Depression is a mental disorder that is associated with sadness, loss of interest or pleasure, feelings of guilt, disrupted sleep, decreased desire for food, fatigue and difficulty concentrating(Reference Khan, Khan and Adnan1). It has become an important public health problem worldwide. The prevalence of depression in adults with end-stage renal disease requiring maintenance haemodialysis (MHD) is as high as 40 % worldwide(Reference Palmer, Vecchio and Craig2), which is amongst the leading causes of disability(3). A multicenter cross-sectional study from northern China showed that the prevalence of depression in haemodialysis (HD) patients was as high as 55 %(Reference Meng, Wu and Niu4). Concomitant depression is associated with worse dialysis treatment, dietary and medication incompliance, lower quality of life, increased hospitalisation rates and higher mortality(Reference Sensky, Leger and Gilmour5–Reference Kurella, Kimmel and Young9). Early prevention and slowing of disease progression are important due to a very high risk of a poor prognosis. According to a previous study, dietary habits and composition of dietary patterns are the best-known and most powerful modifiers of brain health(Reference Marrone and Coccurello10). Notably, HD period is also a point where greater dietary control emerges with respect to dietary source, choice and content(Reference Sahathevan, Khor and Ng11). Hence, this is an important stage of life in which to investigate the association between diet intake and depressive symptoms in a cohort of MHD patients.
Fatty acids (FA) are a nutritional factor that is becoming increasingly important in the occurrence and development of diseases. It plays numerous functions in the body throughout an individual’s lifetime, including providing energy for many body tissues. There are two types of FA: saturated fatty acids (SFA) and unsaturated fatty acids (UFA), which include monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA). In addition, they can be divided into several subtypes depending on the position of the carbon atoms and the number of double bonds. There is evidence that FA, particularly n-3 FA, can influence depression symptoms. According to a prospective cohort study of adults performed by Beydoun et al., higher n-3: n-6 dietary FA ratios were associated with a slower increase in depression symptoms over time, especially in women(Reference Beydoun, Fanelli and Beydoun12). In addition, according to the cross-sectional study conducted by Oddy et al., there is an inverse relationship between n-3 PUFA intake and depressive symptoms in adolescents(Reference Oddy, Hickling and Smith13). A community-based, longitudinal, multicenter and population-based study by Li et al. has found that baseline SFA intake was a predictor of depressive symptoms in middle-aged women after four years(Reference Li, Liang and Tong14). Several previous studies have indicated that dietary FA intake might be related to the brain’s neurochemistry properties, function and inflammation(Reference Lai, Oldmeadow and Hure15,Reference Hryhorczuk, Florea and Rodaros16) . Therefore, we hypothesised that there might be an association between dietary FA intake and depressive symptoms in MHD patients.
To date, no studies have investigated the relationship between dietary FA and depressive symptoms in MHD patients. To address this important issue, a cross-sectional study was carried out to ascertain the correlation, if any, between dietary FA and depressive symptoms in a cohort of MHD patients. The findings of the present study will help provide new dietary management advice and intervention strategies for MHD patients who exhibit depressive symptoms, thereby potentially mitigating depression-associated morbidity and mortality.
Materials and methods
Design and population
A cross-sectional study was performed between December 2021 and January 2022 using HD patients who received regular HD treatment in the Dialysis Center of Dalian Municipal Central Hospital, Dalian, China, which is the largest HD centre in the three provinces of northeast China. The eligibility criteria for participants were as follows: age > 18 years; treatment with conventional HD for at least three months and normal intake of food and water (not receiving enteral or parenteral nutrition). HD patients who declined to participate (n 35), those receiving temporary dialysis (n 4), those with time on dialysis of < 3 months (n 32), patients with mild impaired cognitive function (n 30), those with implausible energy intake (< 700 or > 3700 kcal/d for male and < 600 or > 3000 kcal/d for female,(Reference Wu, Song and Chen17) n 40) and participants with missing key covariates included in the study (n 19) were excluded. A total of 682 MHD patients were included in the final analysis (Fig. 1). All study protocols conformed to the principles of the Declaration of Helsinki and were approved by the Institutional Medical Ethics Committee of Dalian Municipal Central Hospital (protocol number: YN2022-096-04). All study patients signed the written informed consent form. All information were collected in Chinese and experienced staff performed these protocols. We have conducted special training for relatives or nursing staff of some special patients who cannot self-report dietary information and depressive symptoms.
