Diabetes is probably the most common and the fastest growing disease in all the developing countries with high mortality and morbidity. There is increasing evidence that complications related to diabetes are associated with oxidative stress induced by the generation of free radicals(Reference Garg, Ojha and Bansal1). A free radical is any species capable of independent existence that contains one or more unpaired electrons. Thus, free radicals result in the consumption of antioxidant defences which may lead to the disruption of cellular functions and oxidative damage to membranes and enhance susceptibility to lipid peroxidation. Increased generation of reactive oxygen species (ROS) and lipid peroxidation has been found to be involved in the pathogenesis of many diseases of known and unknown aetiology and in the toxic actions of many compounds(Reference Andallu and Varadacharyulu2). Antioxidants thus play an important role to protect the human body against damage caused by ROS(Reference Baynes3). The endogenous antioxidant enzymes (e.g. superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and glutathione peroxidase (GPx)) are primarily involved in the detoxification of deleterious oxygen radicals(Reference Jacob4).
Hyperglycaemia also favours, through the activation of NF-κB, an increased expression of inducible NO synthase (NOS), which is accompanied by an increased generation of NO(Reference Spitaler and Graier5). NO can react with superoxide to produce the strong oxidant peroxynitrite, which in turn can increase lipid peroxidation, protein nitration and LDL oxidation, affecting many signal transduction pathways(Reference Griendling and FitzGerald6). Recent experimental evidence supports the idea of complex roles for NO, ROS and peroxynitrite in the development of early diabetes tissue injury before the evolution of late complications(Reference Stadler, Jenei and von Bolcshazy7).
Several studies have demonstrated that diabetes is a risk factor for developing ocular diseases, increasing their prevalence and severity. Progression to severe eye pathologies with loss of supporting structures is mediated by several factors, including oxidative stress, free radicals, secretion of a broad spectrum of inflammatory and destructive mediators such as cytokines (TNF-α, IL-1β and IL-6), chemokines (IL-8) and PGE2. This is caused by the indirect effects of hyperglycaemia(Reference Soop, Duxbury and Agwunobi8).
The role of nutritional antioxidants in oxidative stress-related diseases has gained immense interest in recent years. Researchers are looking forward in search of protective natural agents in order to combat oxidative stress and inflammation in diabetes. Conjugated fatty acid (FA) is the general term of positional and geometric isomers of PUFA with double bonds in conjugation. The protective effect of conjugated di-enoic FA such as conjugated linoleic acid (CLA) has already been intensively studied. So, it is worth investigating the beneficial activity of conjugated tri-enoic FA against streptozotocin (STZ)-induced diabetes and inflammation. α-Eleostearic acid and punicic acid are two typical conjugated tri-enoic FA isomers of conjugated linolenic acid (CLnA) found in seed oils with systematic structure of cis-9, trans-11, trans-13-octadecatrienoic and cis-9, trans-11, cis-13-octadecatrienoic acid, respectively. Theoretically, α-eleostearic acid consists of 33 % cis and 66 % trans molecular composition and punicic acid has 66 % cis and 33 % trans molecular configuration(Reference Mukherjee, Bhattacharyya and Ghosh9, Reference Fernandez and West10). It has been observed that seed fats of the Cucurbitaceae family, obtained from common vegetables like bitter gourd (Momordica charantia), contain about 30–50 % α-eleostearic acid and seed fats of snake gourd plant (Trichosanthes anguina) contain about 40 % punicic acid(Reference Takagi and Itabashi11). Some researchers(Reference Mukherjee, Bhattacharyya and Ghosh9, Reference Dhar, Ghosh and Bhattacharyya12) showed that α-eleostearic acid and punicic acid could reduce lipid peroxidation and act as efficient antioxidants in the rat model. Dhar et al. (Reference Dhar, Ghosh and Bhattacharyya12, Reference Dhar, Bhattacharyya and Bhattacharyya13) have observed that α-eleostearic acid significantly reduces plasma lipid peroxidation, lipoprotein peroxidation and erythrocyte membrane (EM) lipid peroxidation in both diabetic and non-diabetic blood samples. Some researchers also showed that punicic acid exerts an anti-inflammatory effect through inhibition of TNF-α-induced neutrophil hyperactivation and ROS production and they concluded that this natural dietary compound may provide a novel alternative therapeutic strategy in inflammatory diseases such as inflammatory bowel diseases(Reference Boussetta, Raad and Letteron14).
In our previous studies, it has been observed that different isomers of CLnA are able to combat the induced oxidative stress and genotoxicity generated by the ingestion of sodium arsenite, at different doses by reducing lipid peroxidation, improving lipid profile and antioxidant enzymes level(Reference Saha and Ghosh15–Reference Saha and Ghosh17). The present study has been undertaken to evaluate the antioxidant and anti-inflammatory activity of CLnA isomers against STZ-induced diabetes and also to compare the effectiveness of two isomers of conjugated tri-enoic acid against diabetes.
