Impact statement
Mass extinction events represent the greatest catastrophes in the history of animal life and only five major extinction events have occurred across the past 550 million years. Geological evidence can reveal the physical and chemical processes that caused environmental change, but differences in morphological, ecological, and physiological traits between extinction victims and survivors provide our best record of actual kill mechanisms. In recent years, this field has advanced both through the compilation of experimental data on organismal traits, enabling new insights into extinction patterns, and through the development of mechanistic models for biological response to environmental change, enabling incorporation of physiological tolerance into climate models to predict extinction patterns. Ultimately, mass extinction events are a critical source of data to calibrate the magnitude and rate of biological response to climate change over timescales longer than those of experiments and field studies. In this way, integration of information from the fossil record is becoming essential to the task of predicting and mitigating taxonomic losses due to current environmental change.
Introduction
Earth is currently undergoing a biodiversity crisis on a scale unprecedented in the history of the human species (Barnosky et al., Reference Barnosky, Matzke, Tomiya, Wogan, Swartz, Quental, Marshall, McGuire, Lindsey, Maguire, Mersey and Ferrer2011; Dirzo et al., Reference Dirzo, Young, Galetti, Ceballos, Isaac and Collen2014; McCauley et al., Reference McCauley, Pinsky, Palumbi, Estes, Joyce and Warner2015), but crises of similar or greater magnitude have occurred at least five times across the 600-million-year history of animal life (Figure 1A) (Raup and Sepkoski, Reference Raup and Sepkoski1982; Barnosky et al., Reference Barnosky, Matzke, Tomiya, Wogan, Swartz, Quental, Marshall, McGuire, Lindsey, Maguire, Mersey and Ferrer2011). All major mass extinction events are associated with evidence of rapid environmental change. In some cases, such as the end-Permian (252 million years ago [Mya]) and end-Triassic (201 Mya) mass extinctions, there is evidence for rapid and pronounced climate warming (Kiessling and Simpson, Reference Kiessling and Simpson2011; Payne and Clapham, Reference Payne and Clapham2012; Blackburn et al., Reference Blackburn, Olsen, Bowring, McLean, Kent, Puffer, McHone, Rasbury and 'Et-Touhami2013; Burgess et al., Reference Burgess, Bowring and Shen2014; Bond and Sun, Reference Bond, Sun, Ernst, Dickson and Bekker2021). By contrast, the Late Ordovician (443 Mya) and Late Devonian (372 Mya) extinctions occurred in association with climate cooling (Joachimski and Buggisch, Reference Joachimski and Buggisch2002; Finnegan et al., Reference Finnegan, Bergmann, Eiler, Jones, Fike, Eisenman, Hughes, Tripati and Fischer2011). The end-Cretaceous extinction (66 Mya) was associated with an asteroid impact event whose aftermath resembled the consequences of a hypothetical global thermonuclear war (Pollack et al., Reference Pollack, Toon, Ackerman, McKay and Turco1983; Turco et al., Reference Turco, Toon, Ackerman, Pollack and Sagan1983). Due to the magnitude and global scale of the current “Sixth” extinction, these events from Earth’s past provide historical reference points for predicting the long-term magnitude, ecological impact, and recovery timescale from the current crisis or other, potential, human-mediated catastrophes.
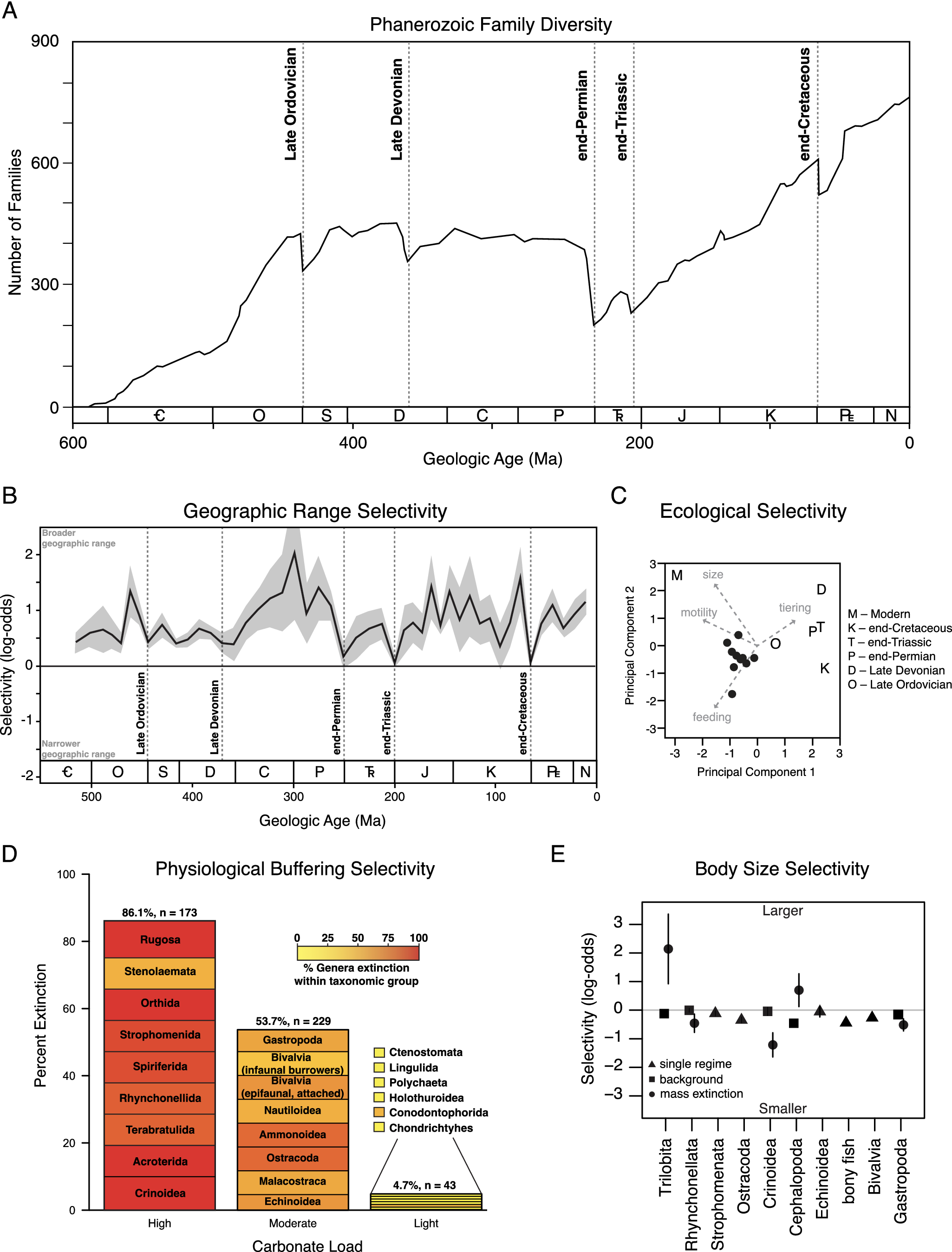
Figure 1. Extinction patterns in the fossil record. (A) Graph of marine animal diversity across the past 600 million years, illustrating the diversity declines associated with the five major mass extinction events (modified from Raup and Sepkoski, Reference Raup and Sepkoski1982). (B) Extinction selectivity with respect to geographic range, illustrating the preferential survival of broadly distributed genera during background intervals and the greatly reduced selectivity during mass extinction events (modified from Payne and Finnegan, Reference Payne and Finnegan2007). (C) Principal components analysis of logistic regression coefficients of ecological traits and body size selectivity of the Big Five mass extinction events and the modern oceans, demonstrating the unique selectivity of the modern extinction threat (modified from Payne et al., Reference Payne, Bush, Heim, Knope and McCauley2016b). (D) Extinction selectivity during the end-Permian mass extinction, illustrating the preferential extinction of heavily calcified marine animal classes with less complex respiratory and circulatory systems (modified from Knoll et al., Reference Knoll, Bambach, Payne, Pruss and Fischer2007; Knoll and Fischer, Reference Knoll, Fischer, Gattuso and Hansson2011). (E) Extinction selectivity with respect to body size for major classes of marine animals, illustrating the general bias of background extinction against smaller-bodied genera versus the variable direction of selectivity for classes that exhibit distinct patterns during mass extinction (modified from Monarrez et al., Reference Monarrez, Heim and Payne2021).
