Due to the scarcity of marine resources, fishmeal (FM) and fish oil (FO) are increasingly being replaced by plant ingredients in feeds for farmed Atlantic salmon(Reference Tacon, Hasan and Subasinghe1, Reference Turchini, Torstensen and Ng2). Atlantic salmon fed high levels of dietary plant ingredients have been reported to have elevated plasma and liver TAG concentrations and increased plasma VLDL compared with fish fed diets based on marine ingredients(Reference Torstensen, Espe and Stubhaug3, Reference Jordal, Lie and Torstensen4). Dietary vegetable oil (VO) together with FM has been reported to affect the expression of genes involved in cholesterol synthesis as well as to reduce plasma LDL(Reference Jordal, Lie and Torstensen4, Reference Leaver, Villeneuve and Obach5).
By replacing FO with VO, dietary cholesterol is reduced and typical plant lipids such as plant sterols, i.e. phytosterols, are introduced into the diet. Cholesterol has an essential role in the cell membrane structure and is indispensable in the processes of growth and as a precursor of steroid hormones and bile acids(Reference Espenshade and Hughes6). Phytosterols are abundant in seeds, legumes and cereals and have backbone structure and function similar to those of cholesterol in animals(Reference Moreau, Whitaker and Hicks7), and their ability to lower plasma cholesterol and LDL-cholesterol in humans has been well documented(Reference Brufau, Canela and Rafecas8). The cholesterol-lowering ability of phytosterols is thought to be due to their effects on proteins involved in the uptake and efflux of sterols in the gut, i.e. Niemann–Pick disease, type C1, gene-like 1 (NPC1L1) and ATP-binding cassette subfamily G members 5 and 8 (ABCG5/8), and possibly also competing with cholesterol for space in the micelles(Reference Amemiya-Kudo, Shimano and Hasty9). The effect of dietary phytosterols on fish cholesterol metabolism, and possibly also on health, is still unclear. However, when provided via water, phytosterols have shown to lower plasma cholesterol and LDL-cholesterol and plasma TAG in brook trout (Salvelinus fontinalis)(Reference Gilman, Leusch and Carl Breckenridge10).
Cellular cholesterol levels are regulated by the nuclear receptors sterol regulatory element-binding protein (srebp) and liver X-receptor (lxr), where srebp increases the uptake and production of cholesterol(Reference Edwards, Tabor and Kast11), while lxr increases the efflux of cholesterol in case of cholesterol accumulation in the cell(Reference Zhao and Dahlman-Wright12). It has been shown in mice that some phytosterols can disrupt cholesterol homeostasis by affecting components in these two pathways(Reference Yang, Yu and Li13). These systems are also connected with lipid metabolism; one isotype of srebp specifically activates fatty acid (FA) synthesis(Reference Horton, Goldstein and Brown14). It is therefore possible that suboptimal dietary sterol supply could affect both lipid and cholesterol levels in the body.
The objective of the present study was thus to elucidate the effects of low dietary cholesterol combined with high dietary phytosterols on cholesterol metabolism in Atlantic salmon. In this respect, two feeding studies with differing plant ingredient inclusions were conducted to isolate the impacts of dietary plant protein (PP), dietary cholesterol and dietary phytosterols on phytosterol tissue accumulation and cholesterol metabolism in Atlantic salmon.
Materials and methods
Experimental design
In the present study, two feeding trials with Atlantic salmon were performed. In the first trial, diets with different levels of both PP and VO were used, allowing for the detection of effects due to the inclusion levels of plant ingredients in the diets. In the second trial, diets in which the protein composition was kept stable and only oil sources varied were used, so that the effects due to different types of oils could be observed. The experimental procedure in both trials was conducted according to the guidelines of the Norwegian State Commission for Laboratory Animals. In the two trials, fish were fed in triplicate (three randomised tanks for each diet) and in excess twice per d with automatic feeders for 0·5 h, followed by feed collection 0·5 h after each feeding. All oils utilised in the diets were unrefined. In trial 1, feeds with different amounts of the marine raw material replaced by PP and a VO blend were fed to Atlantic salmon during 52 weeks in seawater, from the post-smolt stage to the adult fish stage: (1) a control diet with FM and FO (FM-FO) as the sole protein and lipid sources; (2) a diet with an estimated safe maximum replacement of both FM and FO with PP (80 %) and VO (70 %), high PP–high VO (HPP-HVO); (3) a diet with maximum replacement with PP and half the maximum replacement of VO, high PP–medium VO (HPP-MVO); (4) a diet with half the maximum replacement with PP and maximum replacement with VO, medium PP–high VO (MPP-HVO). The VO blend was made from rapeseed, palm and linseed oils (55:30:15, by vol.) to obtain a mixture mimicking capelin (Mallotus villosus) oil in the sum of SFA, MUFA and n-3 PUFA. More detailed information on the trials, as well as growth, nutrient retention, digestibility and lipid accumulation, has been published in the work of Torstensen et al. (Reference Torstensen, Espe and Stubhaug3, Reference Torstensen, Espe and Sanden15). In trial 2, Atlantic salmon were fed diets with a high and constant inclusion of PP (70 % of protein source) and with the lipid portion consisting of either 100 % FO (PP-FO) or one of the three different VO replacing 80 % of the FO during 28 weeks. The VO used were olive oil (PP-OO), rapeseed oil (PP-RO) and soyabean oil (PP-SO). The VO diets were formulated to contain similarly low cholesterol concentrations; RO was chosen for its high phytosterol concentration and having FA composition that is similar to that of the OO; and the OO and SO were chosen for their intermediate and similar concentrations of phytosterols. For further details on the trial set-up and results on growth, digestibility and nutrient retention, refer to the work of Liland et al. (16). The composition and proximate composition of the diets is provided in Tables S1 and S2 (available online).
Sampling
All the experimental feeds were sampled and stored at − 20°C until analysis. Salmon were anaesthetised in a bath with tricaine methanesulfonate (MS-222, 7 g/l; Finquel), killed by a blow to the head, and individually weighed and measured. Blood from five fish per tank was collected in EDTA vacutainers and centrifuged to separate plasma and blood cells. Plasma was pooled to obtain one sample per tank, and then stored on ice until separated into lipoprotein fractions (n 3). Livers from three fish per tank were pooled and homogenised for the analysis of sterols in both trials and for that of total bile acids in trial 2. In addition, white muscle from three fish per tank was pooled and homogenised for sterol analysis in both trials, and adipose tissue from three fish per tank was pooled and homogenised for sterol analysis in trial 1. Also, three other pieces of livers per tank from both trials were pooled, but not homogenised due to the lipolytic reactions occurring when breaking the tissue, and frozen on dry ice for the lipid class analysis. Livers from both trials and mid-intestine from trial 2 were sampled from three fish per tank (n 9 per diet) and flash-frozen separately in liquid N2 for gene expression analyses. All samples were stored at − 80°C. In trial 1, samples were collected at the end of the trial at week 52; plasma was sampled 48 h postprandially, while the other samples were collected 6 h postprandially. In trial 2, the fish were sampled before the start of the trial and after 11 and 28 weeks of feeding. Samplings were done 24 h postprandially at weeks 0 and 11 and 48 h postprandially during the sampling at week 28. This change in postprandial time during the final sampling in trial 2 was due to the feed observed in the digestion tract of the fish during the sampling at week 11.