Experimental methods
Materials
STZ (C8H15N3O7) was purchased from Santa Cruz Biotechnology, Inc. The dose of STZ (60 mg/kg body weight) was chosen on the basis of the previous studies(Reference Weiss18, Reference Ahmed, Adeghate and Cummings19) done by other workers.
All other chemicals used were of analytical grade and procured from Merck India Limited.
Extraction and quantification of conjugated linolenic acid isomers
Authentic bitter gourd seeds and snake gourd seeds were obtained from the local market of Kolkata, India. The seeds were crushed and the oil was extracted with solvent petroleum ether. The extracts of the sample were filtered and then concentrated by a rotary evaporator. NEFA present in the oil were quantified and then were removed by miscella refining process by adding 10 % NaOH solution (20 % excess of the required amount) at 40°C for 30 min(Reference Bhattacharyya, Majumdar and Bhattacharyya20). The soap formed was removed by centrifugation and the organic phase was washed with water. Deacidified oil was recovered by distillation under vacuum and dried. The refined oil was then bleached with tonsil earth (1 %, w/w) obtained from P.T. Sud-Chemic and activated carbon (0·2 %, w/w), supplied by E. Merck India Private Limited, at 60°C under vacuum for 20 min. After the bleaching operation, the oil was recovered by vacuum filtration and stored at − 20°C under N2.
Analysis of fatty acid compositions
The FA compositions of the different dietary oils were determined by GLC (Agilent Technologies India Private Limited) techniques after converting the oils (TAG) into their corresponding FA methyl esters as per Ichihara et al. (Reference Ichihara, Shibahara and Yamamoto21). The amount of cis–trans isomers present in the oil was determined by dissolving the oil in cyclohexane and measuring the absorbance over a wavelength range of 200–300 nm by a UV–Vis Spectrophotometer (Shimadzu Corporation)(22).
Dietary fat blends
Sunflower oil was obtained from Corn Agro Limited. The sunflower oil was mixed with bitter gourd seed oil and snake gourd seed oil to give final oil mixtures with 0·5 % by weight of α-eleostearic acid and punicic acid content, respectively. Table 1 shows the final FA composition of dietary oil mixtures. The dose of CLnA isomers (0·5 % of the total lipid given for each isomer) was used according to the previous studies done in the same laboratory(Reference Mukherjee, Bhattacharyya and Ghosh9, Reference Dhar, Ghosh and Bhattacharyya12, Reference Saha and Ghosh15, Reference Saha and Ghosh16).
Table 1 Fatty acid (FA) composition of dietary oils and oil blends
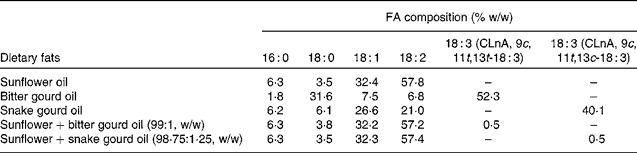
CLnA, conjugated linolenic acid.
Animal experiment
The work was carried out under the supervision of the Animal Ethical Committee of the Department of Chemical Technology (University of Calcutta). Male albino rats of Charles Foster strain (selected for the authenticity of the strain) were housed in individual cages. The animals were divided into four groups (average body weight 120–130 g) consisting of eight animals in each group. The first group served as normal control, and received vehicle (sunflower oil) once per d. Diabetes was induced in rats of groups 2, 3 and 4 by a single intraperitoneal injection of STZ at a dose of 60 mg/kg body weight, dissolved in 25 mm-sodium citrate buffer (pH 4·5)(Reference Weiss18, Reference Ahmed, Adeghate and Cummings19). Group 2 served as treated control and rats serving as vehicle controls were given the same volume of sodium citrate as the STZ-treated groups. STZ induces diabetes within 3 d by destroying the β cells. On day 3 after STZ treatment, whole-blood glucose obtained from mouse tail-vein was detected using a strip-operated blood glucose meter (Ascensia, Entrust; Bayer HealthCare LLC). The animals were considered diabetic if the values of blood glucose level were more than 3000 mg/l. Moreover, rats in groups 3 and 4 were treated with α-eleostearic acid and punicic acid (0·5 % of the total lipid given for each isomer) by oral administration once per d after diabetes induction. Weekly blood glucose levels of the rats of each group were noted.
The rats were fed experimental diets having the following composition: fat-free casein, 18 %; fat, 20 %; starch, 55 %; salt mixture 4 % (composition of salt mixture no. 12 (in g) (Sisco Research Laboratories Private Limited): NaCl, 292·5; KH2PO4, 816·6; MgSO4, 120·3; CaCO3, 800·8; FeSO4.7H2O, 56·6; KCl, 1·66; MnSO4.2H2O, 9·35; ZnCl2, 0·5452; CuSO4.5H2O, 0·9988; CoCl2.6H2O, 0·0476)(Reference Jones and Foster23); cellulose, 3 %; and one multivitamin capsule (vitamin A Indian Pharmacopoeia (I.P.) 10 000 units, thiamine mononitrate I.P. 5 mg, vitamin B I.P. 5 mg, calcium pantothenate USP 5 mg, niacinamide I.P. 50 mg, ascorbic acid I.P. 400 units, cholecalciferol United States Pharmacopoeia (USP) 15 units, menadione I.P. 9·1 mg, folic acid I.P. 1 mg and vitamin E USP 0·1 mg) per kg of diet. The diets were adequate in all nutrients. There was no difference in food intake between the groups because equal amount of food was served and all food was consumed.