While mass extinctions have been identified in the fossil record based largely on the magnitude of diversity loss across many higher taxa (Newell, Reference Newell1963, Reference Newell1967; Raup and Sepkoski, Reference Raup and Sepkoski1982), causal inference has relied more on geological and geochemical evidence of potential triggers (Alvarez et al., Reference Alvarez, Alvarez, Asaro and Michel1980; Svensen et al., Reference Svensen, Planke, Polozov, Schmidbauer, Corfu, Podladchikov and Jamtveit2009; Finnegan et al., Reference Finnegan, Bergmann, Eiler, Jones, Fike, Eisenman, Hughes, Tripati and Fischer2011) and patterns of extinction selectivity interpreted to reflect proximal kill mechanisms (Jablonski, Reference Jablonski1986; Sheehan and Hansen, Reference Sheehan and Hansen1986; Valentine and Jablonski, Reference Valentine and Jablonski1986; Jablonski and Raup, Reference Jablonski and Raup1995; Knoll et al., Reference Knoll, Bambach, Canfield and Grotzinger1996, Reference Knoll, Bambach, Payne, Pruss and Fischer2007; Smith and Jeffery, Reference Smith and Jeffery1998; Finnegan et al., Reference Finnegan, Heim, Peters and Fischer2012; Penn et al., Reference Penn, Deutsch, Payne and Sperling2018). Selectivity patterns have been assessed with respect to a wide range of traits (Figure 1B–E), including geographic range (Jablonski, Reference Jablonski1986; Kiessling and Aberhan, Reference Kiessling and Aberhan2007; Payne and Finnegan, Reference Payne and Finnegan2007; Dunhill and Wills, Reference Dunhill and Wills2015), body size (Jablonski and Raup, Reference Jablonski and Raup1995; Friedman, Reference Friedman2009; Longrich et al., Reference Longrich, Bhullar and Gauthier2012; Allen et al., Reference Allen, Stubbs, Benton and Puttick2019; Payne and Heim, Reference Payne and Heim2020; Monarrez et al., Reference Monarrez, Heim and Payne2021), abundance (Lockwood, Reference Lockwood2003; Payne et al., Reference Payne, Truebe, Nützel, Chang and Nu2011), larval ecology (Valentine and Jablonski, Reference Valentine and Jablonski1986), diet (Wilson, Reference Wilson2013), functional ecology (Bambach et al., Reference Bambach, Knoll and Sepkoski2002; Payne et al., Reference Payne, Bush, Heim, Knope and McCauley2016b; Hughes et al., Reference Hughes, Berv, Chester, Sargis and Field2021), environmental breadth (Jablonski and Raup, Reference Jablonski and Raup1995), respiratory and circulatory anatomy (Knoll et al., Reference Knoll, Bambach, Canfield and Grotzinger1996, Reference Knoll, Bambach, Payne, Pruss and Fischer2007; Clapham, Reference Clapham2017), and shell mineralogy (Clapham and Payne, Reference Clapham and Payne2011; Kiessling and Simpson, Reference Kiessling and Simpson2011).
Extinction selectivity provides our most direct evidence of proximal kill mechanisms (Raup, Reference Raup1986), but to date, most testing of observed extinction patterns against hypothesized kill mechanisms has been semi-quantitative, focused on establishing consistency between predicted and observed directions of selectivity under various hypothesized kill mechanisms. Recently, advances in paleontological databases, geochemical proxies, physiological experiments, and Earth system and ecosystem models have enabled the comparison of observed and predicted extinction patterns within quantitative, self-consistent frameworks (Figure 2) (Penn et al., Reference Penn, Deutsch, Payne and Sperling2018). Although quantitative model-data comparison between observed and predicted extinction patterns is still in its early days, the door for direct comparison of past and future biotic response to climate change is now open, increasing the value of the fossil record in the mitigation of the current biotic crisis.

Figure 2. Workflow illustrating the use of geological and geochemical data to constrain Earth system models (ESMs), physiological experiments to constrain parameters used to populate models with species of different ecophysiotypes, and fossil occurrence data to conduct model-data comparison. Ecosystem structure remains to be incorporated into such models and can be used to predict extinction cascades. Calibration of models against selectivity patterns in ancient extinction events will improve their use in forecasting biotic response to current and future environmental change. Panels on right showing CO2 emissions curves and future biodiversity projections are from Penn and Deutsch (Reference Penn and Deutsch2022).