Plasma and lipoproteins
The separation of lipoproteins was performed by centrifugal flotation(Reference Havel, Eder and Bragdon17), as described by Jordal et al. (Reference Jordal, Lie and Torstensen4), by adding potassium bromide to obtain the density intervals of the different lipoproteins. Plasma and lipoprotein fractions were analysed using a clinical bioanalyser (Maxmat PL analyser; Erba Diagnostics) according to standardised procedures, reagents and controls to determine the fraction cholesterol and TAG concentrations.
Lipid class composition
Lipids from the liver and feed samples were extracted by adding chloroform–methanol (2:1, v/v), 20 × the amount of the sample (v/w). After the extraction of lipids, the samples were filtered and the quantification of lipid class composition in the diets and liver samples was carried out by high performance TLC as described by Torstensen et al. (Reference Torstensen, Espe and Stubhaug3).
Amino acid composition
The diets were hydrolysed in 6 m-HCl at 110°C for 22 h and amino acids were separated on HPLC as described by Espe et al. (Reference Espe, Ruohonen and El-Mowafi18).
Bile acids in liver and plasma
Homogenised liver samples were freeze-dried and bile acids were extracted as described by Suckling et al. (Reference Suckling, Benson and Bond19) and analysed for bile acids on a clinical bioanalyser (Maxmat PL analyser; Erba Diagnostics). Plasma samples were thawed on ice and analysed as done for the prepared liver samples.
Analysis of sterol content
The analysis of phytosterols and cholesterol in liver, white muscle, visceral adipose tissue and feeds was performed with GC as described by Laakso(Reference Laakso20). To each test tube was added 0·3 mg internal standard, 5-β-cholestan-3-α-ol, before weighing in the sample to contain 0·5–1 g sterols per test tube. The control material used was phytosterol-enriched margarine of the brand Vita pro-aktiv (Mills DA). After sample extraction and derivatisation by silylation as described previously(Reference Laakso20), the samples were diluted 20 × in hexane before analysis on GC. The following instrumentation was used: Thermoquest trace GC 2000 with an auto-sampler AS2000 (Thermo Scientific), an on-column injector, a flame ionisation detector and the column Equity-5® of length 30 m and 0·25 mm inner diameter (Supelco). He gas was used as a carrier at 0·9 ml/min, and hydrogen and air were used as the detector gases at 35 and 350 ml/min, respectively. The initial temperature was 100°C, which was increased by 50°C/min to 300°C and maintained for 12 min. The peaks were identified with the software Chromeleon® version 6.8 (Dionex).
Quantitative PCR
mRNA from the flash-frozen liver and mid-intestine samples was extracted using the standard TRIzol extraction method (Invitrogen Limited), and RNA quality was verified and complementary DNA plates were prepared as described by Torstensen et al. (Reference Torstensen, Espe and Stubhaug3). The complementary DNA plates were stored at − 20°C until quantitative PCR was performed. Primers were designed using the program Primer 3(21), and primer sequences are given in Table S3 (available online).
Statistics and data treatment
For the statistical evaluation, the free software environment R(22) was used. Data were analysed for homogeneity in variance using Levene's test and for normality using Shapiro Wilk's test as well as being evaluated graphically by quantile-quantile (QQ) plots. Linear mixed-effects models(23) and one-way ANOVA were used for the growth results and genetic expression (random-effect factor: tank) and liver TAG (random-effect factor: trial). For significantly different data according to ANOVA, Tukey's post hoc test was performed. For data on liver cholesterol, T= 0 was common for all groups, and differences between means for each dietary group at T= 0 and the other samplings were evaluated using a two-sided t test, not assuming homoscedasticity. A two-sided t test was also used for finding differences between groups in plasma cholesterol content and for detecting differences in liver phytosterols between the dietary groups of different trials. A two-way ANOVA was used to find differences in the expression of lxr and cytochrome P450, family 7, subfamily A, polypeptide 1 (cyp7a1) for the dietary groups between the sampling points. Data are given as means with their standard errors, and a significance level of 95 % was used (P< 0·05). Correlation was calculated using Pearson's product–moment correlation, and P value and R 2 are stated where they have been used. The stability of the expression of each reference gene was calculated from the C t values using the program geNorm(Reference Vandesompele, De Preter and Pattyn24) according to the geNorm manual as described by Olsvik et al. (Reference Olsvik, Lie and Jordal25). Liver phytosterol retention in trial 2 was calculated as follows:
Results
Diets
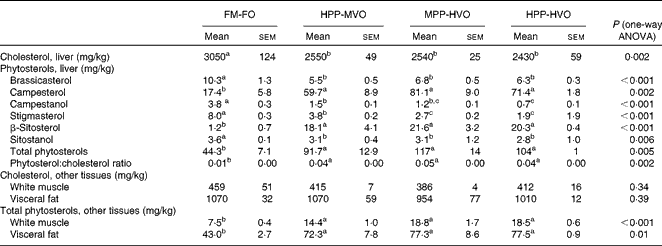
The FA composition of the diets from trials 1 and 2 was according to the planned design (Table S4, available online) and the proximate composition was similar for all diets (Tables S1 and S2, available online). All diets had a similar amino acid composition (results not shown), but taurine concentrations decreased when PP was used to replace the FM (Tables S1 and S2, available online). The diets with the highest concentrations of phytosterols were HPP-HVO, MPP-HVO and PP-RO; the intermediate-phytosterol diets were HPP-MVO, PP-OO and PP-SO; and the FM-FO and PP-FO diets contained only minor amounts of phytosterols (Table 1). The FM-FO and PP-FO diets had the highest cholesterol content (Table 1). Due to a lower cholesterol content in the FO used in trial 2, the phytosterol:cholesterol ratio was highest in the PP-RO feed.