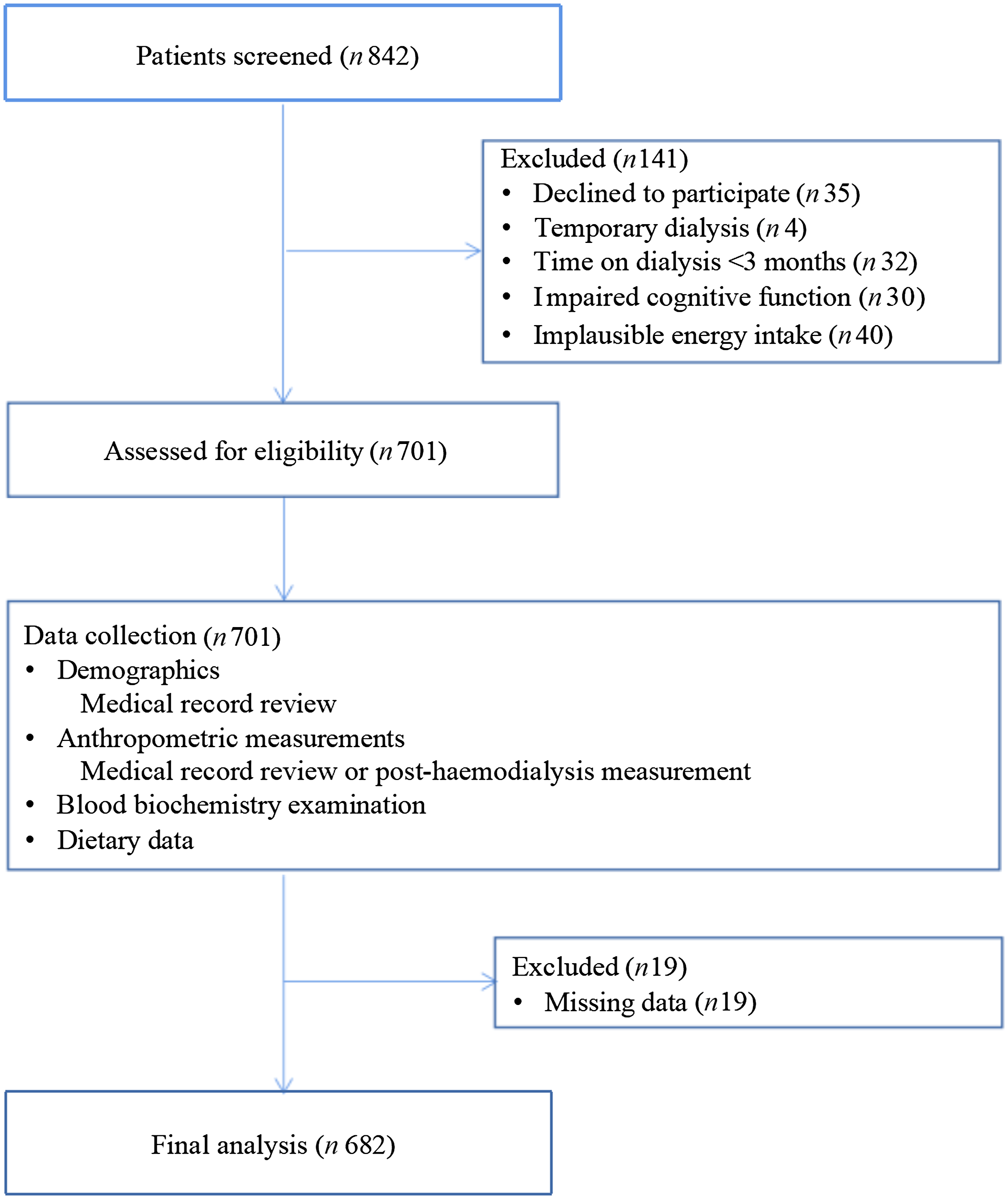
Fig. 1. A flow chart indicates patients’ enrolment.
Data collection
Baseline features were extracted from the home page of electronic medical records of Dalian Municipal Central Hospital or a standardised self-administered questionnaire, including gender, age, start dialysis time, education level, marital status, annual family income, physical activity, anxiety symptoms, smoking status (at least once per day for > 6 months), alcohol consumption (at least once per day for > 6 months) and co-morbidities (diabetes mellitus, hypertension and history of cardiovascular disease [CVD]) within one month before and after blood sample collection. Dialysis age was calculated from the start of dialysis until December 31, 2021. Diabetes was defined as a history of diabetes mellitus or the use of hypoglycaemic drugs. Hypertension was defined as documentation in the medical record or the use of antihypertensive drugs. CVD history was defined as previous angina pectoris, transient ischaemic attack or cerebrovascular incident, congestive heart failure, myocardial infarction, or peripheral arterial disease. A physical examination was conducted after dialysis to measure the patient’s weight. Height was measured by trained staff following a standard protocol. The formula for the calculation of body mass index (BMI) was as follows: BMI = weight in kilograms divided by the square of height in metres (kg/m2). Physical activity was measured using the International Physical Activity Questionnaire-Short Form, which contains seven questions and has been confirmed to be valid in HD patients(Reference Rosa, Gracia-Marco and Barker18). We employed the Montreal Cognitive Assessment (MoCA) to evaluate the cognitive function of patients with HD. The sum of the scale items produces a total MoCA score ranging from 0 to 30, which is positively correlated with the overall cognitive function(Reference Julayanont, Brousseau and Chertkow19). The criteria for mild cognitive impairment is based on the Chinese norms of the MoCA(Reference Lu, Li and Li20): total MoCA score ≤ 13 for illiterate individuals, ≤ 19 for individuals with 1–6 years of education and ≤ 24 for individuals with 7 years of education or more.
Blood samples were collected from patients before starting dialysis in the middle of the week. Biochemical indicator levels, including those of serum Hb, albumin, phosphate, creatinine and urea nitrogen, were determined using the Hemoglobin Assay Kit (SLS-Hb), bromcresol green, phosphomolybdate UV, enzyme method, urease UV rate method in a clinical laboratory, respectively. Urea clearance was measured using standard methods and dialysis adequacy was calculated as follows: Kt/V = -ln(R-0·008 × t) + (4–3·5 × R) × UF/W, where R is the ratio of post-dialysis to pre-dialysis serum urea nitrogen concentration, t is the duration of HD in hours, UF is the amount of ultrafiltration in litres during the HD session, and W is the post-dialysis weight in kilograms(Reference Daugirdas21).