Pattern
Analyses of selectivity for individual mass extinction events date back many decades (Jablonski, Reference Jablonski2005). Studies synthesizing and comparing selectivity patterns across all major mass extinctions (and intervening background intervals) have emerged more recently, alongside publicly available databases of fossil occurrences and other traits (Alroy, Reference Alroy and MacPhee1999; Payne and Finnegan, Reference Payne and Finnegan2007; Peters, Reference Peters2008; Kiessling and Simpson, Reference Kiessling and Simpson2011; Payne et al., Reference Payne, Bush, Heim, Knope and McCauley2016b; Smith et al., Reference Smith, Elliott Smith, Lyons and Payne2018; Payne and Heim, Reference Payne and Heim2020; Monarrez et al., Reference Monarrez, Heim and Payne2021).
Geographic range is one of the traits most commonly hypothesized to correlate with extinction risk due to its influence on the extent to which populations of a given taxon may avoid a regional disturbance or have broad enough physiological tolerance limits or ecological capacities to survive a global one. Analyses of fossil data have confirmed that widely distributed taxa survive preferentially during background intervals (Figure 1C) (Jablonski, Reference Jablonski1986, Reference Jablonski2005; Payne and Finnegan, Reference Payne and Finnegan2007). Broader geographic range is also significantly associated with survival during at least some major mass extinction events (Jablonski and Raup, Reference Jablonski and Raup1995; Finnegan et al., Reference Finnegan, CMØ and DAT2016), but the strength of this association (i.e., the change in odds or probability of extinction per unit change in geographic range) is greatly reduced relative to background intervals (Figure 1C) (Kiessling and Aberhan, Reference Kiessling and Aberhan2007; Payne and Finnegan, Reference Payne and Finnegan2007). Due to the consistency of the association and the expectation of selectivity on total geographic range under most extinction scenarios, these patterns have rarely yielded direct insight into kill mechanisms. By contrast, the biogeography of extinction can be more informative. For example, end-Cretaceous echinoid extinction was significantly more severe in areas proximal to the Chicxulub impact site (Smith and Jeffery, Reference Smith and Jeffery1998), and differences in extinction intensity across latitude often correspond with expectations due to climate change (Finnegan et al., Reference Finnegan, Heim, Peters and Fischer2012; Penn et al., Reference Penn, Deutsch, Payne and Sperling2018; Reddin et al., Reference Reddin, Kocsis and Kiessling2019, Reference Reddin, Kocsis, Aberhan and Kiessling2021). Quantifying the expected magnitude of spatial gradients in extinction intensity and differences in such gradients across higher taxa (or functional groupings) is the key to linking these findings with hypothesized kill mechanisms, and one that is already being partially realized (Penn et al., Reference Penn, Deutsch, Payne and Sperling2018).
The extinctions of large mammals during the Pleistocene (0.0117 Ma) and of large, non-avian dinosaurs during the Maastrichtian (66 Ma) have long prompted speculation that large-bodied animals are at systematically higher risk of extinction during times of environmental change (Raup, Reference Raup1986; Wallace, Reference Wallace1889; Brown, Reference Brown1995). Analyses of the fossil record reveal a more heterogeneous relationship, and one that may differ across taxa and habitats. For example, smaller body size is generally associated with greater extinction risk during background times for many classes of marine animals (Figure 1D) (Payne and Heim, Reference Payne and Heim2020; Monarrez et al., Reference Monarrez, Heim and Payne2021). By contrast, body size was not generally associated with extinction probability for terrestrial mammals until the Pleistocene (Alroy, Reference Alroy and MacPhee1999; Smith et al., Reference Smith, Elliott Smith, Lyons and Payne2018). End-Cretaceous extinctions preferentially eliminated larger-bodied fish, lizards, and snakes (Friedman, Reference Friedman2009; Longrich et al., Reference Longrich, Bhullar and Gauthier2012) but were unbiased in bivalves and gastropods (Jablonski and Raup, Reference Jablonski and Raup1995). End-Permian extinctions preferentially affected larger foraminifera and brachiopods (Schaal et al., Reference Schaal, Clapham, Rego, Wang and Payne2016). Many taxon-size combinations have yet to be examined systematically. In marine animals, size selectivity changes between background and mass extinction in many classes but the direction and magnitude of the size bias during mass extinction differs among classes (Figure 1D) (Payne and Heim, Reference Payne and Heim2020; Monarrez et al., Reference Monarrez, Heim and Payne2021). The differences in responses among classes remain to be explained. Because body size correlates with many ecological and physiological traits (Peters, Reference Peters1983), size bias on its own is insufficient to diagnose proximal kill mechanisms but may be useful in conjunction with other traits or in testing against predictions of specific kill mechanisms (Deutsch et al., Reference Deutsch, Penn, Verberk, Inomura, Endress and Payne2022).
Some mass extinction events exhibit selectivity patterns that can be mapped onto respiratory and circulatory anatomy, potentially reflecting underlying differences in susceptibility to metabolic stress from hypercapnia, anoxia, climate warming, or their interactive effects. For example, the end-Permian mass extinction preferentially affected heavily calcified marine animal genera with limited respiratory and circulatory systems (Figure 1B), suggesting a role for hypercapnia and/or direct and indirect fitness effects of acidification on shell dissolution (Calosi et al., Reference Calosi, Melatunan, Turner, Artioli, Davidson, Byrne, Viant, Widdicombe and Rundle2017) in driving the extinction (Knoll et al., Reference Knoll, Bambach, Canfield and Grotzinger1996). At the same time, the lack of sophisticated oxygen-supply mechanisms would also make these taxa more sensitive to temperature-dependent hypoxia (Deutsch et al., Reference Deutsch, Penn and Seibel2020; Endress et al., Reference Endress, Boag, Burford, Penn, Sperling and Deutsch2022) and metabolic differences among groups likely influence taxonomic selectivity patterns from changes in CO2, temperature, and O2. Similar patterns as seen in the end-Permian apply to other extinction events, including the end-Triassic mass extinction (Clapham, Reference Clapham2017; Kiessling and Simpson, Reference Kiessling and Simpson2011), consistent with shared kill mechanisms. By contrast, the end-Cretaceous mass extinction exhibits the opposite pattern, with taxa thought to be more sensitivity to ocean acidification surviving preferentially (Kiessling and Simpson, Reference Kiessling and Simpson2011), potentially reflecting differences in extinction patterns triggered primarily by volcanism versus impact events. The extent to which these patterns stand out from background extinction remains incompletely studied. A study controlling for differences between benthic versus planktonic and nektonic taxa indicates that many background intervals show the same selectivity, often of similar magnitude (Payne et al., Reference Payne, Bush, Chang, Heim, Knope and Pruss2016a). As discussed below, results of physiological experiments on living relatives of species in the fossil record are enabling quantitative prediction of biological response to past environmental changes inferred from geological and geochemical proxies. This is currently an area of rapid progress.