Table 1 Sterol concentration of the experimental diets from trials 1 and 2*

FM-FO, 100 % fishmeal and 100 % fish oil; HPP-MVO, 80 % plant protein (PP) and 35 % vegetable oil (VO); MPP-HVO, 40 % PP and 70 % VO; HPP-HVO, 80 % PP and 70 % VO; PP-FO, 70 % PP and 100 % FO; PP-OO, 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO, 70 % PP and 80 % rapeseed oil; PP-SO, 70 % PP, 70 % soyabean oil and 10 % palm oil.
* Detection limit: 0·1 mg/kg.
Growth
The fish in trial 1 grew from 355 (sem 17) g (n 30) to 3690 (sem 36) g (n 543). The final weight was significantly lower in the fish fed the high-PP diets than in the other dietary groups from this trial. The fish in trial 2 grew from 814 (sem 5) g (n 600) to 3400 (sem 29) g (n 329). The fish fed the PP-SO diet had a significantly lower final weight than the PP-FO diet-fed fish. The difference in the number of fish sampled from the first to the last weighing in trial 1 is due to thirty fish being randomly sampled and weighed at the beginning of the trial, while during the last sampling all the fish left in all the tanks were weighed. More detailed information on growth has been published elsewhere(Reference Torstensen, Espe and Sanden15, 16).
Tissue sterol content
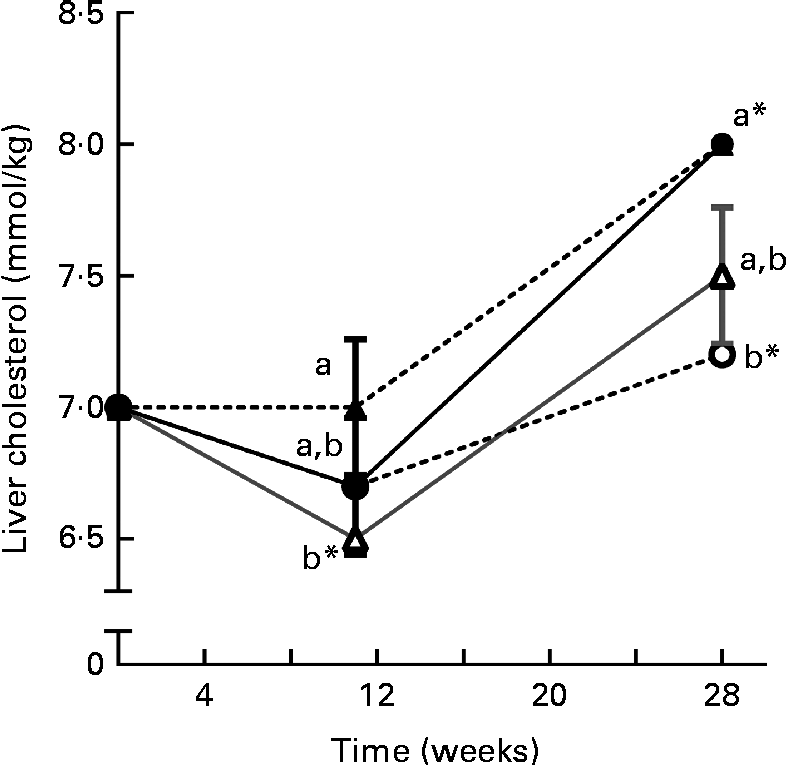
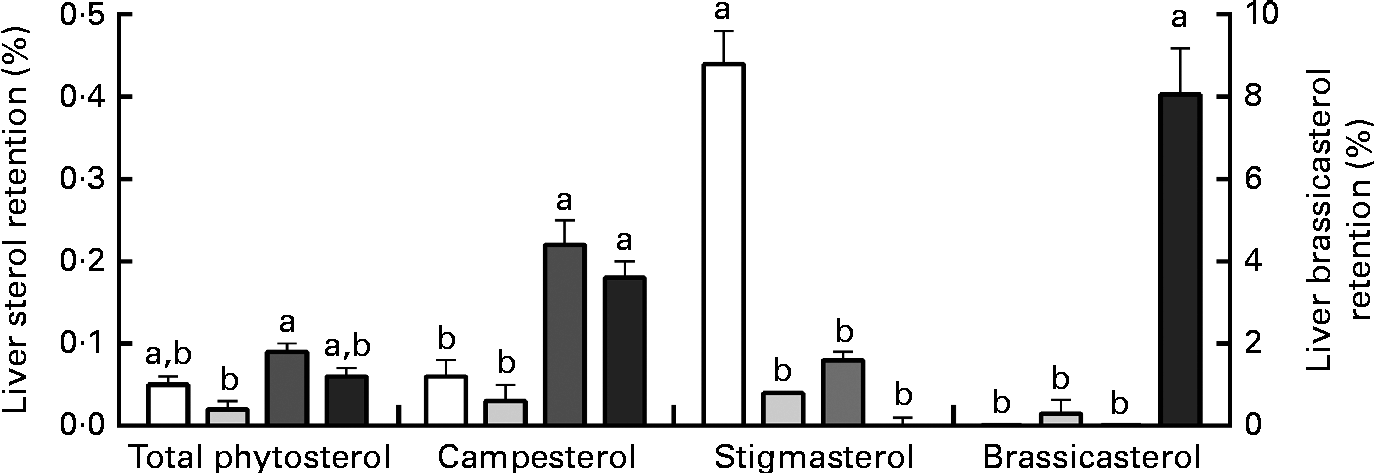
Liver phytosterol concentrations were significantly higher in the fish given the high-phytosterol diets, i.e. PP-RO, MPP-HVO and HPP-HVO, than in the fish given the PP-FO and FM-FO diets (Tables 2 and 3). In addition, the fish fed the PP-RO diet had a higher liver phytosterol content than the fish fed the MPP-HVO and HPP-HVO diets despite containing similarly high dietary phytosterol concentrations (two-sided t test, P< 0·05). The liver was the organ richest in phytosterols; visceral adipose tissue had a medium phytosterol concentration at 66–97 % of the liver concentration; and white muscle had a low concentration of phytosterols at approximately 17 and 24–34 % relative to liver phytosterols in trials 1 and 2, respectively (Tables 2 and 3). The phytosterol composition was similar in all tissues analysed, with campesterol being the most abundant, amounting to 40–78 % of total phytosterols. Prominent differences between the compositions of the feeds and tissues of the fish that consumed them included the amount of campesterol relative to total phytosterols, which was 200–300 % higher in the tissues than in the diets, and stigmasterol that accumulated only in the tissues of the PP-FO diet- and FM-FO diet-fed fish. β-Sitosterol accumulated little in tissues, being found in lower amounts relative to total phytosterols in the tissues than in the diets. Liver cholesterol concentration increased from baseline to week 28 for all dietary groups in trial 2 (P< 0·02), except for the fish fed the PP-RO diet, where liver cholesterol decreased from baseline to week 11 (Fig. 1). Liver total phytosterol retention was significantly higher in the PP-RO diet-fed fish than in the fish given the PP-OO diet (Fig. 2). Liver campesterol retention was significantly higher in the PP-RO diet- and PP-SO diet-fed fish than in the fish fed the PP-FO and PP-OO diets, whereas the PP-FO diet-fed fish showed significantly a higher liver retention of stigmasterol than the fish given the VO diets in trial 2 (Fig. 2). The liver retention of brassicasterol was significantly higher in the PP-SO diet-fed fish than in the fish given the other diets in trial 2 (Fig. 2).