The animals were killed after 4 weeks of STZ injection under anaesthesia and kept in overnight fasting before being killed and the last dose was administered 18 h before killing. Blood was collected from the hepatic vein into heparinised tubes, and the pancreas was immediately excised, blotted, weighed and stored frozen ( − 40°C) for analysis.
Estimation of blood glucose
Blood glucose of the diabetic rats and rats of the control group was estimated by using a strip-operated blood glucose meter (Ascensia, Entrust; Bayer HealthCare LLC) after STZ injection.
Estimation of total antioxidant activity
Total antioxidant activity of plasma was measured by the ferric reducing antioxidant power (FRAP) assay of Benzie & Strain(Reference Benzie and Strain24). The FRAP assay uses antioxidants as reductants in a redox-linked colorimetric method, employing an easily reduced oxidant system present in stoichiometric excess. In this assay, antioxidant activity was measured in terms of reduction of ferric tripyridyl triazine complex to the ferrous form at low pH, which was monitored by measuring the change in absorption at 593 nm.
Preparation of erythrocyte lysate
Heparinised blood (2 ml) was centrifuged for 10 min at 1000 g and the plasma was removed by suction. The erythrocytes were washed twice with PBS, pH 7·4. Distilled water (10 ml) was then added and the erythrocytes were resuspended by agitation and lysed for 2 h at 4°C. A mixture of chloroform–ethanol (3:5, v/v, 0·8 ml) and 0·3 ml of water were added to the lysate so as to precipitate the Hb, which was centrifuged at 3000 g for 10 min at 4°C(Reference Dani and Dhawan25).
Measurement of antioxidative parameters
Superoxide dismutase activity
Whole pancreas tissue was homogenised in 0·1 m-Tris buffer (pH 7·0) and the homogenate was centrifuged at 105 000 g for 1 h. The supernatant portion of pancreas homogenate, plasma and erythrocyte lysate was measured spectrophotometrically by calculating the rate of inhibition of auto-oxidation of haematoxylin for the assay of SOD according to the method as previously described(Reference Martin, Dailey and Sugarman26) and expressed as units/mg of protein.
Catalase activity
The CAT activity in the pancreas homogenate, plasma and erythrocyte lysate was measured spectrophotometrically by calculating the rate of degradation of H2O2, the substrate of the enzyme(Reference Yumoto, Ichihashi and Iwata27), and expressed as mmol of H2O2 decomposed/min per mg of protein.
Reduced glutathione assay
The intracellular GSH contents in the pancreas homogenate, plasma and erythrocyte lysate were quantified spectrophotometrically according to the method of Beutler et al. (Reference Beutler, Duron and Kelly28) and expressed as μg/mg of protein.
Glutathione peroxidase activity
The activity of GPx in the pancreas homogenate, plasma and erythrocyte lysate was assayed by the method of Mohandas et al. (Reference Mohandas, Marshall and Duggin29). Enzymatic reduction of H2O2 by GPx through consumption of reduced GSH was measured in this assay. GSH was restored from oxidised glutathione in a coupled enzymatic reaction by glutathione reductase. Glutathione reductase reduced oxidised glutathione to GSH using NADPH as a reducing agent. Disappearance of NADPH was measured immediately at 340 nm against a blank containing all the components except the enzyme source, and the activity of GPx was expressed as units/mg of protein.
Measurement of pro-oxidative enzyme activity
NOS activity in the pancreas homogenate and plasma homogenate, quantified by the accumulation of nitrite in the tissues, was measured spectrophotometrically according to the method of Hevel & Marletta(Reference Hevel and Marletta30) based on the Greiss reaction with sodium nitrite as the standard and expressed as nmol/mg of protein.
Measurement of protein content
Protein content was measured by Lowry's method(Reference Lowry, Rosebrough and Farr31).
Preparation and oxidative sensitivity of erythrocyte membrane ghost
After plasma separation, the erythrocytes were washed three times by centrifugation at 3000 g for 10 min with 3 vol of a cooled isotonic solution containing 0·15 m-NaCl and 10− 5 m-EDTA. The erythrocytes were haemolysed using hypotonic solution and centrifuged at 20 000 g for 40 min at 4°C. The supernatant was removed carefully with a Pasteur pipette. The process was repeated two more times. After the last wash step, the supernatant was removed as much as possible and the loosely packed milky-looking membrane pellet was resuspended at the bottom of the tube using 0·89 % NaCl solution. The concentrated membrane solution was taken into a 2 ml screw-capped vial and stored at − 40°C(Reference Rose and Oklander32).
