyfus2016; Snaman et al., Reference Snaman, Kaye and Lu2017; Chong et al., Reference Chong, De Castro Molina and Teo2018; Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019; Zernikow et al., Reference Zernikow, Szybalski and Hübner-Möhler2019). Of the 14 cohort studies, outcomes were adjusted for demographic and clinical variables in the analyses in only five of them (Smith et al., Reference Smith, Andrews and Bratton2015; Osenga et al., Reference Osenga, Postier and Dreyfus2016; Fraser et al., Reference Fraser, Fleming and Parslow2018; Widger et al., Reference Widger, Sutradhar and Rapoport2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019). Possible confounding by family preference was not controlled for in any of the included studies. The duration of time from receiving PC to death was stated in only one study (Ullrich et al., Reference Ullrich, Lehmann and London2016); therefore, there were concerns as to whether the follow-up time of the PC involvement in the intervention group was sufficiently long enough for outcomes to occur. This review was unable to reveal differences in the impact of PC among children, adolescents, and young adults referred to PC based on whether it occurred early and late in their illness trajectory.
There were concerns about the representativeness of samples. Subjects in seven of the 15 studies (46.7%) comprised only patients who had been hospitalized and died in hospital in order to collect accurate EoL care data (Keele et al., Reference Keele, Keenan and Sheetz2013; Osenga et al., Reference Osenga, Postier and Dreyfus2016; Ullrich et al., Reference Ullrich, Lehmann and London2016; Snaman et al., Reference Snaman, Kaye and Lu2017; Chong et al., Reference Chong, De Castro Molina and Teo2018; Revon-Rivière et al., Reference Revon-Rivière, Pauly and Baumstarck2019; Spraker-Perlman et al., Reference Spraker-Perlman, Tam and Bardsley2019). Subjects in seven of the 15 studies (46.7%) used a single-site design (Friedrichsdorf et al., Reference Friedrichsdorf, Postier and Dreyfus2015; Smith et al., Reference Smith, Andrews and Bratton2015; Vern-Gross et al., Reference Vern-Gross, Lam and Graff2015; Osenga et al., Reference Osenga, Postier and Dreyfus2016; Ullrich et al., Reference Ullrich, Lehmann and London2016; Snaman et al., Reference Snaman, Kaye and Lu2017; Spraker-Perlman et al., Reference Spraker-Perlman, Tam and Bardsley2019). The primary diagnoses of the subjects varied, with the majority having neonatology/chromosome disorders, respiratory diseases, and hematology/oncology diseases. The distribution of diagnosis among the groups of subjects may have been different from the general pediatric population in different locations. There was a concentration of studies from the USA. Although studies involving Chinese and Bahasa populations were included, and Chinese and Indonesian databases were searched, no studies conducted using these languages were found. EoL care reflects child and family preferences, which is highly relationship-based (Hinds et al., Reference Hinds, Drew and Oakes2005) and culturally related. Therefore, more population-based cohort studies assessing the impact of PC are needed in non-Western countries.
Strengths and limitations of the review
This was the first systematic review to demonstrate the impact of PC on EoL care and PoD in children, adolescents, and young adults with life-limiting conditions. Since this review focused on studies published from 2010 to 2020, this increased the homogeneity of the progress of curative treatments and the recognition of the need for PC in the pediatric population. Only studies comparing those who actually received and did not receive PC were included, as opposed to comparing those with and without access to such services. This ensured that all subjects in the intervention groupf did receive PC. No included studies compared groups using a before–after design. This decreased the potential impact on the EoL care outcomes due to the progression of the illness. This review was not restricted to a specific PC model in order to allow a comparison of impacts across different components of intervention while also limiting the outcome of hospital admission and LoS to the last year of life. This widened the scope of the included studies and enriched the data synthesis, but the outcome may not be generalizable to a population at the early diagnosis stage.
Outcomes in this review may have been influenced by differences in the characteristics of the intervention and control groups. Patients who died due to any cause were considered as being in life-limiting conditions and were included in this review. For those who had better prognoses and received a significant amount of intensive care, but subsequently died with iatrogenic complications, caregivers and healthcare providers may have been less aware of the availability of specialist PC resources, or patients and caregivers may have been less likely to be referred for such care (Ullrich et al., Reference Ullrich, Lehmann and London2016; Widger et al., Reference Widger, Sutradhar and Rapoport2018). These groups may have increased ICU admissions, HI-EoL, been denied PC team involvement, or died in the hospital, which led to a concern related to possible referral bias.
Implications for clinical practice and research
Our results provide insights into PC resource allocation throughout the last year of life. To the best of our knowledge, there were only two articles to date comparing HI-EoL care between oncology pediatric patients with and without PC. One randomized controlled trial examining the impact of PC on infants with prenatal single-ventricle diagnoses was excluded from this review because the outcomes did not meet the last year of life criteria (Hancock et al., Reference Hancock, Pituch and Uzark2018). More research is needed related to developing suitable indicators for the purpose of investigating the intensity of EoL care among non-cancer pediatric patients. Due to concerns about the heterogeneity of the populations, interventions, outcome measures, and study designs, it was not appropriate to conduct a further meta-analysis.
Conclusion
In this work, PC was associated with a reduction in ICU admissions, ICU deaths, and HI-EoL care in the last month of life. The findings reveal an enormous ongoing gap in the understanding of whether pediatric PC is generally useful in reducing hospital admissions and LoS during EoL, reducing futile CPR, and avoiding hospital death. Based on this review, we make the following recommendations: first, specialist and multidisciplinary PC as well as the continuity of PC are required to reduce HI-EoL care. Second, careful attention should be paid to the need for a longer LoS in a medical setting late in life, and earlier EoL care discussion should take place with patients/caregivers especially in regard to attempting CPR in toddlers, adolescents, and the young adult population. Third, a lack of robust evidence led to identifying a gap in rigorous, comprehensive, and multisite prospective studies of data collection focusing on the impact of PC on EoL care and PoD for pediatric patients between 0 and 25 years of age. In addition, there may be reason to believe the patterns of utilization vary in different areas around the world, which necessitates the inclusion of cultural factors in future data collection.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1478951520001455.
Funding
This research was supported by National Cheng Kung University Hospital (NCKUH-10909026).
Conflict of interest
None declared.