Table 2 Sterol composition in liver and total phytosterol and cholesterol concentrations of liver, muscle tissue and visceral fat from Atlantic salmon fed diets with different levels of plant ingredients in trial 1, sampled at week 52* (Mean values with their standard errors of three tanks per diet with three pooled fish from each tank, n 3)

FM-FO, 100 % fishmeal and 100 % fish oil; HPP-MVO, 80 % plant protein (PP) and 35 % vegetable oil (VO); MPP-HVO, 40 % PP and 70 % VO; HPP-HVO, 80 % PP and 70 % VO.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Detection limit: 0·1 mg/kg.
Table 3 Phytosterol composition in liver and total phytosterol and cholesterol concentrations of muscle tissue from Atlantic salmon fed diets with high plant protein (PP) and different lipid sources in trial 2, sampled at week 28* (Mean values and standard deviations of three tanks per diet with three pooled fish from each tank, n 3)

PP-FO, 70 % PP and 100 % fish oil; PP-OO, 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO, 70 % PP and 80 % rapeseed oil; PP-SO, 70 % PP, 70 % soyabean oil and 10 % palm oil.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Detection limit: 0·1 mg/kg.

Fig. 1 Liver cholesterol concentration over time for Atlantic salmon fed diets with high plant protein (PP) and different lipid sources in trial 2, sampled at T= 0 and at weeks 11 and 28. T= 0 was before the fish had been separated into separate tanks for the feeding trial and value is therefore common for all groups. Values are means (three tanks per diet with three pooled fish from each tank, n 3), with their standard errors represented by vertical bars. PP-FO (![]() ), 70 % PP and 100 % FO; PP-OO (
), 70 % PP and 100 % FO; PP-OO (![]() ), 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO (
), 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO (![]() ), 70 % PP and 80 % rapeseed oil; PP-SO (
), 70 % PP and 80 % rapeseed oil; PP-SO (![]() ), 70 % PP, 70 % soyabean oil and 10 % palm oil. a,bMean values between the different dietary groups at each sampling were significantly different (P= 0·003). * Mean value was significantly different from that at T= 0 (P< 0·04).
), 70 % PP, 70 % soyabean oil and 10 % palm oil. a,bMean values between the different dietary groups at each sampling were significantly different (P= 0·003). * Mean value was significantly different from that at T= 0 (P< 0·04).

Fig. 2 Liver phytosterol retention in Atlantic salmon fed diets with high plant protein (PP) and different lipid sources in trial 2, sampled at week 28. Values are means (three tanks per diet with three pooled fish from each tank, n 3), with their standard errors represented by vertical bars. PP-FO (□), 70 % PP and 100 % fish oil; PP-OO (![]() ), 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO (
), 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO (![]() ), 70 % PP and 80 % rapeseed oil; PP-SO (■), 70 % PP, 70 % soyabean oil and 10 % palm oil. a,bMean values with unlike letters were significantly different (P< 0·05).
), 70 % PP and 80 % rapeseed oil; PP-SO (■), 70 % PP, 70 % soyabean oil and 10 % palm oil. a,bMean values with unlike letters were significantly different (P< 0·05).
Plasma lipoprotein cholesterol and TAG
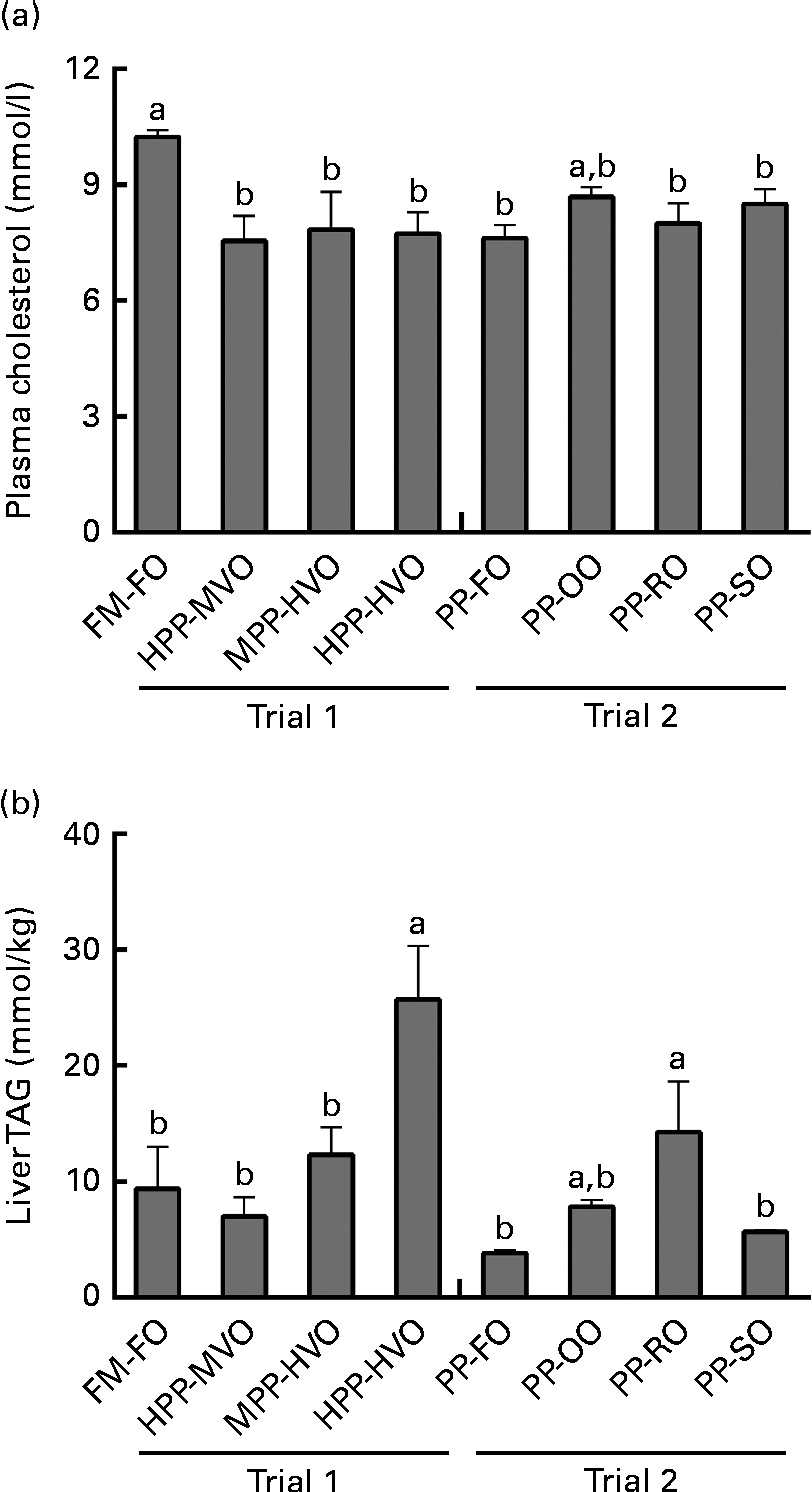
Plasma cholesterol was significantly lower in the fish fed diets with ≥ 40 % PP during 52 and 28 weeks in trials 1 and 2, respectively, than in the fish fed the FM-FO diet (Fig. 3). No correlation between dietary cholesterol and plasma cholesterol was observed (R 2 0·14, P= 0·08). VLDL-cholesterol did not differ between the groups in either of the trials. There was, however, a tendency of lower LDL-cholesterol in the groups given the high-VO diets in trial 1 than in the groups given 35 % VO or no VO diet, as well as a significantly higher HDL-cholesterol in the PP-OO diet- and PP-RO diet-fed fish than in the PP-FO diet-fed fish in trial 2 (Tables S5 and S6, available online). Plasma TAG was significantly higher in the fish fed the PP-RO diet than in the PP-FO diet- and PP-SO diet-fed fish at week 28 in trial 2 (Table 4).