Dietary assessment
Dietary consumption was assessed using a 110-item semi-quantitative food frequency questionnaire (FFQ) at the time of recruitment, which was demonstrated to have rational reliability and validity. The reproducibility coefficients median > 0·50 for energy and nutrients and reached 0·60 for food groups. The validity coefficients between the FFQ and weighed diet records (WDR) median were 0·46, 0·52 and 0·52 for the energy, nutrients and food groups, respectively. The triad method indicated that validation coefficients for the FFQ were above 0·3 for most nutrients, which indicated a moderate or high level of validity(Reference Cui, Xia and Liu22). The FFQ was also self-reported during a routine HD treatment within one month before and after blood sample collection. The standard food portion size was used to calculate the daily food intake in grams based on the recommendations in the four-day WDR. Food item consumption was assessed by asking patients the following questions: How often did you eat any food in the past year? Response choices ranged from ‘never’ to ‘twice or more a day’. The intake of each food item in grams/day was calculated by multiplying consumption frequencies per day by fitted portion sizes (g/time). The calculation of dietary SFA, UFA, MUFA, PUFA, α-Linolenic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), docosahexaenoic acid (DHA), linoleic acid, arachidonic acid intake was derived using the China Food Composition Tables (sixth edition)(23). Dietary total FA were calculated by summing the amounts of all aforementioned FA. Total n-3 FA were calculated by summing the amounts of α-Linolenic acid, EPA, DPA and DHA. Total n-6 FA were calculated by summing the amounts of linoleic acid and arachidonic acid.
Depressive symptom assessment
Depressive symptom information was self-reported within one month before and after blood sample collection. The Patient Health Questionnaire-9 (PHQ-9) score was used in conjunction with the lifestyle questionnaire to assess depressive symptoms(Reference Kroenke, Spitzer and Williams24). PHQ-9 is one of the most common self-assessment screening tools for depression that has shown significant screening efficacy in a variety of populations, including Chinese patients in primary care settings(Reference Chen, Chiu and Xu25–Reference Zhang, Ting and Lam27). A total of 27 points are assigned in the PHQ-9 score, which consists of nine questions rated from 0 to 3 according to the frequency of symptoms(Reference Kroenke, Spitzer and Williams24). When PHQ-9 scores are greater than or equal to 5, subjects are considered depressed(Reference Zhang, Li and Yang28).
Covariates
The data included the dietary information for the respondents and general demographic characteristics, such as age (continuous, years), gender (categorical, male/female), time on dialysis (continuous, months), BMI (continuous, kg/m2), physical activity (continuous, metabolic equivalent of task/min/week), smoking status (categorical, never/ever), alcohol intake (categorical, never/ever), marital status (categorical, unmarried/married/divorced/widowed), anxiety symptoms (categorical, yes or no), education level (categorical, junior high school or below, senior high school/secondary specialised school/junior college/university or above), co-morbidities (categorical, yes or no) and total energy intake (continuous, kcal/day).
Statistical analysis
The residual method was used to adjust the dietary intake of FA. All continuous variables were tested for normality using the Kolmogorov–Smirnov test. Normally distributed continuous variables were represented as means with standard deviation (SD). Skewed continuous variables were represented as medians (quartile1–quartile3), while frequency and percentage were used for categorical variables. Variance analysis and the Kruskal–Wallis H test were used to determine the differences in continuous variables. The chi-square test was utilised for categorical variables. Just as in several previous studies(Reference Lin, Qin and Yang29,Reference Narasaki, Okuda and Kalantar30) , dietary FA were categorised based on the tertile distribution. The lowest tertile was considered to be the reference group. An unconditional multiple logistic regression model was used to estimate the odds ratios (ORs) and the corresponding 95 % confidence intervals (CIs) of depressive symptoms (binary dependent variable). For the covariates in each model, we mainly refer to previous studies(Reference Li, Liang and Tong14,Reference Zhang, Li and Yang28) and clinical experience. The first model adjusted for age, gender and time on dialysis. The second model additionally adjusted for BMI, physical activity, smoking status, alcohol intake, education level, co-morbidities, marital status and anxiety symptoms on the basis of model 1. The third model additionally adjusted for dietary total energy intake on the basis of model 2. Logistic regression analysis was used to determine linear trends by allocating the median dietary FA intake for each tertile as a continuous variable. The interaction test was carried out by adding corresponding multiplication terms to the model at the same time. Consistent with the previous study(Reference Lin, Qin and Yang29), to further control the interference of potential confounding factors, subgroup analysis was also conducted on the basis of gender (male/female), age (≤ 60/ > 60 years), diabetes status (yes/ no), CVD status (yes/ no), BMI (< 24/ ≥ 24 kg/m2)(Reference Di Iorio, Cirillo and Bellizzi31–Reference Tsigalou, Chalikias and Kantartzi33) and dialysis age (< 36/ ≥ 36 months). The dose–response relationship between dietary FA and depression syndrome was determined using a restricted cubic spline (RCS) regression model with four knots at the 5th, 35th, 65th and 95th percentiles(Reference Gong, Li and Wang34) combined with logistic regression adjustment for the aforementioned covariates. Sensitivity analyses were conducted only on patients who had no change in dietary behaviour in the past year in order to test the robustness of the relationship between dietary FA intake and depression syndrome. All analyses were performed using SAS (version 9·4; SAS Institute Inc.). The level of statistical significance was set at P < 0·05 and was based on a two-sided test.
Results
MHD patients with higher dietary FA intake were characterised by longer dialysis duration, more physical activity and less drinking alcohol (Table 1). Compared with patients with lower FA intake, those with a higher intake tended to consume more SFA, UFA, MUFA, PUFA, total n-3 FA, α-linolenic acid, EPA, DPA, DHA, total n-6 FA, linoleic acid and arachidonic acid but consumed a lower amount of total caloric energy (Table 2).
Table 1. Baseline characteristics of study patients.