Simultaneous analysis of extinction selectivity across multiple traits and time intervals enables quantitative comparison of selectivity patterns between background and mass extinction as well as among mass extinction events (Figure 1E). Such analyses generally confirm that mass extinction events differ in selectivity from background patterns (Figure 1C, E) (Payne and Finnegan, Reference Payne and Finnegan2007; Kiessling and Simpson, Reference Kiessling and Simpson2011; Finnegan et al., Reference Finnegan, Heim, Peters and Fischer2012; Payne et al., Reference Payne, Bush, Heim, Knope and McCauley2016b; Monarrez et al., Reference Monarrez, Heim and Payne2021) and that the pronounced size bias of the modern extinction makes it an outlier relative to major mass extinctions as well as to recent background intervals (Figure 1D) (Payne et al., Reference Payne, Bush, Heim, Knope and McCauley2016b; Smith et al., Reference Smith, Elliott Smith, Lyons and Payne2018).
Overall, selectivity patterns accord with geological and geochemical data, indicating that mass extinction events are typically associated with large and rapid environmental perturbations rather than intensification of background extinction processes (Alvarez et al., Reference Alvarez, Alvarez, Asaro and Michel1980; Hallam and Wignall, Reference Hallam and Wignall1997; Finnegan et al., Reference Finnegan, Bergmann, Eiler, Jones, Fike, Eisenman, Hughes, Tripati and Fischer2011). Testing hypothesized kill mechanisms requires simultaneous consideration of selectivity across multiple variables because physiological and ecological traits are often linked in complex ways. For example, body size is related to the supply and demand of oxygen (Deutsch et al., Reference Deutsch, Ferrel, Seibel, Pörtner and Huey2015, Reference Deutsch, Penn, Verberk, Inomura, Endress and Payne2022) and food (Gearty et al., Reference Gearty, McClain and Payne2018) as well as to trophic level (Romanuk et al., Reference Romanuk, Hayward and Hutchings2011).
Process
Introduction
Understanding the causes of extinction selectivity in the fossil record requires additional information about the patterns of environmental change, the sensitivity of species to those changes, and disruptions in ecological networks. The interpretation of extinction selectivity thus relies on geochemical reconstructions of climate, understanding of the ecological and physiological traits of living taxa and, increasingly, on models that incorporate all these aspects of ecological and Earth system dynamics into an internally consistent, quantitative framework (Figure 2).
Patterns of extinction selectivity can arise simply from the fact that environmental changes can be highly variable in strength or even direction across space. Extinction selectivity could also arise from taxonomic or geographic differences in physiological sensitivity to environmental change, even if climate trends were globally uniform. In general, these factors are likely to be connected, as the tolerance limits of taxa to environmental conditions will shape the pre-extinction geographic distribution, which may confer greater or lesser sensitivity to environmental change in certain regions. Contemporary studies have advanced a mechanistic approach to investigating the causes of selectivity in mass extinctions by integrating many of these elements, from geochemical proxies of climate change, the modern diversity of ecophysiological traits, and the climate dynamics of Earth system models. In ocean studies, emphasis has been on integrating climate and physiological constraints (Penn et al., Reference Penn, Deutsch, Payne and Sperling2018; Stockey et al., Reference Stockey, Pohl, Ridgwell, Finnegan and Sperling2021). Terrestrial studies, by contrast, have tended to focus on ecological (food web) mechanisms largely missing from marine analyses (Roopnarine, Reference Roopnarine2006; Roopnarine and Angielczyk, Reference Roopnarine and Angielczyk2015). These dichotomous approaches have made significant advances in their respective domains, paving the way for more unified marine and terrestrial studies.
Example: Metabolic Index
One promising avenue for examining physiological kill mechanisms for ancient extinction events is the Metabolic Index, which was initially developed to test whether the biogeographic distributions of species are physiologically limited by O2 supply and demand in the modern ocean (Deutsch et al., Reference Deutsch, Ferrel, Seibel, Pörtner and Huey2015). This ecophysiological model quantifies habitat viability for a species, in terms of its ability to carry out aerobic respiration, by taking a ratio of environmental oxygen supply to biological oxygen demand as a function of temperature and taxon-specific metabolic and O2 supply traits (Eq. (1)). The metabolic energy demands of water-breathing marine animals increase with water temperature and body size (Gillooly et al., Reference Gillooly, Brown, West, Savage and Charnov2001), raising corresponding biological O2 requirements. Temperature and body size also impact the rates of organismal O2 supply through diffusion, ventilation, and internal circulation (Deutsch et al., Reference Deutsch, Penn, Verberk, Inomura, Endress and Payne2022; Endress et al., Reference Endress, Boag, Burford, Penn, Sperling and Deutsch2022), while warmer water holds less ambient O2. The ratio of temperature and body size (B)-dependent rates of potential O2 supply and organismal metabolic demand, termed the Metabolic Index (ɸ), quantifies the metabolic viability of a habitat for a given species:
where A
0 (atm−1) is the ratio of O2 supply to resting demand rate coefficients, or hypoxia tolerance at a reference temperature and body size (B), with allometric scaling exponent
![]() $ \varepsilon $
and Arrhenius temperature sensitivity, E
0 (eV), and pO2 and T are the oxygen partial pressure and temperature of ambient water, respectively (Figure 3) (Deutsch et al., Reference Deutsch, Ferrel, Seibel, Pörtner and Huey2015, Reference Deutsch, Penn and Seibel2020). These physiological traits and their distributions across taxa can be estimated from critical oxygen thresholds in respirometry experiments conducted for diverse marine biota over the past half century (Rogers et al., Reference Rogers, Urbina, Reardon, McKenzie and Wilson2016; Chu and Gale, Reference JWF and KSP2017). Critical oxygen thresholds define the Metabolic Index to be 1 (i.e., ɸ = 1), allowing the traits to be estimated for organisms in a resting state under laboratory conditions. In the environment, O2 requirements are elevated by more strenuous activities important for population persistence, such as growth, reproduction, feeding, defense, or motion. These additional energy demands require the O2 supply to be raised by a factor, ɸcrit, corresponding to sustained metabolic scope (Peterson et al., Reference Peterson, Nagy and Diamond1990). Stable aerobic habitat barriers thus arise in ocean regions where the Metabolic Index falls below ɸcrit, while the geographic positions of these barriers depend on the species’ traits (Deutsch et al., Reference Deutsch, Penn and Seibel2020). The habitability of any given parcel of water can therefore be determined from the temperature and oxygen partial pressure given the species values of A
0, E
0, and ɸcrit. Earth system models can be populated with hypothetical species by drawing combinations of values from the trait distributions (Figure 3). The promise of this framework for paleontological application is that trait distributions can be used to predict the patterns of biodiversity, providing a means for testing the model against the fossil record. Indeed, the observed tropical dip in marine species richness observed for diverse animal groups in the modern ocean (Chaudhary et al., Reference Chaudhary, Richardson, Schoeman and Costello2021) can be explained by aerobic habitat limitation implied by modern species Metabolic Index traits (Penn and Deutsch, Reference Penn and Deutsch2022). Environmental temperature and oxygen concentration can be quantified using geochemical proxies for ancient events to calibrate Earth system models and body size can be measured from fossil specimens. In principle, ecological interactions can be further incorporated to model, allowing extinction cascades to be accounted for alongside direct, climate-driven habitat loss (Figure 4).