Fig. 3 (a) Plasma cholesterol and (b) liver TAG of Atlantic salmon fed diets with different levels of fishmeal (FM) and fish oil (FO) replacement or high-plant protein (PP) diets with different lipid sources, sampled at weeks 52 and 28 in trials 1 and 2, respectively, collected 48 h postprandially. Liver TAG from trial 1 adapted with permission from Torstensen et al. (Reference Torstensen, Espe and Stubhaug3). Values are means (three tanks per diet with three pooled fish from each tank, n 3), with their standard errors represented by vertical bars. FM-FO, 100 % FM and 100 % FO; HPP-MVO, 80 % PP and 35 % vegetable oil (VO); MPP-HVO, 40 % PP and 70 % VO; HPP-HVO, 80 % PP and 70 % VO; PP-FO, 70 % PP and 100 % FO; PP-OO, 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO, 70 % PP and 80 % rapeseed oil; PP-SO, 70 % PP, 70 % soyabean oil and 10 % palm oil. a,bMean values with unlike letters were significantly different for plasma cholesterol (P< 0·05, two-sided t test) and liver TAG (P= 0·004, nested ANOVA).
Table 4 Plasma TAG and liver bile acids from Atlantic salmon fed diets with high plant protein (PP) and different lipid sources in trial 2, sampled at week 28 (Mean values and standard deviations of three tanks per diet with three pooled fish from each tank, n 3)

PP-FO, 70 % PP and 100 % fish oil; PP-OO, 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO, 70 % PP and 80 % rapeseed oil; PP-SO, 70 % PP, 70 % soyabean oil and 10 % palm oil.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Liver TAG
Liver TAG content in trial 1 has been published by Torstensen et al. (Reference Torstensen, Espe and Stubhaug3), but it is included here to allow for comparison with that of trial 2 to be able to separate the effects due to dietary PP from the effects of phytosterols. The HPP-HVO diet-fed fish had significantly higher liver TAG than all other dietary groups from both trials except those fed the PP-OO and PP-RO diets. In trial 2, the fish fed the PP-RO diet had higher liver TAG than the fish fed the PP-FO and PP-SO diets (Fig. 3).