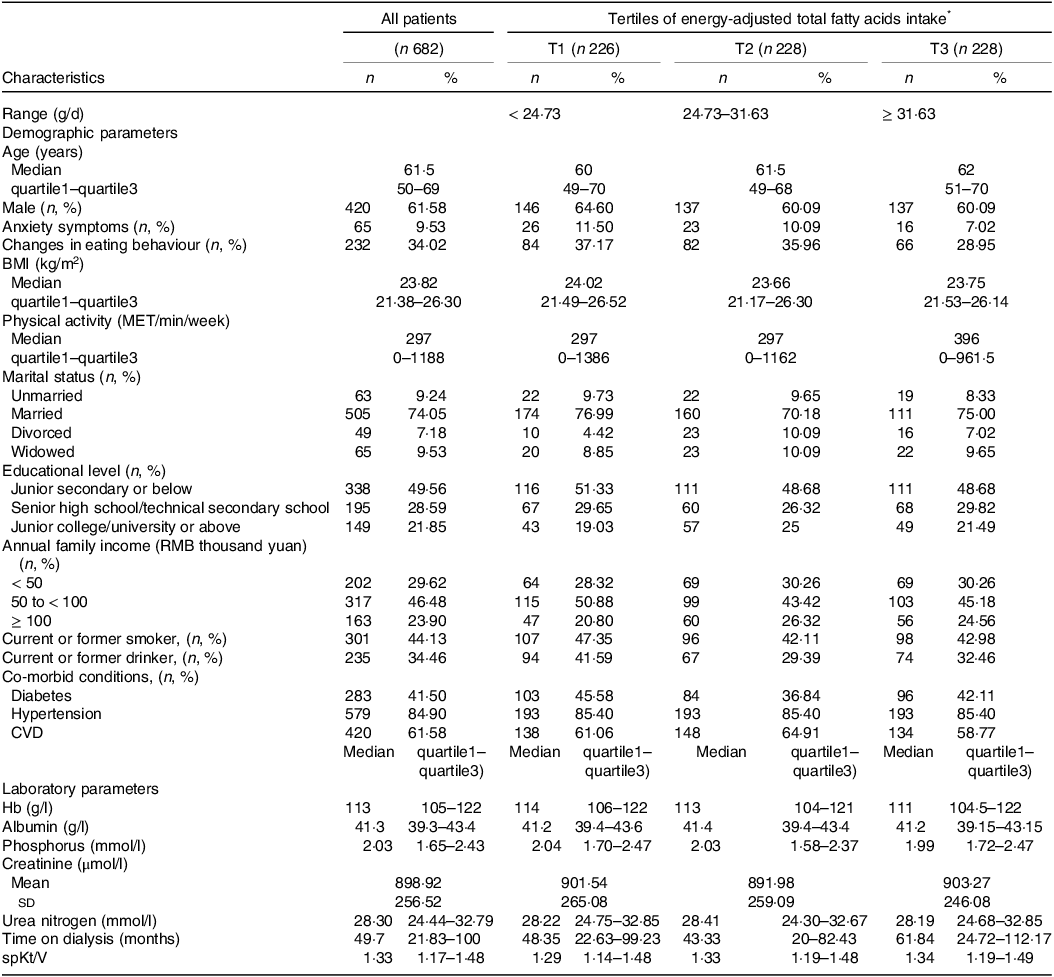
Abbreviations: BMI: body mass index; CVD, cardiovascular disease; Hb: hemoglobin; MET, metabolic equivalents of task; spKt/V, Single-pool Kt/Vurea.
Data presented as mean ± sd (standard deviation) or median (quartile1–quartile3) (continuous variables) or number (%) (categorical variables).
* Energy adjusted according to the residual method.
Table 2. Diet intake characteristics of study patients.
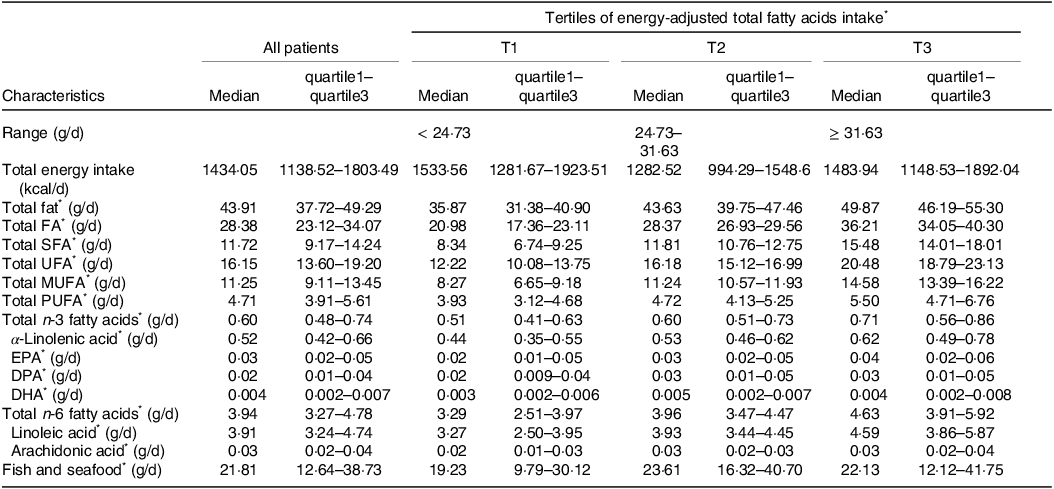
Abbreviations: DPA: docosapentaenoic acids; DHA: docosahexaenoic acids; EPA: eicosapentaenoic acids; FA, fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; SFA: saturated fatty acids; UFA: unsaturated fatty acids.
Data presented as median (quartile1–quartile3) (continuous variables).