$ \varepsilon $
and Arrhenius temperature sensitivity, E
0 (eV), and pO2 and T are the oxygen partial pressure and temperature of ambient water, respectively (Figure 3) (Deutsch et al., Reference Deutsch, Ferrel, Seibel, Pörtner and Huey2015, Reference Deutsch, Penn and Seibel2020). These physiological traits and their distributions across taxa can be estimated from critical oxygen thresholds in respirometry experiments conducted for diverse marine biota over the past half century (Rogers et al., Reference Rogers, Urbina, Reardon, McKenzie and Wilson2016; Chu and Gale, Reference JWF and KSP2017). Critical oxygen thresholds define the Metabolic Index to be 1 (i.e., ɸ = 1), allowing the traits to be estimated for organisms in a resting state under laboratory conditions. In the environment, O2 requirements are elevated by more strenuous activities important for population persistence, such as growth, reproduction, feeding, defense, or motion. These additional energy demands require the O2 supply to be raised by a factor, ɸcrit, corresponding to sustained metabolic scope (Peterson et al., Reference Peterson, Nagy and Diamond1990). Stable aerobic habitat barriers thus arise in ocean regions where the Metabolic Index falls below ɸcrit, while the geographic positions of these barriers depend on the species’ traits (Deutsch et al., Reference Deutsch, Penn and Seibel2020). The habitability of any given parcel of water can therefore be determined from the temperature and oxygen partial pressure given the species values of A
0, E
0, and ɸcrit. Earth system models can be populated with hypothetical species by drawing combinations of values from the trait distributions (Figure 3). The promise of this framework for paleontological application is that trait distributions can be used to predict the patterns of biodiversity, providing a means for testing the model against the fossil record. Indeed, the observed tropical dip in marine species richness observed for diverse animal groups in the modern ocean (Chaudhary et al., Reference Chaudhary, Richardson, Schoeman and Costello2021) can be explained by aerobic habitat limitation implied by modern species Metabolic Index traits (Penn and Deutsch, Reference Penn and Deutsch2022). Environmental temperature and oxygen concentration can be quantified using geochemical proxies for ancient events to calibrate Earth system models and body size can be measured from fossil specimens. In principle, ecological interactions can be further incorporated to model, allowing extinction cascades to be accounted for alongside direct, climate-driven habitat loss (Figure 4).

Figure 3. Graphs illustrating the key species traits of the Metabolic Index (ɸ) along with how ɸ relates to temperature and oxygen partial pressure. (A–C) Frequency distributions of the Metabolic Index parameters for marine animals. (D, E) Graphs of variation in ɸ as a function of temperature and oxygen for species with negative (D) and positive (E) temperature sensitivities (E o) of hypoxia tolerance (A o), which is the inverse of the critical oxygen threshold (red circle) at a reference temperature (T ref), as derived from respirometry experiments. For species in a resting state, the aerobic habitat limit occurs when ɸ = 1, but in the environment a species’ activity level or sustained metabolic scope (SMS) elevates the habitat limit to ɸcrit. For species with negative E o, aerobic habitat availability increases with temperature, whereas for those with positive E o (i.e., most species; panel B), aerobic habitat declines with warming. Changes in PO2 has the potential to lower aerobic habitat availability, and thus the amount of warming a species can withstand, as exemplified for two scenarios of with different fractions of present atmospheric levels of O2 (PAL; yellow dots and arrows). A change in CO2 also has the potential to alter hypoxia tolerance, but the magnitude and direction of this effect is unknown across marine biota and is illustrated here from experimental data for a single species under ∆ pH = +0.5 (Rosa et al., Reference Rosa, Trübenbach, Repolho, Pimentel, Faleiro, Boavida-Portugal, Baptista, Lopes, Dionísio, Leal, Calado and Pörtner2013). Arrows in A–C denote species traits in D and E.