Quantitative PCR
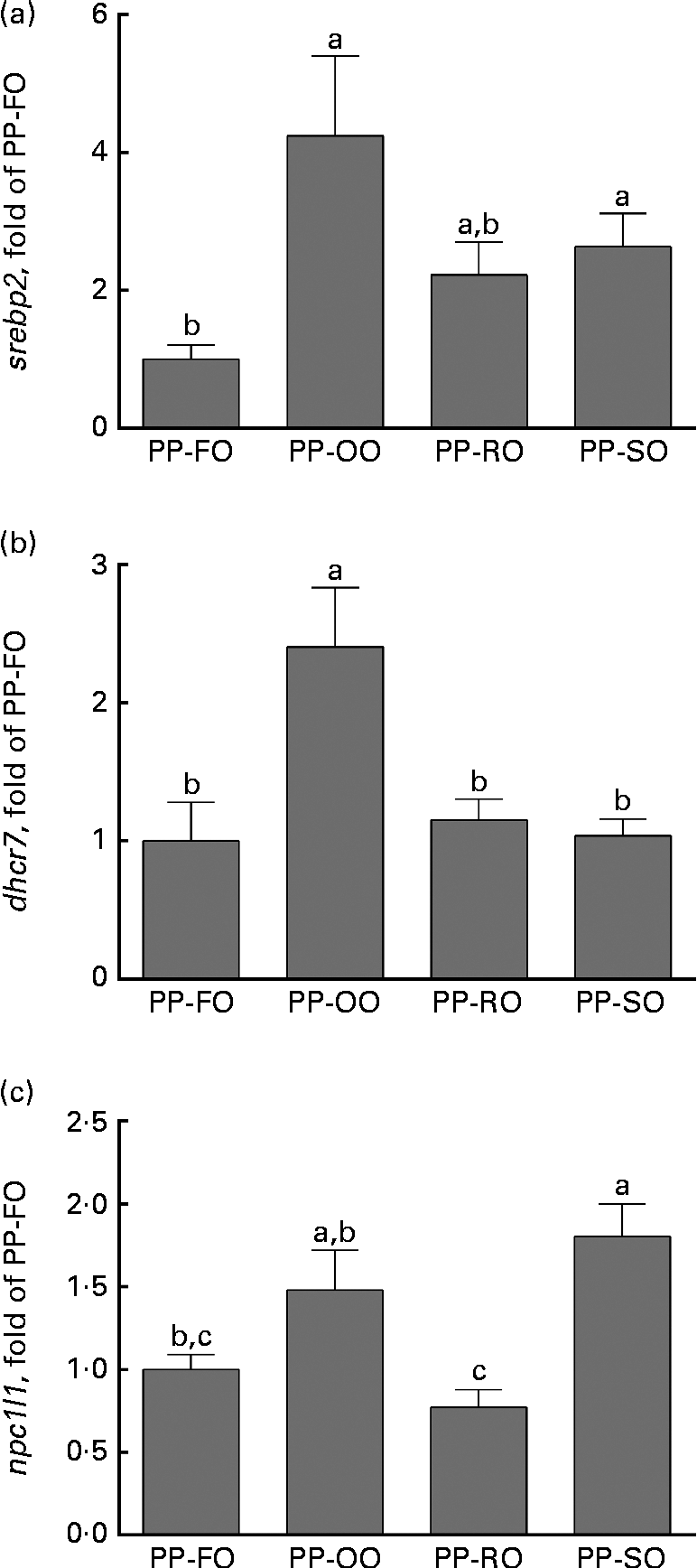
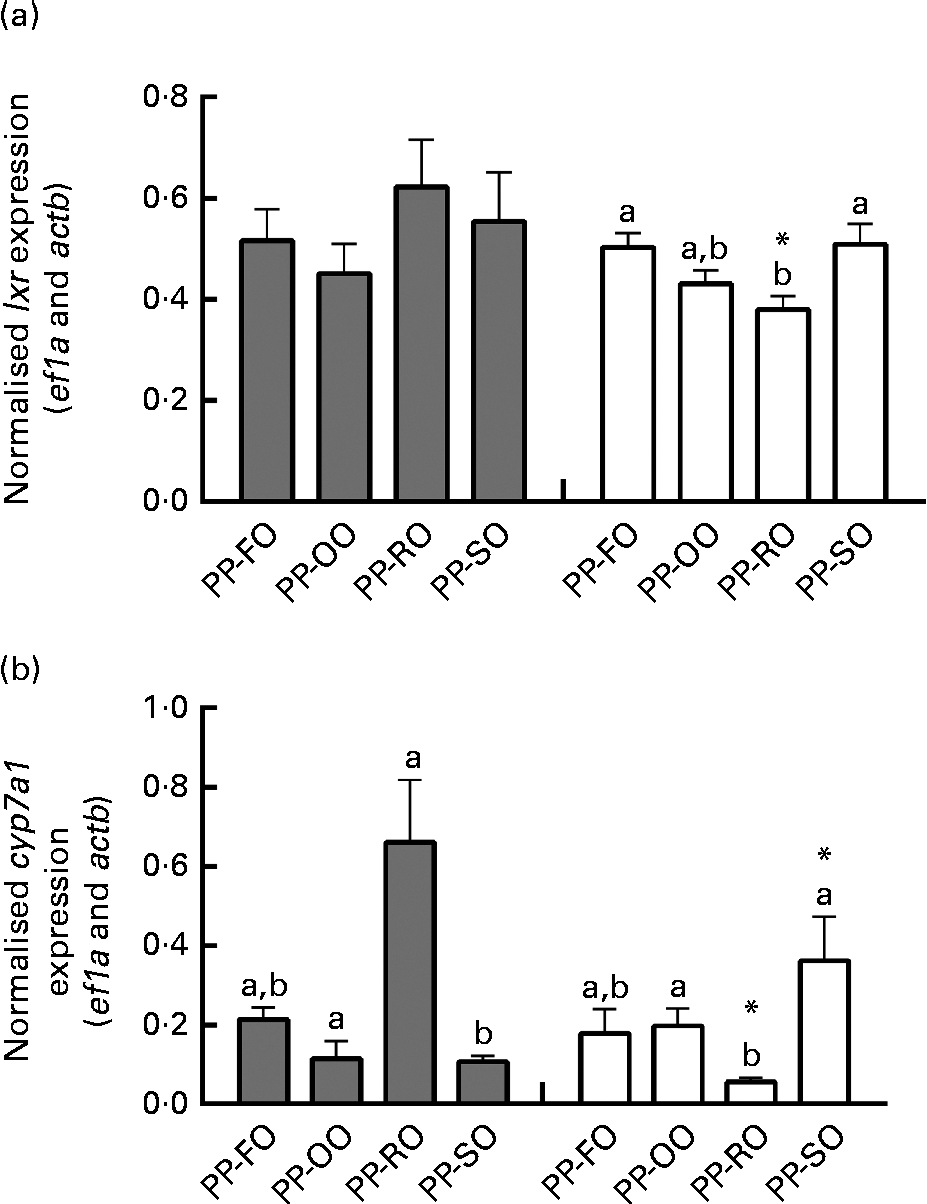
At week 28 in trial 2, two genes involved in cholesterol biosynthesis, srebp2 and dhcr7 (7-dehydrocholesterol reductase), were significantly highly expressed in the liver of the fish fed the PP-OO diet than in that of the fish fed the PP-FO diet (Fig. 4). The expression of fas (FA synthase), srebp1, apob100 and abcb11 (ATP-binding cassette, subfamily B, member 11) in the liver of the fish from trial 2 was unaffected by the treatments (data not shown). Liver expression of lxr (liver X receptor-α) was similar for all the dietary groups at week 11, while liver cyp7a1 expression was significantly greater in the fish fed the PP-RO diet than in the fish fed the PP-OO and PP-SO diets during the same sampling (Fig. 5). At week 28, however, both lxr and cyp7a1 were expressed in lower amounts in the fish fed the PP-RO diet than in the other groups (Fig. 5). Liver expression of srebp2 and dhcr7 in the fish from trial 1 did not show differences between the groups (data not shown). At week 28, the expression of sterol absorber npc1l1 in the mid-intestine was greater in the fish fed the PP-SO diet than in the fish fed the PP-FO and PP-RO diets (Fig. 4).

Fig. 4 Normalised expression of genes involved in lipid uptake and metabolism in the liver ((a) sterol regulatory element-binding protein-2 (srebp2) and (b) 7-dehydrocholesterol reductase (dhcr7)) and intestine ((c) Niemann–Pick disease, type C1, gene-like 1 (npc1l1)) of Atlantic salmon fed diets with high plant protein (PP) and different lipid sources at week 28 in trial 2. Values are mean fold change compared with the 70 % PP and 100 % fish oil (PP-FO) diet-fed fish (three tanks per diet with three non-pooled fish from each tank, n 9), with their standard errors represented by vertical bars. PP-OO, 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO, 70 % PP and 80 % rapeseed oil; PP-SO, 70 % PP, 70 % soyabean oil and 10 % palm oil. a,b,cMean values with unlike letters were significantly different (P< 0·05).