A modification of the 2-thiobarbituric acid test(Reference Mezick, Settlemire and Brierley33) was used to measure the lipid peroxides. A 0·5 ml aliquot of the EM suspension was mixed with 1·0 ml of 10 % TCA and 2·0 ml of 0·67 % 2-thiobarbituric acid. The mixture was heated at 95°C for 15 min, cooled and centrifuged. The absorbance of the supernatant was measured at 534 nm in a spectrophotometer (Shimadzu), and the relative amounts of lipid peroxides were expressed in absorbance units, A 534 nm(Reference Brownlee, Huttner and Panganamala34).
Western analysis of hepatic p65 activity
Liver tissue was homogenised in lysis buffer and the concentration of total protein was estimated by Lowry's method(Reference Lowry, Rosebrough and Farr31). Then, 50 μg of protein were loaded in SDS-PAGE and transferred to the polyvinylidene fluoride membrane. The membranes were blocked in 5 % milk in TBST (20 mm-Tris–HCl (pH 7·6), 1377 mm-NaCl, 0·05 % Tween 20), incubated with mouse polyclonal antibodies to NF-κB (p65) (1:1000-fold dilution in blocking buffer with 0·05 % Tween 20), and then incubated with horseradish peroxidase-linked goat anti-mouse Ig (Imgenex). The membranes were washed in TBST between steps. Immune-complexed NF-κB (p65) proteins were detected in X-ray film using luminol reagent-mediated chemiluminescence.
Analysis of plasma TNF-α and IL-6 by sandwich ELISA
Microtitre plates were coated with 1:500 dilution of primary antibodies to TNF-α and IL-6 (Imgenex) overnight at 4°C. The plates were washed three times with PBS and blocked with PBS/3 % bovine serum albumin for 60 min at room temperature. The samples and standard (TNF-α) were diluted in PBS to achieve a measurement in the linear range of the ELISA. After further washes, the samples and standard were applied in duplicate for 4 h at 37°C. The plates were washed three times with PBS. After three washes with PBS, 1:5000 dilution of alkaline phosphatase tagged secondary antibody in PBS/0·5 % bovine serum albumin (Imgenex) was added for 1 h at room temperature. The plates were rinsed three times with PBS. Subsequently, para-nitrophenyl phosphate (1 mg/ml) was used to generate a colour reaction that was measured at 405 nm. The TNF-α concentration in the plasma was calculated from the standard curve(Reference Saalbach, Wetzig and Haustein35).
Statistical analysis
The data were expressed as means with their standard errors and in some cases means and standard deviations. A one-way ANOVA was also used for statistical analysis between groups. The F ratio of one-way ANOVA is significant when the P value < 0·05. Tukey's multiple-range method(Reference Scheffe36) was used for comparison.
Results
Effect on blood sugar level
The oral administration of bitter gourd and snake gourd oil containing CLnA lowered significantly the blood sugar level of STZ-induced diabetic rats as compared to the diabetic control group (Fig. 1), although the administration of experimental oil was unable to reduce blood sugar level to the normal control value. Results showed that the effectiveness of bitter gourd oil was slightly more than snake gourd oil in terms of reduction in blood sugar level.

Fig. 1 Effect of conjugated linolenic acid isomers on blood sugar level of diabetic rats fed blended oils. Control (![]() ); STZ-D, streptozotocin (60 mg/kg body weight)-treated diabetic group (
); STZ-D, streptozotocin (60 mg/kg body weight)-treated diabetic group (![]() ); STZ-D+0·5 % EA, streptozotocin-treated diabetic group fed sunflower oil containing 0·50 % α-eleostearic acid (
); STZ-D+0·5 % EA, streptozotocin-treated diabetic group fed sunflower oil containing 0·50 % α-eleostearic acid (![]() ); STZ-D+0·5 % PA, streptozotocin-treated diabetic group fed sunflower oil containing 0·50 % punicic acid (
); STZ-D+0·5 % PA, streptozotocin-treated diabetic group fed sunflower oil containing 0·50 % punicic acid (![]() ). Values are means with their standard errors represented by vertical bars. * Mean values were significantly different from those of STZ-D (P < 0·05). † Mean values were significantly different from those of STZ+0·5 % PA (P < 0·05).
). Values are means with their standard errors represented by vertical bars. * Mean values were significantly different from those of STZ-D (P < 0·05). † Mean values were significantly different from those of STZ+0·5 % PA (P < 0·05).
Effect on total antioxidant activity of plasma
Total antioxidant activity was measured by the FRAP assay. Results showed that the FRAP value of diabetes-induced rats (Table 2) decreased significantly compared to the control group due to oxidative stress induced by STZ treatment. The FRAP value of plasma was restored to the control value with the administration of bitter gourd and snake gourd seed oils due to the presence of α-eleostearic acid and punicic acid, respectively. The results also showed that the FRAP value of the α-eleostearic acid-treated group was significantly higher (P < 0·05) than that of the punicic acid-treated group.