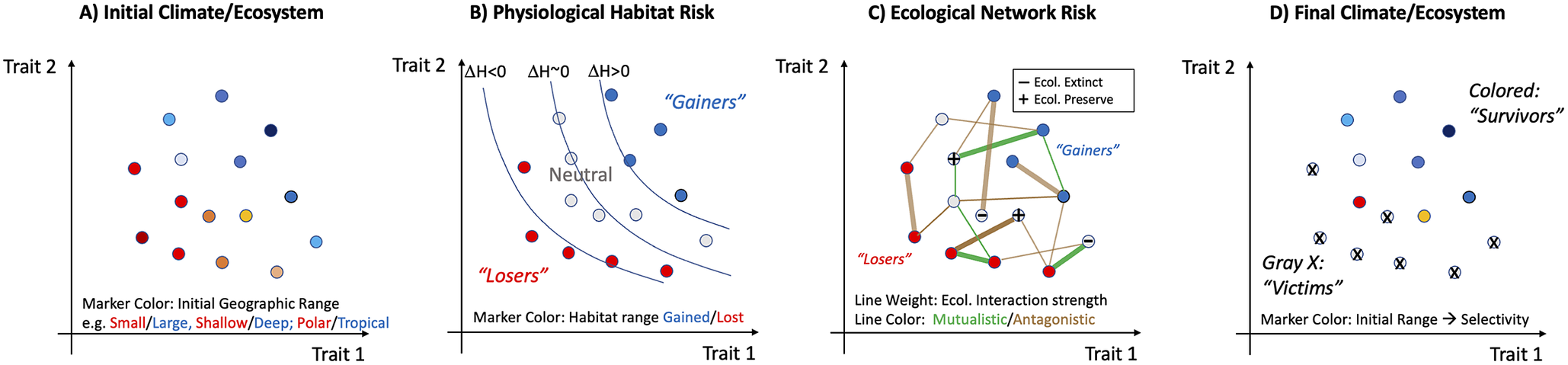
Figure 4. Hypothetical progression of a mass extinction highlighting sources of trait-based and geographic selectivity and potential ecological amplification. (A) An initial distribution of species (or “ecophysiotypes”) defined by traits under selection by large-scale environmental conditions will likely result in systematic correlations between traits and geographic range. The range metric here can be considered overall range size (area and volume), or centroid (e.g., low-latitude versus high-latitude, shallow versus deep). (B) The initial biota are subjected to climate perturbation that poses a direct stress through a reduction in fitness whose magnitude depends on species traits and on local climate trends. The resulting change in available habitat (ΔH; contours) presents an ecophysiological extinction risk that is geographically selective because it is trait selective (but may also be caused by climate patterns themselves). In this hypothetical case, habitat loss (ΔH < 0) selects against species with high values of two traits (habitat “Losers”) and may even benefit species with low values of those traits (habitat “Gainers”; ΔH > 0). (C) Physiological extinction poses further ecological risks (or advantages) depending on the mutualistic or adversarial interactions with ecophysiotypes (nodes in graph) that are under trait-selective risk. Ecological risk is complex and for any particular species will depend on the physiological risk faced by the other species with which it interacts, which may be positive (green lines) or negative (brown lines), and strong (thick lines) or weak (thin lines). The results of these associations, which may be multiple and indirect, could alter extinction risk by either preserving ecological fitness (“+” symbol) or reducing it (“−” symbol). Changes in extinction risk are likely to be most pronounced for those in the neutral zone whose antagonists go extinct or who are buoyed by prey/mutualists that are under positive selection. (D) Post-extinction ecosystem, equal to the initial one (A) minus the ecotypes that have gone extinct from either primary (B) or secondary (C) effects.
During periods of climate warming, rising water temperatures can drive the metabolic O2 demand above a supply declining from ocean deoxygenation, leading to the loss of available aerobic habitat, and eventually species extinctions at local and global scales (Penn et al., Reference Penn, Deutsch, Payne and Sperling2018; Reddin et al., Reference Reddin, Nätscher, Kocsis, Pörtner and Kiessling2020). At regional scales, such as in the California Current System, aerobic habitat changes have been linked to multi-decadal fluctuations of anchovy populations, including near-extirpation of larvae from portions of their range (Howard et al., Reference Howard, Penn, Frenzel, Seibel, Bianchi, Renault, Kessouri, Sutula, McWilliams and Deutsch2020). At global scales, aerobic habitat loss under the climate change simulated for the end-Permian mass extinction predicted a geographic selectivity of extinction consistent with the fossil record (Figure 5A): Extinction risk was greater for species inhabiting higher latitudes. This geographic selectivity arises because species previously occupying the tropics would already have been adapted to warm, low-O2 conditions that became more widespread, whereas polar habitat niches disappeared more completely (Penn et al., Reference Penn, Deutsch, Payne and Sperling2018). In contrast to the geographic selectivity predicted for warming, periods of global cooling, such as during the Late Ordovician, are expected to generate extinctions focused on the low latitudes (Saupe et al., Reference Saupe, Qiao, Donnadieu, Farnsworth, Kennedy-Asser, Ladant, Lunt, Pohl, Valdes and Finnegan2020), consistent with the patterns observed for that mass extinction (Finnegan et al., Reference Finnegan, Heim, Peters and Fischer2012) and may also occur through aerobic habitat loss if accompanied by deoxygenation (Finnegan et al., Reference Finnegan, CMØ and DAT2016) or due to declining hypoxia tolerance in cold water in species with thermal optima (Boag et al., Reference Boag, Stockey, Elder, Hull and Sperling2018; Endress et al., Reference Endress, Boag, Burford, Penn, Sperling and Deutsch2022). Aerobic habitat loss is also predicted to select against large-bodied species, with a strong variability within size classes that depends on a species’ temperature sensitivity (Deutsch et al., Reference Deutsch, Penn, Verberk, Inomura, Endress and Payne2022). Extinctions driven by aerobic habitat loss may also explain the amplified background extinction rates observed for the early Phanerozoic, because of dramatically lower atmospheric O2 levels and thus species living closer to their ecophysiological limits (Stockey et al., Reference Stockey, Pohl, Ridgwell, Finnegan and Sperling2021). Trait adaption to different past climate states (Bennett et al., Reference Bennett, Sunday, Calosi, Villalobos, Martínez, Molina-Venegas, Araújo, Algar, Clusella-Trullas, Hawkins and Keith2021) has the potential to buffer or amplify predicted extinction risks. The role of differences in ecophysiological traits across taxonomic groups in explaining observed patterns of extinction selectivity across higher taxa (Knoll et al., Reference Knoll, Bambach, Canfield and Grotzinger1996, Reference Knoll, Bambach, Payne, Pruss and Fischer2007) remains an open area of research.