Fig. 5 Normalised expression of (a) liver X-receptor (lxr) and (b) cytochrome P450, family 7, subfamily A, polypeptide 1 (cyp7a1) mRNA in the liver of Atlantic salmon fed diets with high plant protein (PP) and different lipid sources at weeks 11 and 28 in trial 2. Values are mean normalised expression (three tanks per diet with three non-pooled fish from each tank, n 9), with their standard errors represented by vertical bars. PP-FO, 70 % PP and 100 % fish oil; PP-OO, 70 % PP, 68 % olive oil, 6 % soyabean oil and 6 % linseed oil; PP-RO, 70 % PP and 80 % rapeseed oil; PP-SO, 70 % PP, 70 % soyabean oil and 10 % palm oil; actb, actin, beta; ef1a, elongation factor, alpha. a,bMean values with unlike letters were significantly different between groups at each sampling (P< 0·05). * Mean values were significantly different for a dietary group from week 11 (![]() ) to week 28 (□) (P< 0·05).
) to week 28 (□) (P< 0·05).
Bile acids in liver and plasma
At week 11 in trial 2, there were no differences between the groups in liver bile acid content, which was 262 (sem 15) mmol/kg (n 11). At week 28, the group fed the PP-RO diet had a higher liver bile acid concentration than the other groups in trial 2 (Table 4). Corresponding plasma bile acids from trial 2, with a mean value of 3·6 (sem 0·7) μmol/l (n 10), did not show differences between the dietary groups.
Discussion
Accumulation of phytosterols in tissues
Liver phytosterol content of the PP-RO diet-fed fish was 50 % greater than that of both the HPP-HVO diet- and MPP-HVO diet-fed fish, even though dietary phytosterols of all the three diets were similar and the experimental period was longer for the two latter dietary groups. The ratio of dietary phytosterols:cholesterol has been shown to be important for liver lipid metabolism in rats when the ratio of phytosterols:cholesterol exceeds 1(Reference Laraki, Pelletier and Mourot26), which was the case for all the high-VO diets except for the PP-SO diet. This further implies a threshold in the phytosterol:cholesterol ratio, increasing the tissue level of phytosterols when the ratio is as high as that in the PP-RO diet (i.e. 1·7). The retention of phytosterols in the liver differed between the different types of phytosterols as well as between the dietary groups. The lower relative accumulation of β-sitosterol when compared with campesterol was also confirmed, reported as less digestible than campesterol in Atlantic salmon parr(Reference Miller, Nichols and Carter27). The type of phytosterol could possibly also have an effect, as the high levels of campesterol in the PP-RO diet; however, it is difficult to conclude on the effects of separate phytosterols in the present experimental design. Data from Miller et al. (Reference Miller, Nichols and Carter27) and trial 1 indicate a relationship between liver phytosterols and liver lipid accumulation, while this was not observed in trial 2, thus rejecting the hypothesis that phytosterols accumulate passively and are determined by the liver lipid concentration alone. It is confirmed that Atlantic salmon of a commercial harvest size does not accumulate enough phytosterols in the muscle tissue to be a phytosterol-rich seafood(Reference Miller, Nichols and Carter27), as 100 g of Atlantic salmon fillet from fish fed the high-phytosterol diet PP-RO only contained about 6 mg phytosterols, which is approximately 2 % of a normal daily phytosterol intake for humans(Reference Gylling, Miettinen, Lasztity, Pfannhauser, Simon-Sarkadi and Tömöközi28).
Plasma cholesterol
No correlation between dietary cholesterol and plasma cholesterol was observed. However, dietary PP decreased plasma cholesterol concentrations even at intermediate inclusion levels (40 % of protein source), consistent with the findings in mammalian trials(Reference Wergedahl, Liaset and Gudbrandsen29). Reduced dietary taurine concentrations following the replacement of FM with PP have been shown to reduce cholesterol levels in rats by an increased expression of cyp7a1 and thus to increase the excretion of sterols as bile acids(Reference Yokogoshi, Mochizuki and Nanami30). Dietary taurine levels did not correlate with the expression of cyp7a1 mRNA in our data, but there could be a difference in the protein abundance of CYP7A1 not reflected in the mRNA expression of the protein. Also, other non-protein components such as taurine and polyamines or their precursor amino acids may be involved in the cholesterol-lowering effect reported for PP(Reference Espe, Rathore and Du31, Reference Pirinen, Gylling and Itkonen32). These possible cholesterol-lowering effects and their mechanisms of action need to be studied in more detail in new trials. Also, the low-phytosterol diet PP-FO lowered plasma cholesterol compared with the FM-FO diet; increasing dietary phytosterols thus did not contribute significantly towards lowering plasma cholesterol.
Liver and plasma TAG
Earlier reported effects of replacing dietary FO with VO on plasma TAG and lipoproteins in salmonids are inconclusive, showing no effect(Reference Torstensen, Lie and Frøyland33) or decreased plasma lipids and plasma LDL(Reference Jordal, Lie and Torstensen4) in Atlantic salmon when replacing 100 % of FO with VO. Published results from trial 1(Reference Torstensen, Espe and Stubhaug3) have shown higher levels of TAG in liver and plasma as well as more visceral fat in fish fed the HPP-HVO diet than in the other groups from trial 1, indicating an interaction effect on adiposity when both PP and VO are included at high levels in the diet. From trial 1, we could thus observe increased liver TAG when increased amounts of VO were added to the diet, but due to the design we could not determine whether this was due to phytosterols or PP in the diet. When combining data from trial 1 and those from trial 2, we could, because of the different design of the diets, separate these effects. The presence of a direct effect of the type of VO used on liver TAG is supported by the higher liver TAG content in the fish fed the PP-RO diet than in the other groups given the VO diet in trial 2. Methionine limitation in Atlantic salmon leads to increased fas activity and accumulation of TAG in liver(Reference Espe, Rathore and Du31), and taurine supplementation reduces the body lipid:protein ratio(Reference Espe, Ruohonen and El-Mowafi18). However, in both trials, the fish were fed diets containing adequate methionine (>2·2 g/16 g N(34)), and the differences in liver TAG did not correlate with dietary taurine. Low levels of taurine in addition to low n-3 PUFA content in the diets with high VO inclusion might, however, have contributed towards increasing liver TAG together with other dietary components.