* Energy adjusted according to the residual method.
The associations between dietary FA intake and depressive symptoms in a cohort of MHD patients are presented in Table 3. Higher intake amounts of total FA and SFA were significantly associated with higher prevalence of depressive symptoms compared with the reference group in models 2 and 3. In the fully adjusted model, a statistically significant positive association between the third tertile consumption of total FA and depressive symptoms was detected compared with the reference group (OR = 1·59, 95 % CI = 1·04, 2·46; P trend = 0·03). Similarly, a higher SFA intake was associated with an increased prevalence of depressive symptoms (OR = 1·83, 95 % CI = 1·19, 2·84; P trend < 0·01). In addition, an sd increment in SFA intake was significantly associated with an increased prevalence of depressive symptoms (OR = 1·20, 95 % CI = 1·01, 1·43).
Table 3. Adjusted odds ratio (OR) and 95 % confidence interval (CI) for the association between dietary fatty acids intake * and the prevalence of depressive symptoms.
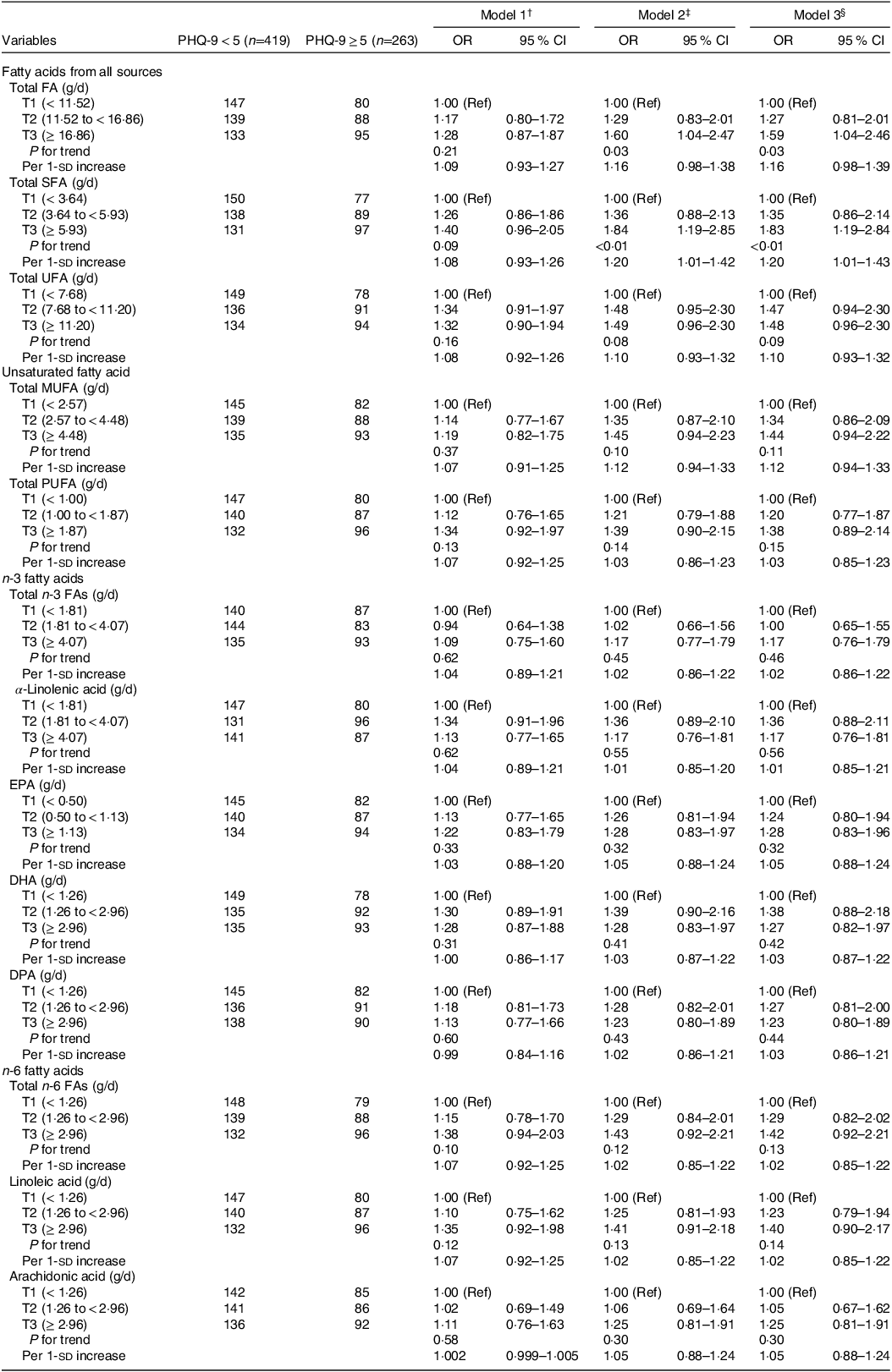
Abbreviations: CI, confidence interval; OR, odds ratio; SD, standard deviation; T, tertiles; Ref, reference; DPA: docosapentaenoic acids; DHA: docosahexaenoic acids; EPA: eicosapentaenoic acids; FA, fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; SFA: saturated fatty acids; UFA: unsaturated fatty acids; PHQ-9: Patient Health Questionnaire-9.
* Energy adjusted according to the residual method.
† Model 1: adjusted for gender, age and time on dialysis.
‡ Model 2: adjusted for gender, age, time on dialysis, BMI, physical activity, smoking status, drinking consumption, education level, marital status, anxiety state and co-morbidities.