Table 2 Effect of conjugated linolenic acid isomers on plasma, pancreatic, erythrocyte membrane (EM) lipid peroxidation and ferric reducing antioxidant power (FRAP) value of plasma of rats fed blended oils
(Mean values with their standard errors; n 8)
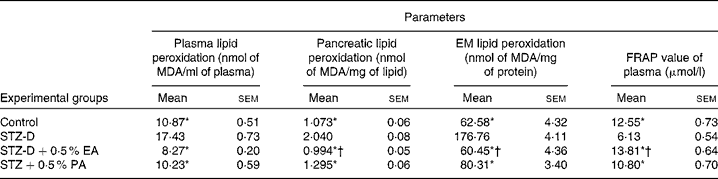
MDA, malondialdehyde; STZ-D, streptozotocin (60 mg/kg body weight) treated diabetic group; STZ-D+0·5 % EA, streptozotocin treated diabetic group fed sunflower oil containing 0·50 % α-eleostearic acid; STZ-D+0·5 % PA, streptozotocin treated diabetic group fed sunflower oil containing 0·50 % punicic acid.
* Mean values were significantly different from those of STZ-D (P < 0·05).
† Mean values were significantly different from those of STZ-D+0·5 % PA (P < 0·05).
Effect on plasma, erythrocyte membrane and pancreatic lipid peroxidation
Results showed that the administration of STZ increased lipid peroxidation significantly (P < 0·05) in plasma, EM and pancreatic lipid (Table 2) compared to the respective control. Supplementation of CLnA isomers caused significant reduction in lipid peroxidation and restored them almost to the control value. The results showed that lipid peroxidation was significantly lower in the case of the 0·5 % α-eleostearic acid-treated group than the 0·5 % punicic acid-treated group.
Effect on antioxidant and pro-oxidant enzyme activity
In the present study, the activities of antioxidant enzymes such as SOD, CAT and GPx in plasma, pancreas homogenate and erythrocyte lysate were found to be significantly (P < 0·05) decreased in STZ-induced diabetic rats as compared to the control due to oxidative stress generated by STZ administration. The decreased antioxidant enzyme activity in plasma, pancreas homogenate and erythrocyte lysate appeared to be improved after CLnA supplementation (Tables 3 and 4). All the antioxidant enzyme activities were significantly higher in the case of the 0·5 % α-eleostearic acid-treated group than the 0·5 % punicic acid-treated group in most of the observations. STZ-induced diabetic rats showed a significant reduction in cellular GSH levels in the plasma, pancreas homogenate and erythrocyte lysate compared to the respective controls but with the administration of oils containing CLnA, there was an increase in the levels of GSH content (Tables 3 and 4). GSH content in plasma and erythrocyte lysate was significantly (P < 0·05) higher in the case of the 0·5 % α-eleostearic acid-treated rats than the 0·5 % punicic acid-treated rats, but the difference in GSH content in the pancreas homogenate was not significant. The pro-oxidant enzyme NOS activity was significantly (P < 0·05) increased in the plasma and pancreas homogenate as compared to the respective control due to STZ administration. Increased NOS activity was significantly restored to normal value in the plasma and pancreas homogenate due to administration of both the oils containing CLnA isomers while the difference in NOS activity between the α-eleostearic acid- and punicic acid-treated rats was not significant in the plasma and pancreas (Table 3). Overall increase in antioxidant enzyme activity was more significant in the case of α-eleostearic acid than punicic acid.
Table 3 Effect of conjugated linolenic acid isomers on antioxidant and prooxidant enzyme activity of plasma and pancreatic homogenate of rats fed blended oils
(Mean values with the standard errors; n 8)

SOD, superoxide dismutase, CAT, catalase, GPx, glutathione peroxidase, GSH, reduced glutathione; NOS, NO synthase; STZ-D, streptozotocin (60 mg/kg body weight) treated diabetic group; STZ-D+0·5 % EA, streptozotocin treated diabetic group fed sunflower oil containing 0·50 % α-eleostearic acid; STZ-D+0·5 % PA, streptozotocin treated diabetic group fed sunflower oil containing 0·50 % punicic acid.
* Mean values were significantly different from those of STZ-D (P < 0·05).
† Mean values were significantly different from those of STZ-D+0·5 % PA (P < 0·05).
Table 4 Effect of conjugated linolenic acid isomers on antioxidant enzyme activity in erythrocyte lysate of rats fed blended oils
(Mean values with their standard errors; n 8)
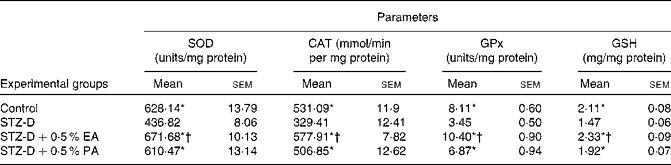
SOD, superoxide dismutase, CAT, catalase, GPx, glutathione peroxidase, GSH, reduced glutathione; STZ-D, streptozotocin (60 mg/kg body weight) treated diabetic group; STZ-D+0·5 % EA, streptozotocin treated diabetic group fed sunflower oil containing 0·50 % α-eleostearic acid; STZ-D+0·5 % PA, streptozotocin treated diabetic group fed sunflower oil containing 0·50 % punicic acid.