Sterol regulatory element-binding protein system
The PP-RO diet-fed fish had higher plasma and liver TAG than the fish fed any of the other diets in trial 2, indicating increased fat retention and/or higher liver FA synthesis in these fish. FA synthesis is controlled mainly by different types of srebp, which activate FA and cholesterol synthesis(Reference Horton, Goldstein and Brown14). In mammalian systems, three isotypes of srebp are described, where srebp1 C mainly activates TAG production, srebp2 cholesterol production, and srebp1 A both cholesterol synthesis and TAG synthesis(Reference Espenshade and Hughes6, Reference Horton, Goldstein and Brown14). srebp2 activates dhcr7, a rate-determining enzyme for cholesterol synthesis(Reference Horton, Goldstein and Brown14). The higher liver expression of both srebp2 and dhcr7 in the fish fed the PP-OO diet compared with the fish fed the PP-FO diet thus indicates increased cholesterol synthesis in the PP-OO diet-fed fish. This increase was not observed in the PP-RO diet- and PP-SO diet-fed fish. Intestinal npc1l1 is known to be important for sterol uptake in mammals(Reference Altmann, Davis and Zhu35). Provided that the function of npc1l1 in Atlantic salmon is similar to the function of that in mammals, the data from the present study demonstrate a lower ability to absorb sterols in the fish fed the PP-RO diet than in the other VO diet-fed fish in trial 2. This could be due to the high phytosterol content in this diet, consistent with the findings showing that β-sitosterol lowers npc1l1 expression as well as NPC1L1 protein levels in human enterocytic cells(Reference Jesch, Seo and Carr36).
Low cellular cholesterol concentrations activate srebp2, triggering the transcription of genes involved in the synthesis of cholesterol(Reference Horton, Goldstein and Brown14). Since the molecular structure of phytosterols is similar to that of cholesterol(Reference Moreau, Whitaker and Hicks7), it has been suggested that accumulated phytosterols in the tissues may act as cholesterol mimics and prevent the activation of srebp2. This theory is supported by the work showing that some phytosterols inhibit srebp2 processing, and thus also cholesterol production, when accumulated in the mouse adrenal gland(Reference Yang, Yu and Li13). The PP-RO diet- and PP-SO diet-fed fish had a higher liver phytosterol:cholesterol ratio than the PP-OO diet-fed fish, which could explain why the PP-RO diet- and PP-SO diet-fed fish did not exhibit increased cholesterol production to counteract the low cholesterol concentrations in the diet as observed in the fish fed the PP-OO diet. The dietary cholesterol concentration was 33 % lower in the PP-FO diet compared with the FM-FO diet. However, no increase in the expression of genes involved in the uptake or synthesis of cholesterol indicates that no cholesterol limitation was experienced by the group given the PP-FO diet. A similar connection between the replacement of FO with VO and the expression of srebp2 was, however, not observed in trial 1.
It thus appears that the fish fed the PP-OO and PP-SO diets regulate their cholesterol homeostasis by absorbing more and/or producing more cholesterol via the npc1l1 and srebp2 systems. High tissue concentrations of phytosterols may inhibit the maturation of the srebp2 protein in the PP-RO diet- and PP-SO diet-fed fish, thus preventing an increase in endogenous cholesterol synthesis. In addition, the high dietary phytosterol in the PP-RO diet prevents the up-regulation of npc1l1, probably leading to lower cholesterol uptake. The ratio of phytosterol:cholesterol in the liver could also be important for regulating sterol metabolism.
Regulation of cholesterol levels
While plasma cholesterol concentrations were maintained stable throughout the trials, liver cholesterol varied over time in trial 2. The high expression of cyp7a1, an enzyme important for converting cholesterol into bile acids, in the liver of the PP-RO diet-fed fish at week 11 coincided with the depletion of cholesterol in the same organ, and an accumulation of bile acids was still visible 17 weeks later. High levels of bile acids in the liver coincided with a very low cyp7a1 expression in the PP-RO diet-fed fish, indicating a negative feedback similar to that found in mammals, where bile acids inhibit cyp7a1 expression(Reference Chiang37). The main controller of cholesterol efflux, lxr, showed the same pattern, although not as markedly as cyp7a1. The highly varying expression of lxr and cyp7a1 in the fish fed the PP-RO diet could imply that these fish are struggling to maintain stable cholesterol levels. In addition, lxr is an activator of srebp1 C(Reference Repa, Liang and Ou38), which promotes FA synthesis, and could therefore have contributed to the increased TAG accumulation in the liver of the PP-RO diet-fed fish. The two isotypes of srebp1 mRNA (srebp1 A and srebp1 C) have not yet been described in Atlantic salmon and the primer used was a section of mRNA common for both isomers. This makes it difficult to say whether the increased TAG accumulation in the PP-RO diet-fed fish really is due to the activity of srebp1 C.
Conclusions
Cholesterol and lipid metabolism in Atlantic salmon was clearly affected by the replacement of 40 % of FM with PP together with dietary VO. The mammalian metabolism of cholesterol and lipids is connected through pathways such as the srebp and lxr systems(Reference Espenshade and Hughes6, Reference Janowski, Willy and Devi39), which also seems to be the case for salmonids according to the present and other studies performed(Reference Leaver, Villeneuve and Obach5, Reference Minghetti, Leaver and Tocher40, Reference Cruz-Garcia, Sanchez-Gurmaches and Gutierrez41). We have demonstrated here similarities between the accumulation and excretion of cholesterol in Atlantic salmon and those of cholesterol in the mammalian system. Tissue cholesterol and plasma cholesterol are regulated in Atlantic salmon by inhibiting the production and excretion of bile acids in the liver in case of low cholesterol, thereby increasing the retention of cholesterol. When the supply of dietary cholesterol is low, as observed in the high-phytosterol diet PP-RO, the regulation is more active. The activation of cholesterol metabolism could also affect the lxr system and thus affect the production and accumulation of TAG in the tissues. The effect of dietary phytosterols on cholesterol uptake and metabolism is clearly demonstrated, and has to be taken into account when determining optimal dietary cholesterol for Atlantic salmon in future studies.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513001347
Acknowledgements
Trial 1 was funded by the IP-EU project ‘AQUAMAX’ (016249-2). Trial 2 was funded by the Research Council of Norway (RCN) (‘SAFE FEED, SAFE AND HEALTHY SEAFOOD’, project no. 199626). We would like to thank Arnor Gullanger (IMR, Matre Aquaculture Research Station) and Thommy Holmvåg (Skretting ARC) for excellent fish husbandry. The technical assistance of Jacob Wessels (NIFES) is greatly appreciated. Technical staffs at NIFES are thanked for their excellent assistance with the chemical analysis. The authors' contributions were as follows: B. E. T., M. E., Ø. L., R. F., R. W. and G. R. designed the ideas and projects; B. E. T., M. E., N. S. L., G. R. and J. I. H. conducted the experiment and analyses; N. S. L., M. E., R. W. and B. E. T. wrote the paper; N. S. L. had primary responsibility for the final content. All authors contributed to the writing process and read and approved the final version of the paper. There are no conflicts of interest to report.