§ Model 3: adjusted for gender, age, time on dialysis, BMI, physical activity, smoking status, drinking consumption, education level, marital status, anxiety state, co-morbidities and total energy intake.
Stratified analyses were carried out to evaluate the relationship between dietary FA intake and depressive symptoms in different subgroups. Although we observed a significant interaction between dialysis age and total PUFA and n-6 FA (P interaction < 0·05), the results showed that no variable significantly modified the aforementioned correlation (online Supplementary Tables 1–3). In sensitivity analyses, a similar association between dietary FA intake and depressive symptoms was also observed when restricting the patients who experienced no dietary behaviour change over the course of the last year (online Supplementary Table 4). This also verified that the associations between dietary FA intake and depressive symptoms in the main findings were reliable.
The results of dose–response relationship between dietary FA and the prevalence of depression after adjusting for aforementioned covariates are shown in Figs 2 and 3. Consuming dietary total FA, UFA, MUFA, PUFA, n-3 FA and n-6 FA were positively correlated with the prevalence of depression and in a positive linear pattern (P nonlinear > 0·05), but the amount of SFA was also positively associated with depression in an inverted U-shape (P nonlinear = 0·042).
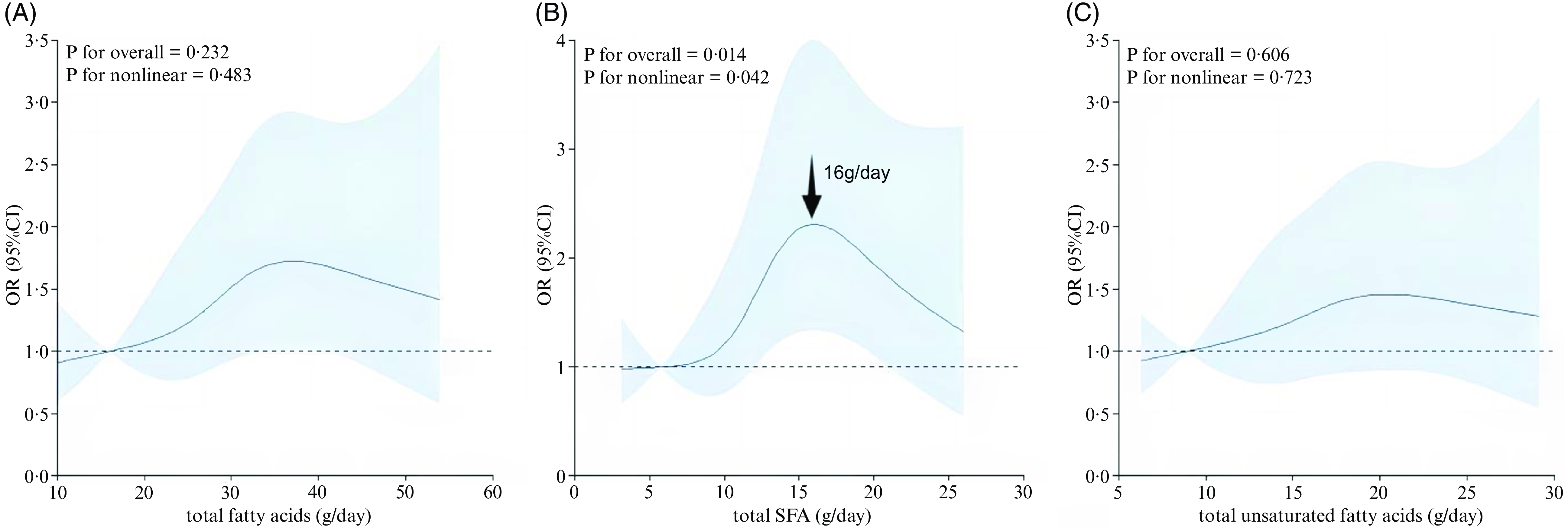
Fig. 2. The dose–response curve of the relationship between (a) total fatty acids, (b) total saturated fatty acids and (c) total unsaturated fatty acids intake and depressive symptoms. The dark blue line and shaded area represent the estimated OR and 95 % CI.