Figure 5. Geographic patterns of extinction and ocean changes in Earth system model simulations of the end-Permian climate transition (left column) and under anthropogenic greenhouse gas forcing (C) to 2300 C.E. (middle column). Line plot comparisons of end-Permian and potential future environmental changes versus latitude are shown in panels on the right (F, I, L, O). Model extinctions (A, B) are driven by ocean warming (D, E) and O2 loss (G, H), as quantified through the Metabolic Index, and in (A) reproduce the latitudinal pattern from the fossil record of the end-Permian (red points). These primary extinctions have the potential to be amplified by other environmental stressors like changes in net primary productivity (NPP) (J, K) or pH (M, N) or through secondary extinctions via the food web. Shaded region in (A) shows uncertainty in end-Permian extinction magnitudes across a range of potential extinction threshold parameters. Solid line in (B) shows future extinction risk averaged across Earth system models, using an extinction threshold calibrated from the end-Permian (same as the solid line in A) (see Penn et al., Reference Penn and Deutsch2022 for calibration details), while the shading in (B) shows the inter-model range. Future changes are projected under a high greenhouse gas emissions scenario, leading to a net radiative forcing of 8.5 W m−2 in 2100 C.E. (C) and are relative to the pre-industrial era (1850–1900). Model fields are averaged over the upper 500 m, and for the future projections, they are averaged across Earth system models (n = 5). Model details are provided in Penn et al. (Reference Penn, Deutsch, Payne and Sperling2018, Reference Penn and Deutsch2022). Panels A–C are modified from Penn et al. (Reference Penn, Deutsch, Payne and Sperling2018, Reference Penn and Deutsch2022), respectively.
Primary extinctions driven by the loss of aerobic habitat have the potential to be amplified by secondary extinctions arising from food web effects (Figure 4) or co-occurring environmental stressors that exacerbate direct aerobic habitat loss (Figure 5J–O). Aerobically tolerant species could still be lost if they are ecologically tied to vulnerable ones, for example, through the food web (Figure 4) or other critical interactions. Ocean acidification (Figure 5M–O) has the potential to further deplete aerobic habitat through direct CO2 effects on critical oxygen thresholds, but the magnitude and direction of this effect is uncertain and variable across limited available experimental studies (Figure 3E) (Rosa et al., Reference Rosa, Trübenbach, Repolho, Pimentel, Faleiro, Boavida-Portugal, Baptista, Lopes, Dionísio, Leal, Calado and Pörtner2013; Lefevre et al., Reference Lefevre, Watson, Munday and Nilsson2015). On its own, the magnitude of primary extinction from climate warming and associated physiological stresses depends on the amount of habitat loss beyond which a species can no longer sustain a viable population (i.e., the extinction threshold) (Urban, Reference Urban2015; Penn et al., Reference Penn, Deutsch, Payne and Sperling2018; Penn and Deutsch, Reference Penn and Deutsch2022), even if population decline takes a long time to occur. Extinction thresholds may vary across species, but the average value at the global ecosystem level has been estimated from comparison of end-Permian model simulations to the fossil record, and assuming a similar loss of habitat that drove extinctions in the past would apply in the modern ocean (Penn and Deutsch, Reference Penn and Deutsch2022). Calibration of this parameter from the fossil record has recently been used to project future extinction risk from climate changes resembling those of the end-Permian, which are arising today due to accelerating anthropogenic greenhouse gas emissions (Figure 5).
Example: Food webs
Terrestrial paleo-community dynamics are usually modeled according to trophic ecology and body size to investigate the role of food-web topology in the propagation of disruptions caused by environmental change. Models of extinction cascades suggest that responses can be complex, resulting from both bottom-up and top-down effects (Kaneryd et al., Reference Kaneryd, Borrvall, Berg, Curtsdotter, Eklöf, Hauzy, Jonsson, Münger, Setzer, Säterberg and Ebenman2012), with debate about whether simple or complex communities are more susceptible to such cascades and whether trophic versus other ecological interactions are most important (Eklöf and Ebenman, Reference Eklöf and Ebenman2006; Donohue et al., Reference Donohue, Petchey, Kéfi, Génin, Jackson, Yang and O’Connor2017). Explicit consideration of extinction cascades during mass extinctions has generally focused on the consequences of collapse in primary production (Tappan, Reference Tappan1968; Vermeij, Reference Vermeij2004). Bottom-up models predict extinction of smaller-bodied species in both the marine and terrestrial realms, due to the correlation of body size with trophic level, and exacerbated paleo-community instability post-extinction, which are consistent with investigations conducted on patterns of selectivity in relation to body size (Dunne et al., Reference Dunne, Williams and Martinez2002; Roopnarine, Reference Roopnarine2006; Roopnarine et al., Reference Roopnarine, Angielczyk, Wang and Hertog2007; Dunne and Williams, Reference Dunne and Williams2009; de Visser et al., Reference de Visser, Freymann and Olff2011; Lotze et al., Reference Lotze, Coll and Dunne2011). Interestingly, the end-Cretaceous mass extinction, for which we have the strongest evidence for collapse of primary production, is associated with preferential extinction of larger-bodied species in some clades (Friedman, Reference Friedman2009; Longrich et al., Reference Longrich, Bhullar and Gauthier2012) but not with the preferential extinction of smaller-bodied species, suggesting that physiology or other ecological factors (including top-down extinction cascades) were important in determining survivorship.
Two challenges remain in the modeling of extinction via networks of ecological interactions. First, evidence that “primary” extinctions may often occur via environmental change that exceeds the physiological tolerance limits of species at many positions in the food web creates a need for further investigation of how food webs respond to such losses. Are extinction cascades more, or less, extensive when driven by primary extinctions occurring simultaneously at multiple trophic levels? Second, there is the challenge of integrating physiological and ecological models such that the full response of the marine or terrestrial ecosystem could be predicted in an integrated manner from the modeling of climate change to the loss of species that cannot physiologically tolerate the modified world, to the loss of species that depended on ecological interactions with species lost via primary extinctions (Figure 4). Differences in timescale and level of biological organization at which physiological and ecological processes dominate add to this challenge.