* Mean values were significantly different from those of STZ-D (P < 0·05).
† Mean values were significantly different from those of STZ-D+0·5 % PA (P < 0·05).
Effect on hepatic p65 activity
Western analysis of hepatic NF-κB (p65) activity (Fig. 2) showed that the expression of p65 was higher in the case of STZ-induced diabetic rats as compared to control rats due to oxidative stress which was significantly (P < 0·05) restored after the administration of vegetable oils containing CLnA isomers. In terms of restoration of hepatic p65 expression, there was no difference between the effectiveness of these two isomers.

Fig. 2 Effect of conjugated linolenic acid isomers on expression of hepatic NF-κB (p65) of diabetic rats fed blended products. (A) Western blots. (B) Quantitative presentation of expression of hepatic NF-κB of experimental groups in terms of arbitrary units. STZ-D, streptozotocin (60 mg/kg body weight)-treated diabetic group; STZ-D+0·5 % EA, streptozotocin-treated diabetic group fed sunflower oil containing 0·50 % α-eleostearic acid; STZ-D+0·5 % PA, streptozotocin-treated diabetic group fed sunflower oil containing 0·50 % punicic acid. Values are means with their standard errors represented by vertical bars. * Mean values were significantly different to STZ-D (P < 0·05).
Effect on plasma TNF-α and IL-6 activity
Sandwich ELISA analysis of plasma pro-inflammatory cytokines showed that the activities of TNF-α and IL-6 in blood were increased significantly (P < 0·05) due to the oxidative stress-induced inflammatory response in STZ-induced diabetic rats, and that levels were restored by CLnA administration (Table 5). Punicic acid was slightly more efficient in the restoration of TNF-α and IL-6 level in blood than α-eleostearic acid although the difference was not significant.
Table 5 Effect of conjugated linolenic acid isomers on expression of TNF-α and IL-6 in plasma of diabetic rats fed blended oils
(Mean values with their standard errors; n 8)
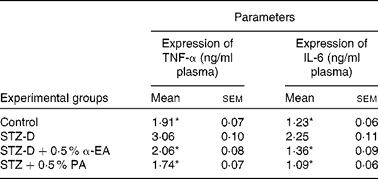
STZ-D, streptozotocin (60 mg/kg body weight) treated diabetic group; STZ-D+0·5 % EA, streptozotocin treated diabetic group fed sunflower oil containing 0·50 % α-eleostearic acid; STZ-D+0·5 % PA, streptozotocin treated diabetic group fed sunflower oil containing 0·50 % punicic acid.
* Mean values were significantly different from those of STZ-D (P < 0·05).
Discussion
The present study was conducted to evaluate the beneficial effects of bitter gourd and snake gourd seed oil containing CLnA isomers against oxidative stress and inflammation in STZ-induced diabetic rats. Pancreatic β-cell death underlies the pathogenesis of type 1 (insulin-dependent) diabetes mellitus. STZ-induced experimental diabetes is a valuable model for type 1 diabetes. Studies reveal that STZ-induced diabetic rats exhibit most of the diabetic complications mediated through oxidative stress and also suggest free-radical involvement in pancreatic cell destruction(Reference Tomlinson, Gardiner and Hebden37, Reference Sun, Yang and Zhao38). Low-dose STZ-induced diabetes shares many clinical and histological features of human type 1 diabetes, affecting both T-cells and macrophages(Reference Herold, Vezys and Sun39). Thus, multiple low-dose STZ treatment induces an autoimmune insulitis that leads to insulin insufficiency and diabetes(Reference Kolb-Bachofen, Epstein and Kiesel40). ROS has been implicated in the induction of pancreatic cell damage(Reference Hotta, Yamato, Miyazaki, Packer, Rosen, Tritschler, King and Azzi41). The cellular mechanisms involved in the hypoglycaemic effects of CLnA isomers are not yet established. They seem to stimulate peripheral glucose utilisation or may inhibit key gluconeogenic enzymes(Reference Sathishsekar and Subramanian42). These isomers may cause some elevation in endogenous insulin release by delaying the toxic effect of STZ on β cells due to their antioxidant nature and also by protecting them. Results show that although CLnA isomers do not normalise the glucose level in STZ-induced diabetic rats, they possess some sort of protection against STZ-induced diabetes. α-Eleostearic acid showed to some extent higher blood sugar-reducing activity due to its higher antioxidant efficacy than punicic acid(Reference Saha and Ghosh15).