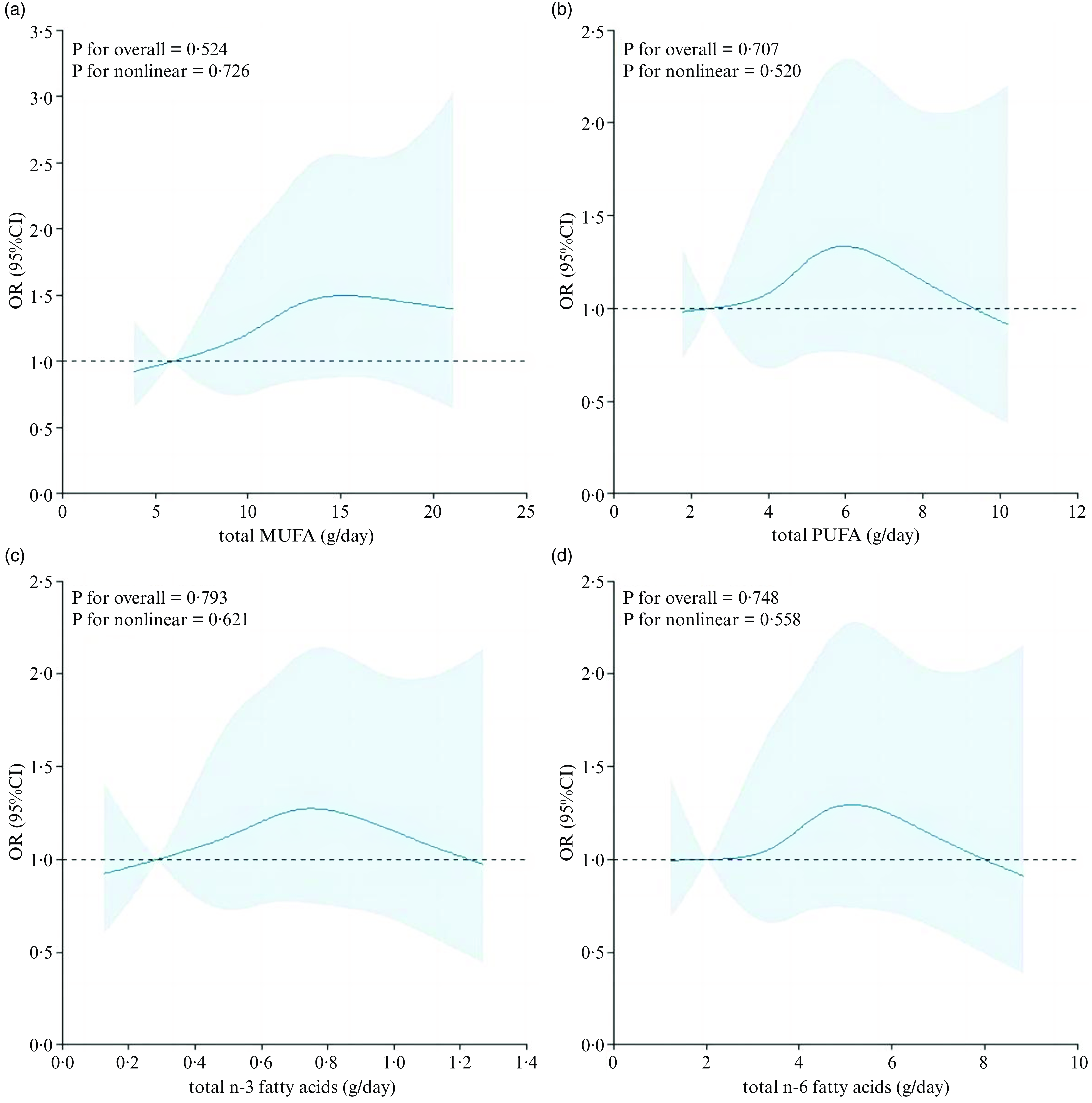
Fig. 3. The dose–response curve of the relationship between (a) total MUFA, (b) total PUFA, (c) total n-3 fatty acids and (d) total n-6 fatty acids intake and depressive symptoms. The dark blue line and shaded area represent the estimated OR and 95 % CI.
Discussion
In this cross-sectional study, we discussed the relationship between dietary FA intake and depressive symptoms in a cohort of MHD patients. Generally, after adjusting for confounding factors, the total FA and SFA intake are associated with higher depressive symptom prevalence. In addition, the same association was observed in the sensitivity analysis. RCS analysis showed an inverse U-shaped association between SFA intake and the prevalence of depression, and the highest prevalence of depression for HD patients was 16 g/d.
Several previous observational studies have investigated the association between dietary FA intake and depression in general population. A cohort study from China, including 2400 participants, demonstrated that baseline dietary SFA intake was positively associated with depressive symptoms in middle-aged women(Reference Li, Liang and Tong14). Additionally, a longitudinal study enrolling adults aged 55–85 years showed that SFA intake was positively associated with depression in both sexes(Reference Lai, Oldmeadow and Hure15). These findings were consistent with the present results. However, one randomised crossover study of 37 university students found no significant difference in depression scores after consuming SFA for four days(Reference Lai, Oldmeadow and Hure15), which may be related to the small sample size of the study. In a population-based prospective cohort study carried out in Japan and containing 1181 subjects, Matsuoka et al. found a reduced risk of major depressive disorder in the third quartile for fish intake, the second quartile for EPA and the third quartile for DPA in aged Japanese individuals(Reference Matsuoka, Sawada and Mimura35). Similarly, a recent meta-analysis consisting of 31 studies and including 255 076 individuals conducted by Grosso et al. has suggested that dietary fish or n-3 PUFA intake is associated with a lower risk of depression(Reference Grosso, Micek and Marventano36). In addition, a cross-sectional study including 453 men and 400 women conducted by Panagiotakos et al. in Attica has showed that dietary PUFA and MUFA intake were inversely associated with depression risk score(Reference Panagiotakos, Mamplekou and Pitsavos37). However, similar results were not observed in the present study patients, which might be attributed to study’s small sample size and the fact that a sufficiently long observation period was not utilised.
Subgroup analyses revealed that dietary total FA, SFA and UFA were positively associated with depression prevalence for patients over 60 years old, although no significant interaction was observed (P interaction > 0·05), which might be limited by small sample size. Several previous studies also indicated that the increased prevalence of depressed mood over the last decades has been associated with an excessive consumption of saturated fats in the elderly people(Reference Aihara, Minai and Aoyama38,Reference Payne, Hybels and Bales39) . This may be due to the weakened gastrointestinal function and decreased richness of the microbiota in elderly people, which can easily lead to brain-gut axis disorders(Reference Tsai, Chuang and Wang40). According to Carabotti et al. (Reference Carabotti, Scirocco and Maselli41), there is a bidirectional and reciprocal communication between the nervous system and gut microbiota. The age-associated decline of the immune system accompanied by chronic low-grade inflammation(Reference Franceschi, Capri and Monti42) seems to have a close relationship with both structure and composition of the gut microbiota(Reference Leung and Thuret43). Given that depression and age-related neurodegenerative illnesses are linked to inflammation(Reference Brites and Fernandes44), it follows that age-related depression affects the makeup of the gut microbiota. It is interesting to note that a relationship between gut microbiota and hippocampus neurogenesis has been found by Ogbonnaya and colleagues(Reference Ogbonnaya, Clarke and Shanahan45).