Application to the sixth extinction
Mass extinction events provide our best source of information regarding the response of the biosphere to planetary-scale environmental disruption and the timescales and mechanisms of subsequent recovery. This information may be particularly important for the oceans, where observing biological response to environmental change is challenging and where the fossil record is particularly complete and diverse. Since the industrial revolution, the oceans have experienced substantial changes in ocean biogeochemistry, mainly because of rapid injection of CO2 into the atmosphere from anthropogenic sources. Under the accelerating future anthropogenic emissions scenario consistent with historical trends (Figure 5C), the oceans are expected to warm by 4–5°C and pH is expected to decrease, on average, by 0.44 pH units by the end of the 21st century, with changes increasing even further over the next few centuries (Figure 5E, N) (Kwiatkowski et al., Reference Kwiatkowski, Torres, Bopp, Aumont, Chamberlain, Christian, Dunne, Gehlen, Ilyina, John, Lenton, Li, Lovenduski, Orr, Palmieri, Santana-Falcón, Schwinger, Séférian, Stock, Tagliabue, Takano, Tjiputra, Toyama, Tsujino, Watanabe, Yamamoto, Yool and Ziehn2020). High temperatures are also expected to reduce the ocean’s oxygen content while also altering nutrient cycles (Sweetman et al., Reference Sweetman, Thurber, Smith, Levin, Mora, Wei, Gooday, Jones, Rex, Yasuhara, Ingels, Ruhl, Frieder, Danovaro, Würzberg, Baco, Grupe, Pasulka, Meyer, Dunlop, Henry and Roberts2017). Unabated anthropogenic emissions could drive the oceans toward widespread oxygen deficiency over the rest of the 21st century and beyond (Figure 5H) (Breitburg et al., Reference Breitburg, Levin, Oschlies, Grégoire, Chavez, Conley, Garçon, Gilbert, Gutiérrez, Isensee, Jacinto, Limburg, Montes, Naqvi, Pitcher, Rabalais, Roman, Rose, Seibel, Telszewski, Yasuhara and Zhang2018).
Such changes would have drastic consequences for marine ecosystems as evident from declining fish stocks, expansion of marine dead zones, and reduced primary productivity across different parts of the globe (Figure 5K) (Blanchard et al., Reference Blanchard, Jennings, Holmes, Harle, Merino, Allen, Holt, Dulvy and Barange2012). Efforts are already underway to project changes in species’ ranges and abundances in response to climate change on land and in the oceans (Thuiller, Reference Thuiller2004; Cheung et al., Reference Cheung, Lam, Sarmiento, Kearney, Watson and Pauly2009; Chen et al., Reference Chen, Hill, Ohlemüller, Roy and Thomas2011; Pinsky et al., Reference Pinsky, Selden and Kitchel2020). Extrapolating results from experiments and field observations over days or years to timescales of centuries, millennia, and beyond is challenging because different processes may dominate the biospheric response on different timescales, although there is emerging evidence that responses to some stresses are concordant across timescales (Reddin et al., Reference Reddin, Nätscher, Kocsis, Pörtner and Kiessling2020). Furthermore, the primary phase of extinction, dominated by physiology, may give way over time to a secondary phase of extinction, dominated by the effects of changing ecological interactions. Connecting the physiological and ecological processes driving extinction remains a research frontier.
Studies from the fossil record show that the ecophysiological constraints on marine taxa due to global warming and ocean deoxygenation will exert a key role in determining their risk to extinction under current and future emissions scenarios. The fossil record can even be used to calibrate the Earth system models used to predict future extinctions and changes in geographic range, just as paleoclimate records are used to calibrate models providing climate projections (Zhu et al., Reference Zhu, Otto-Bliesner, Brady, Gettelman, Bacmeister, Neale, Poulsen, Shaw, McGraw and Kay2022). Under a high emissions scenario (Figure 5C), the marine biological richness could be reduced to 65% of its current state due to global warming and oxygen loss from oceans by 2,300 (Penn and Deutsch, Reference Penn and Deutsch2022). The combined climate-ecophysiological models indicate that the local loss of species is expected to be the highest in tropical to temperate regions where taxa are expected to undergo a significant loss of aerobic habitat at their warm/low-O2 range boundaries. In contrast, in terms of global habitat loss and extinction risk, the equatorial taxa are expected to fare better overall in low oxygen and warmer oceans compared to polar species due to their higher tolerance limits to warm climates and opportunities to expand their available habitats as the poles become more like the present-day tropics. This scenario has precedent in the fossil record with the end-Permian mass extinction where a similar latitudinal extinction pattern unfolded (Figure 5A, B) (Penn et al., Reference Penn, Deutsch, Payne and Sperling2018; Reddin et al., Reference Reddin, Kocsis and Kiessling2019). Further work to integrate the effects of changes in pH, pCO2, salinity, and other key environmental variables into physiological performance models has the potential to make these models more general and accurate in reconstructing the causes of past extinction and predicting the consequences of future global change.
The ecological functions disrupted by global warming and marine defaunation are also bound to have cascading effects which could lead to extinction of vulnerable taxa. Modeling such effects is challenging due to the complexity of the interactions involved. The fossil record is our only source of data on the effects of major environmental disturbance at global scale. Fortunately, calibration of environmental change to physiologically expected extinction is becoming possible due to parallel advances in geochemistry, Earth system modeling, and physiological experimentation. The next decade will require integration of food webs and other types of ecosystem models to extract the full value of the lessons from Earth’s past in forecasting and guiding its future.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/ext.2023.10.
Data availability statement
No data were collected or analyzed as part of this review paper.
Acknowledgments
The authors thank G. Wilson Mantilla, P. Calosi, C. Rasmussen, and an anonymous reviewer for their constructive feedback on the manuscript.
Author contribution
All authors contributed to the conceptualization, original draft preparation, review, and editing of the manuscript.
Financial support
This work was supported by the National Science Foundation (J.L.P., grant number EAR-2121392; C.D., grant number EAR-2121466).
Competing interest
The authors declare no competing interests exist.










Comments
September 15, 2022
Dear Profs. Alroy and Brook,
Please find attached a manuscript entitled Selectivity of mass extinction: patterns, processes, and future directions, submitted for publication in Cambridge Prisms: Extinction. This manuscript is submitted in response to your invitation and is intended as a review of the topic with a focus on progress over the past two decades. To produce the review, I have collaborated with several advisees in my research group as well as two scientists at the forefront of applying Earth system models to the study of biological response to climate change in the modern and ancient worlds. We have addressed, in particular, the use of Earth system models and data from physiological experiments as quantitative bridges between geochemical constraints on environmental change and paleontological data on extinction selectivity during mass extinctions. We have briefly addressed the use of the fossil record for calibrating model forecasts of future extinction in response to climate change. We have attempted to write the manuscript in a way that will be accessible to the broad readership of this new journal. We hope that you will find it acceptable for publication and appreciate the opportunity to contribute.
Best,
Jonathan Payne and co-authors