STZ induces diabetes via oxidative damage by generating a large amount of ROS and increasing lipid peroxidation in plasma and tissue. Albumin contains most of the protein thiol groups in plasma. These thiol groups act as antioxidants. being oxidised by electron-deficient free radicals, and the FRAP value is the measurement of ‘antioxidant power’(Reference Benzie and Strain24, Reference Koduru, Tejaswini and Thakur43). In this study, the FRAP value decreased significantly after STZ treatment due to oxidative stress and this is in agreement with the previous findings of Koduru et al. (Reference Koduru, Tejaswini and Thakur43). CLnA isomers also acted as potent antioxidants and caused complete restoration of the FRAP value. SOD, CAT and GPx constitute the first line of cellular antioxidant defence enzymes. SOD is one of the most important enzymes in the enzymatic antioxidant defence system which catalyses the dismutation of superoxide radicals to produce H2O2 and molecular oxygen(Reference Venarucci, Venarucci and Vallese44). The decreased SOD activity in the organs, blood and erythrocyte lysate is due to the accumulation of superoxide anion radicals and the glycation of enzymes. Accumulation of superoxide anions might be responsible for increased lipid peroxidation following diabetes induction by STZ(Reference Sozmen, Sozmen and Delen45).
Reduced activity of CAT after exposure to STZ in the present study could be correlated to the increased generation of H2O2. The significant decrease in SOD and CAT activities of rats treated with STZ is in agreement with the previous findings of Mahesh & Menon(Reference Mahesh and Menon46). CAT and GPx are responsible for the elimination of H2O2 at high and low concentrations, respectively. In diabetes, the activities of CAT and GPx are significantly decreased by superoxide radicals and by glycation reactions(Reference Sankaranarayanan and Pari47).
GSH, a tripeptide, has a multifactorial role in antioxidant defence. GSH functions as a direct scavenger of free radicals and also reduces the toxicity of peroxide by acting as a co-substrate for GPx(Reference Sathishsekar and Subramanian42). GSH may be oxidised due to the interaction with the free radicals induced by STZ. NADPH is involved in GSH regeneration. The polyol pathway, predominant in hyperglycaemic conditions, also utilises NADPH and thus it causes depletion of GSH, resulting in lowered GPx activity(Reference Lorenzi48). Low GPx activity is associated with diabetes and other diseases(Reference Faure49).
During the metabolic action of GSH, its sulphydryl group becomes oxidised resulting in the formation of a corresponding disulphide compound, oxidised glutathione. GSH depletion in erythrocytes leads to the production of free radicals. These free radicals interact with membrane lipids leading to the production of lipid hydroperoxides(Reference Radabaugh and Aposhian50). Thus, to eliminate free radicals, these cellular antioxidants play an important role and equilibrium exists between these enzymes under normal physiological conditions.
α-Eleostearic acid and punicic acid, two typical isomers of CLnA, exhibit protective effects against lipid oxidative damage in plasma, pancreas and EM lipid induced by STZ. These two CLnA isomers caused improvement in antioxidant enzyme activity by reducing lipid peroxidation and also by scavenging free radicals which are formed due to the action of STZ. Moreover, the administration of CLnA to diabetic rats alleviated stress and ameliorated SOD and CAT levels. In the present investigation of exposure to STZ, the possible accumulation of H2O2 in the liver as a result of diminished activity of CAT, particularly on CLnA administration circumvented by the increased GSH concentrations, leads to the stimulation of the GPx-mediated reduction of H2O2 and organic hydroperoxides. This finding is in agreement with the previous studies of Dhar et al. (Reference Dhar, Ghosh and Bhattacharyya12) and Saha et al. (Reference Saha and Ghosh16). Thus, it may be possible that in the in vivo conditions, these conjugated linolenic FA may have reduced the formation of hydroperoxides by scavenging the free radicals and the peroxidation of PUFA occurs in EM and other lipids(Reference Dhar, Ghosh and Bhattacharyya12). Another possible explanation may be that the biohydrogenation or free-radical addition to one of the conjugated double bonds of CLnA might have taken place, resulting in the formation of conjugated dienes that could have possibly acted as antioxidants(Reference Ip, Scimeca and Thompson51). The possible mechanism concludes in hydroperoxide formation and biohydrogenation of CLnA. It appears to act as a chain-breaking antioxidant by trapping chain-propagating free radicals. Some researchers(Reference Yu, Adams and Gabel52) showed that CLA could play its antioxidative roles by directly acting with free radicals to terminate the radical chain reaction or chelating transition metals to suppress the initiation of radical formation.
NO is an important marker of oxidative stress and induced inflammation and therefore the activity of NOS has been included in the study by measuring the nitrite (![]() ) level. Stadler et al. (Reference Stadler, Jenei and von Bolcshazy53) has revealed that NO levels are increased in STZ-induced diabetic rats at the initial stage and increased expression of inducible NOS in diabetic liver has also been reported previously(Reference Madar, Kalet-Litman and Stark54). Thus, NO may produce the highly reactive oxidant species peroxynitrite by reacting with ROS such as the superoxide radical and causes enormous oxidative and nitrosative stress(Reference Dias, Porawski and Alonso55). The present study established the role of NO in diabetes and which is significantly reduced by the administration of CLnA. Studies also showed that through the activation of PPARγ, CLA decreased inducible NOS(Reference Yu, Correll and vanden Heuvel56).