The nonlinear relationship between variables and outcomes was analysed using RCS, which is a widely used method. RCS is essentially a polynomial, but it is usually necessary to have continuous points and second-order derivatives to guarantee the curve’s smoothness. In the current study, the results of RCS analysis showed that there is a positive association between the SFA intake and depression prevalence. It is worth emphasising that when the intake of SFA is 16 g/d, HD patients have the highest prevalence of depression. Therefore, it is very necessary to control the SFA in a certain range, and our results may provide new ideas for health policymakers to prevent depression in HD patients.
Mechanically, several studies have shown that dietary SFA can influence the brain’s neurochemistry and function(Reference Marrone and Coccurello10,Reference Li, Liang and Tong14) . It is worth noting that SFA has been shown to damage many brain circuits related to emotion regulation, such as those involved in neuroinflammation and feeding behaviour(Reference Hryhorczuk, Florea and Rodaros16). For example, there is evidence that SFA can induce neuroinflammation by stimulating the release of proinflammatory cytokines and inducing apoptosis of astrocytes (supporting cells of the central nervous system)(Reference Gupta, Knight and Gupta46). Several studies into the chemical processes by which SFA affect emotional behaviour in rodents have shown that consuming these fats can decrease the action of the brain’s dopamine system(Reference Cone, Chartoff and Potter47,Reference Sharma and Fulton48) . Additionally, eating a diet that is relatively heavy in fat (21 per cent of energies from fat, with about 62 % of that coming from SFA) decreased the levels of brain-derived neurotrophic factor (BDNF) in rodents(Reference Sharma, Zhuang and Gomez-Pinilla49). Conversely, in a placebo-controlled study, plasma BDNF levels were increased by EPA plus DHA dietary supplementation in first-episode psychotic patients(Reference Pawelczyk, Grancow-Grabka and Trafalska50). This is significant because antidepressant therapy has increased BDNF levels and reduced depressive symptoms, whereas decreased BDNF levels in the human brain have been linked to depression(Reference Lee and Kim51).
There are several strengths in the present study. To the best of our knowledge, this is the first exploration of the association between dietary FA intake and depressive symptoms in a cohort of MHD patients, which provides certain insights for follow-up research. Second, a validated FFQ was used to assess dietary information via face-to-face interviews, which provided reliable estimates of dietary FA consumption. Third, a wide array of exposures of interest was considered, including total n-3 and total n-6 FA, while stratifying the analysis by age, gender, diabetes status, CVD status, BMI and dialysis age. Fourth, some known and suspected potential confounding factors have been adjusted for in the analyses. The present study also had some limitations. First, the study had a cross-sectional design, so it was impossible to demonstrate the causal relationship between dietary FA intake and depression prevalence. Thus, future prospective studies need to confirm the present findings. Second, FFQ was used to evaluate dietary FA intake and other dietary factors and covariates at a single time point, which unavoidably resulted in misclassification. Third, the effect of the cooking method on dietary FA was not considered. However, no studies have distinguished dietary FA consumption in relation to the food preparation method. Therefore, future research should concentrate on whether the association with dietary FA consumption may be affected by the cooking techniques. Fourth, because of the limitations in the original data, although several potential confounding variables were adjusted for in the multivariable model, the effect of unmeasured or residual confounding (residual kidney function, e.g.) could not be controlled. Fifth, due to differences in patient compliance and the degree of emphasis placed on dietary nutrition by attending physicians for HD patients, patients enrolled in this study were from a single dialysis centre, thus their representability was weak. As a result, the findings may not be generalisable to the overall HD population, and further verification is needed with additional studies in the future. Sixth, we did not assay blood samples for FA composition, which may not accurately determine the intake of dietary FA in these patients. The collection of dietary information only depended on the participants’ memories, which might lead to recall bias. In addition, memory may be affected by depression, which might result in a potentially differential assessment error. Finally, due to the limited number of patients, the sample size was relatively small, which could have led to some analyses being underpowered.
Conclusion
Higher intake of total FA and SFA was associated with increased prevalence of depressive symptoms in Chinese MHD patients. However, statistically significant associations between other types of FA and depressive symptoms were not observed. Future randomised controlled trials assessing the impact of dietary FA on health outcomes should be conducted to examine their long-term effects on depression.
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving patients were approved by the Ethics Committee of Dalian Municipal Central Hospital (protocol code YN2022-096-04). Written informed consent was obtained from all patients.
This work was supported by the Dalian Key Medical Specialty Dengfeng Project (No.2022ZZ231 and No.2022ZZ243) to Shu-Xin Liu; Applied Basic Research Project of Liaoning Province, China (No.2023JH2/101300091) to Shu-Xin Liu; the Dalian Key Medical Specialty Dengfeng Project (No.2022ZZ236) to Shuang Zhang; Dalian Medical Science Research Program (No.2211008) to Shuang Zhang; Dalian Science and Technology Talent Innovation Support Policy Implementation Plan Project (2023RQ037) to Shuang Zhang.
Conceptualisation: S-X. L. and Q-J. W.; methodology and formal analysis: S. Z.; performed the study and collected data: Z-H. W., H. L., P. X., Y. L., C. D., Q-M. M.; writing original draft preparation: S. Z.; funding acquisition: S. Z. and S-X. L.; writing – review & editing: S. Z. and S-X. L., and all authors had read and approved the final manuscript.
There are no conflicts of interest.
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524001570