) level. Stadler et al. (Reference Stadler, Jenei and von Bolcshazy53) has revealed that NO levels are increased in STZ-induced diabetic rats at the initial stage and increased expression of inducible NOS in diabetic liver has also been reported previously(Reference Madar, Kalet-Litman and Stark54). Thus, NO may produce the highly reactive oxidant species peroxynitrite by reacting with ROS such as the superoxide radical and causes enormous oxidative and nitrosative stress(Reference Dias, Porawski and Alonso55). The present study established the role of NO in diabetes and which is significantly reduced by the administration of CLnA. Studies also showed that through the activation of PPARγ, CLA decreased inducible NOS(Reference Yu, Correll and vanden Heuvel56).
Some researchers(Reference Cardozo, Heimberg and Heremans57) have revealed that the activation of Rel/NF-κB may be a critical determinant in β-cell death leading to type 1 diabetes. Hyperglycaemia indirectly induces several factors, including oxidative stress, free radicals, secretion of a broad spectrum of inflammatory and destructive mediators such as cytokines (TNF-α, IL-1β and IL-6), chemokines (IL-8) and PGE2(Reference Soop, Duxbury and Agwunobi8). An increased level of TNF-α in STZ-induced diabetes may act through mitogen-activated protein kinase signal pathways to activate NF-κB, which in turn causes elevation in NO level by activating inducible NOS. Increased levels of all these inflammatory mediators ultimately induce apoptosis(Reference Graves, Liu and Alikhani58). It has also been reported that in the absence of an appropriate compensatory response from the endogenous antioxidant network, the system becomes overwhelmed (redox imbalance), leading to the activation of stress-sensitive signalling pathways, such as NF-κB, and others(Reference Dias, Porawski and Alonso55). The increase in NO production in diabetic rats as reflected by the activity of NOS in plasma, pancreas and EM lysate is most probably due to the overexpression of NF-κB (p65). In the present investigation, CLnA-induced suppression of the release of NF-κB is accompanied by down-regulation of the expression of NOS and this is due to the free-radical scavenging activity of CLnA isomers. In this study, the administration of vegetable oils containing CLnA isomers significantly restored elevated TNF-α and IL-6 level in the plasma of STZ-induced diabetic rats. CLnA isomers might act as potent anti-inflammatory agents by suppressing proinflammatory cytokine production by themselves or by converting into CLA through biohydrogenation. It has been reported that CLA might cause a decrease in the production of downstream eicosanoid products such as PGE2 by modulating the accumulation of arachidonate in phospholipids and interfering with the synthesis of arachidonic acid from linoleic acid, resulting in a reduced arachidonate pool(Reference Belury59). A decrease in PGE2 causes suppression in the production of IL-1β, IL-6, TNF-α, IL-2 and interferon-γ(Reference Calder60). However, the anti-inflammatory effect of CLnA via suppressing the synthesis of proinflammatory cytokines (i.e. IL-6 and TNF-α) seems to be related to PPARγ expression, which is supported by the findings of Changhua et al. (Reference Changhua, Jindong and Defa61). They have reported that the synthesis of IL-6 and TNF-α is decreased simultaneously in CLA-fed pigs after an inflammatory challenge due to the up-regulation of PPARγ expression. It has been reported that the activation of PPARγ in the colon inhibits mucosal production of IL-1β and TNF-α by down-regulation of the NF-κB and mitogen-activated protein kinase signal pathways(Reference Desreumaux, Dubuquoy and Nutten62). Therefore, our observations regarding the antioxidant and anti-inflammatory role of vegetable oils containing CLnA isomers against STZ-induced diabetes have been well supported by the aforementioned studies.
Conclusions
In conclusion, the data from the present study all along support the antioxidant and anti-inflammatory role of vegetable oil containing CLnA isomers. All the biochemical and inflammatory parameters strongly suggest that though there are some differences in the effectiveness of α-eleostearic acid and punicic acid due to the difference in their structural cis–trans configuration, both can effectively act as stress-reducing antioxidant and anti-inflammatory agents against STZ-induced diabetes. α-Eleostearic acid has showed better antioxidant activity due to its high trans content (cis-9, trans-11, trans-13-octadecatrienoic). In terms of the inhibitory effect on NOS activity, expression of hepatic NF-κB, plasma TNF-α and IL-6 level, these two CLnA isomers have a potent anti-inflammatory role and in this respect, punicic acid is more efficient than α-eleostearic acid due to its high cis content (cis-9, trans-11, cis-13-octadecatrienoic acid). Although it would have been better to compare the beneficial effects of CLnA isomers with non-conjugated tri-enoic acids such as α-linolenic acid, the different CLnA isomers present in these two experimental oils have been established as natural antioxidant and anti-inflammatory agents against STZ-induced diabetes by the present study.
Acknowledgements
This work was funded by the Indian Council of Medical Research (ICMR), Government of India. The authors have no conflicts of interest to declare. M. G. supervised the study and S. S. S. conducted the experiments and analysis. Both the authors made equal contribution in the drafting of the manuscript. The authors sincerely acknowledge the assistance received from Dr Santinath Ghosh, Department of Chemical Technology, University of Calcutta.









